Efferocytosis during Skeletal Muscle Regeneration
Abstract
:1. Introduction
2. Pleiotropic Roles of Macrophages during Muscle Regeneration
2.1. Overview of Skeletal Muscle Regeneration
2.2. Cell Interactions Established by Macrophages to Sustain Skeletal Muscle Regeneration
3. The Resolution of Inflammation, through Efferocytosis, Is Necessary for Skeletal Muscle Regeneration
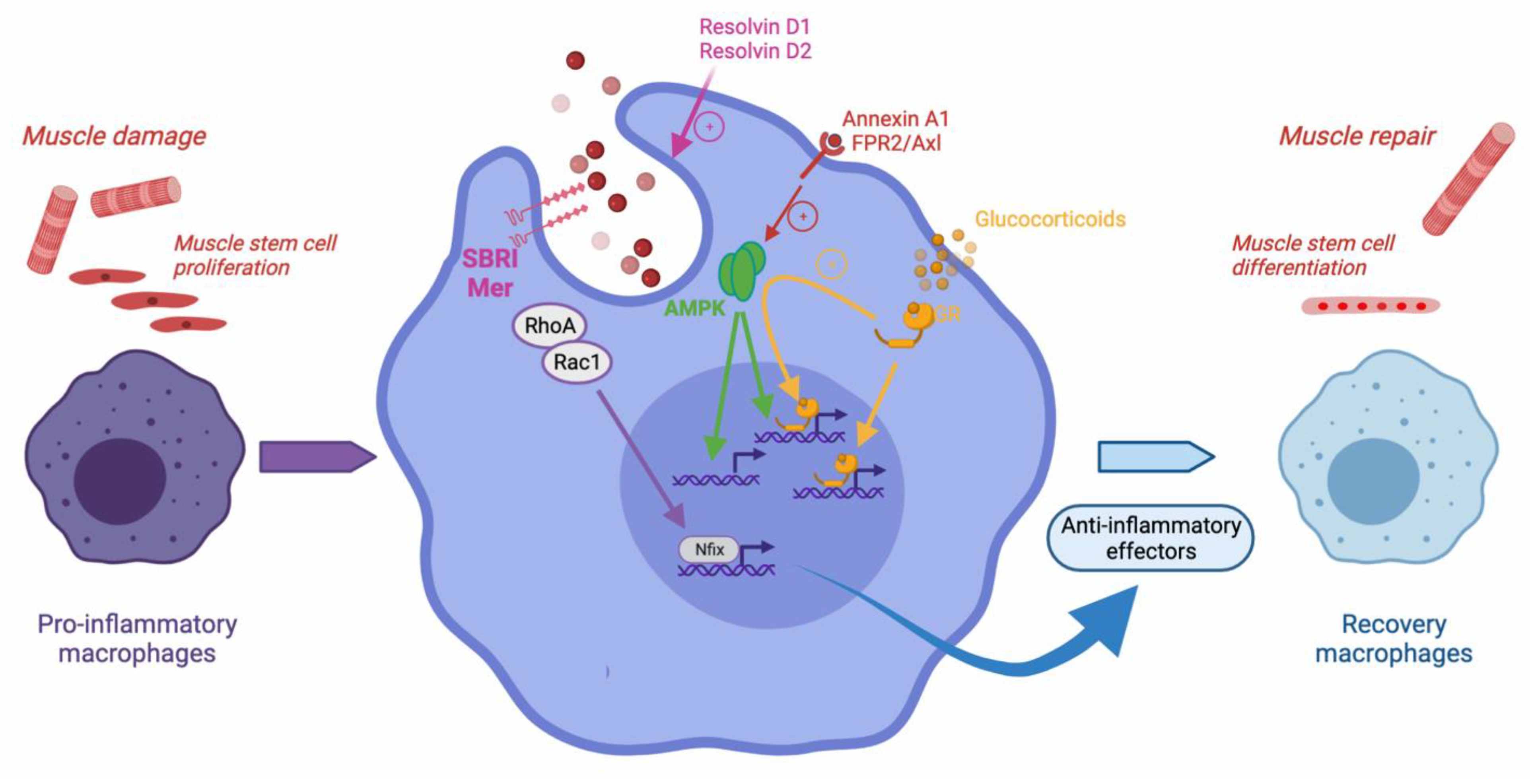
3.1. Macrophage Inflammatory Profile Shift and the Resolution of Inflammation during Skeletal Muscle Regeneration
3.2. Efferocytosis-Induced Phenotypic Shift of Macrophages during Muscle Regeneration
4. The Link between Efferocytosis and Cellular Metabolism
4.1. Metabolic Pathways Linked to Efferocytosis
4.2. Disposal/Recycling of Metabolites from Engulfed Cells
5. Immunometabolism, Efferocytosis, and Skeletal Muscle Regeneration
5.1. Efferocytosis and Metabolic Circuits in Macrophages during Muscle Regeneration
5.2. Modulation of Metabolism and Efferocytosis during Muscle Regeneration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fadok, V.A.; Bratton, D.L.; Guthrie, L.; Henson, P.M. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: Role of proteases. J. Immunol. 2001, 166, 6847–6854. [Google Scholar] [CrossRef]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving tgf-beta, pge2, and paf. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef] [Green Version]
- McDonald, P.P.; Fadok, V.A.; Bratton, D.; Henson, P.M. Transcriptional and translational regulation of inflammatory mediator production by endogenous tgf-beta in macrophages that have ingested apoptotic cells. J. Immunol. 1999, 163, 6164–6172. [Google Scholar]
- Godman, G.C. On the regeneration and redifferentiation of mammalian striated muscle. J. Morphol. 1957, 100, 27–81. [Google Scholar] [CrossRef]
- McLennan, I.S. Resident macrophages (ed2- and ed3-positive) do not phagocytose degenerating rat skeletal muscle fibres. Cell Tissue Res. 1993, 272, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, M.B.; Tajbakhsh, S. Regulation and phylogeny of skeletal muscle regeneration. Dev. Biol. 2018, 433, 200–209. [Google Scholar] [CrossRef]
- Mashinchian, O.; Pisconti, A.; Le Moal, E.; Bentzinger, C.F. The muscle stem cell niche in health and disease. Curr. Top. Dev. Biol. 2018, 126, 23–65. [Google Scholar] [PubMed]
- Panci, G.; Chazaud, B. Inflammation during post-injury skeletal muscle regeneration. Semin. Cell Dev. Biol. 2021, 119, 32–38. [Google Scholar] [CrossRef]
- Varga, T.; Mounier, R.; Horvath, A.; Cuvellier, S.; Dumont, F.; Poliska, S.; Ardjoune, H.; Juban, G.; Nagy, L.; Chazaud, B. Highly dynamic transcriptional signature of distinct macrophage subsets during sterile inflammation, resolution, and tissue repair. J. Immunol. 2016, 196, 4771–4782. [Google Scholar] [CrossRef] [Green Version]
- Saclier, M.; Yacoub-Youssef, H.; Mackey, A.L.; Arnold, L.; Ardjoune, H.; Magnan, M.; Sailhan, F.; Chelly, J.; Pavlath, G.K.; Mounier, R.; et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 2013, 31, 384–396. [Google Scholar] [CrossRef] [Green Version]
- Mackey, A.L.; Kjaer, M. The breaking and making of healthy adult human skeletal muscle in vivo. Skelet Muscle 2017, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1071–1081. [Google Scholar] [CrossRef] [Green Version]
- Varga, T.; Mounier, R.; Gogolak, P.; Poliska, S.; Chazaud, B.; Nagy, L. Tissue lyc6- macrophages are generated in the absence of circulating lyc6- monocytes and nur77 in a model of muscle regeneration. J. Immunol. 2013, 191, 5695–5701. [Google Scholar] [CrossRef] [Green Version]
- Baht, G.S.; Bareja, A.; Lee, D.E.; Rao, R.R.; Huang, R.; Huebner, J.L.; Bartlett, D.B.; Hart, C.R.; Gibson, J.R.; Lanza, I.R.; et al. Meteorin-like facilitates skeletal muscle repair through a stat3/igf-1 mechanism. Nat. Metab. 2020, 2, 278–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McArthur, S.; Juban, G.; Gobbetti, T.; Desgeorges, T.; Theret, M.; Gondin, J.; Toller-Kawahisa, J.E.; Reutelingsperger, C.P.; Chazaud, B.; Perretti, M.; et al. Annexin a1 drives macrophage skewing to accelerate muscle regeneration through ampk activation. J. Clin. Investig. 2020, 130, 1156–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mounier, R.; Theret, M.; Arnold, L.; Cuvellier, S.; Bultot, L.; Goransson, O.; Sanz, N.; Ferry, A.; Sakamoto, K.; Foretz, M.; et al. Ampkalpha1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013, 18, 251–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdiguero, E.; Sousa-Victor, P.; Ruiz-Bonilla, V.; Jardi, M.; Caelles, C.; Serrano, A.L.; Munoz-Canoves, P. P38/mkp-1-regulated akt coordinates macrophage transitions and resolution of inflammation during tissue repair. J. Cell Biol. 2011, 195, 307–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruffell, D.; Mourkioti, F.; Gambardella, A.; Kirstetter, P.; Lopez, R.G.; Rosenthal, N.; Nerlov, C. A creb-c/ebpbeta cascade induces m2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. USA 2009, 106, 17475–17480. [Google Scholar] [CrossRef] [Green Version]
- Saclier, M.; Lapi, M.; Bonfanti, C.; Rossi, G.; Antonini, S.; Messina, G. The transcription factor nfix requires rhoa-rock1 dependent phagocytosis to mediate macrophage skewing during skeletal muscle regeneration. Cells 2020, 9, 708. [Google Scholar] [CrossRef] [Green Version]
- Odaka, C.; Mizuochi, T.; Yang, J.; Ding, A. Murine macrophages produce secretory leukocyte protease inhibitor during clearance of apoptotic cells: Implications for resolution of the inflammatory response. J. Immunol. 2003, 171, 1507–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St Pierre, B.A.; Tidball, J.G. Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. J. Appl. Physiol. 1994, 77, 290–297. [Google Scholar] [CrossRef] [PubMed]
- McLennan, I.S. Degenerating and regenerating skeletal muscles contain several subpopulations of macrophages with distinct spatial and temporal distributions. J. Anat. 1996, 188, 17–28. [Google Scholar] [PubMed]
- Arnold, L.; Perrin, H.; de Chanville, C.B.; Saclier, M.; Hermand, P.; Poupel, L.; Guyon, E.; Licata, F.; Carpentier, W.; Vilar, J.; et al. Cx3cr1 deficiency promotes muscle repair and regeneration by enhancing macrophage apoe production. Nat. Commun. 2015, 6, 8972. [Google Scholar] [CrossRef] [Green Version]
- Grainger, D.J.; Reckless, J.; McKilligin, E. Apolipoprotein e modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein e-deficient mice. J. Immunol. 2004, 173, 6366–6375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Qu, C.; Li, T.; Cui, W.; Wang, X.; Du, J. Phagocytosis mediated by scavenger receptor class bi promotes macrophage transition during skeletal muscle regeneration. J. Biol. Chem. 2019, 294, 15672–15685. [Google Scholar] [CrossRef]
- Al-Zaeed, N.; Budai, Z.; Szondy, Z.; Sarang, Z. Tam kinase signaling is indispensable for proper skeletal muscle regeneration in mice. Cell Death Dis. 2021, 12, 611. [Google Scholar] [CrossRef]
- Van den Bossche, J.; O’Neill, L.A.; Menon, D. Macrophage immunometabolism: Where are we going? Trends Immunol. 2017, 38, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.D.; Chandel, N.S. Immunometabolism of pro-repair cells. J. Clin. Investig. 2019, 130, 2597–2607. [Google Scholar] [CrossRef] [Green Version]
- Juban, G. Transcriptional control of macrophage inflammatory shift during skeletal muscle regeneration. Semin. Cell Dev. Biol. 2021, 119, 82–88. [Google Scholar] [CrossRef]
- Morioka, S.; Perry, J.S.A.; Raymond, M.H.; Medina, C.B.; Zhu, Y.; Zhao, L.; Serbulea, V.; Onengut-Gumuscu, S.; Leitinger, N.; Kucenas, S.; et al. Efferocytosis induces a novel slc program to promote glucose uptake and lactate release. Nature 2018, 563, 714–718. [Google Scholar] [CrossRef]
- Park, D.; Han, C.Z.; Elliott, M.R.; Kinchen, J.M.; Trampont, P.C.; Das, S.; Collins, S.; Lysiak, J.J.; Hoehn, K.L.; Ravichandran, K.S. Continued clearance of apoptotic cells critically depends on the phagocyte ucp2 protein. Nature 2011, 477, 220–224. [Google Scholar] [CrossRef]
- Wang, Y.; Subramanian, M.; Yurdagul, A., Jr.; Barbosa-Lorenzi, V.C.; Cai, B.; de Juan-Sanz, J.; Ryan, T.A.; Nomura, M.; Maxfield, F.R.; Tabas, I. Mitochondrial fission promotes the continued clearance of apoptotic cells by macrophages. Cell 2017, 171, 331–345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Weinberg, S.; DeBerge, M.; Gainullina, A.; Schipma, M.; Kinchen, J.M.; Ben-Sahra, I.; Gius, D.R.; Yvan-Charvet, L.; Chandel, N.S.; et al. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab. 2019, 29, 443–456.e445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidani, Y.; Bensinger, S.J. Liver x receptor and peroxisome proliferator-activated receptor as integrators of lipid homeostasis and immunity. Immunol. Rev. 2012, 249, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Kiss, R.S.; Elliott, M.R.; Ma, Z.; Marcel, Y.L.; Ravichandran, K.S. Apoptotic cells induce a phosphatidylserine-dependent homeostatic response from phagocytes. Curr. Biol. 2006, 16, 2252–2258. [Google Scholar] [CrossRef] [Green Version]
- Varga, T.; Mounier, R.; Patsalos, A.; Gogolak, P.; Peloquin, M.; Horvath, A.; Pap, A.; Daniel, B.; Nagy, G.; Pintye, E.; et al. Macrophage ppargamma, a lipid activated transcription factor controls the growth factor gdf3 and skeletal muscle regeneration. Immunity 2016, 45, 1038–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukundan, L.; Odegaard, J.I.; Morel, C.R.; Heredia, J.E.; Mwangi, J.W.; Ricardo-Gonzalez, R.R.; Goh, Y.P.; Eagle, A.R.; Dunn, S.E.; Awakuni, J.U.; et al. Ppar-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat. Med. 2009, 15, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Holst, D.; Luquet, S.; Nogueira, V.; Kristiansen, K.; Leverve, X.; Grimaldi, P.A. Nutritional regulation and role of peroxisome proliferator-activated receptor delta in fatty acid catabolism in skeletal muscle. Biochim. Biophys. Acta 2003, 1633, 43–50. [Google Scholar] [CrossRef]
- Giannakis, N.; Sansbury, B.E.; Patsalos, A.; Hays, T.T.; Riley, C.O.; Han, X.; Spite, M.; Nagy, L. Dynamic changes to lipid mediators support transitions among macrophage subtypes during muscle regeneration. Nat. Immunol. 2019, 20, 626–636. [Google Scholar] [CrossRef]
- Bae, H.B.; Zmijewski, J.W.; Deshane, J.S.; Tadie, J.M.; Chaplin, D.D.; Takashima, S.; Abraham, E. Amp-activated protein kinase enhances the phagocytic ability of macrophages and neutrophils. FASEB J. 2011, 25, 4358–4368. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Park, D.W.; Stigler, W.S.; Creighton, J.; Ravi, S.; Darley-Usmar, V.; Zmijewski, J.W. Mitochondria and amp-activated protein kinase-dependent mechanism of efferocytosis. J. Biol. Chem. 2013, 288, 26013–26026. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.; Min, C.; Kim, G.; Kim, D.; Kim, K.; Lee, S.A.; Moon, B.; Yang, S.; Lee, J.; Yang, S.J.; et al. Crbn modulates calcium influx by regulating orai1 during efferocytosis. Nat. Commun. 2020, 11, 5489. [Google Scholar] [CrossRef] [PubMed]
- Gronski, M.A.; Kinchen, J.M.; Juncadella, I.J.; Franc, N.C.; Ravichandran, K.S. An essential role for calcium flux in phagocytes for apoptotic cell engulfment and the anti-inflammatory response. Cell Death Differ. 2009, 16, 1323–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, R.M.; Warunek, J.; Wohlfert, E.A. Chronic infection stunts macrophage heterogeneity and disrupts immune-mediated myogenesis. JCI Insight 2018, 3, e121549. [Google Scholar] [CrossRef] [Green Version]
- Dadgar, S.; Wang, Z.; Johnston, H.; Kesari, A.; Nagaraju, K.; Chen, Y.W.; Hill, D.A.; Partridge, T.A.; Giri, M.; Freishtat, R.J.; et al. Asynchronous remodeling is a driver of failed regeneration in duchenne muscular dystrophy. J. Cell Biol. 2014, 207, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Desgeorges, T.; Caratti, G.; Mounier, R.; Tuckermann, J.; Chazaud, B. Glucocorticoids shape macrophage phenotype for tissue repair. Front. Immunol. 2019, 10, 1591. [Google Scholar] [CrossRef]
- Michlewska, S.; Dransfield, I.; Megson, I.L.; Rossi, A.G. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: Key role for tnf-alpha. FASEB J. 2009, 23, 844–854. [Google Scholar] [CrossRef] [Green Version]
- Heasman, S.J.; Giles, K.M.; Rossi, A.G.; Allen, J.E.; Haslett, C.; Dransfield, I. Interferon gamma suppresses glucocorticoid augmentation of macrophage clearance of apoptotic cells. Eur. J. Immunol. 2004, 34, 1752–1761. [Google Scholar] [CrossRef]
- Giles, K.M.; Ross, K.; Rossi, A.G.; Hotchin, N.A.; Haslett, C.; Dransfield, I. Glucocorticoid augmentation of macrophage capacity for phagocytosis of apoptotic cells is associated with reduced p130cas expression, loss of paxillin/pyk2 phosphorylation, and high levels of active rac. J. Immunol. 2001, 167, 976–986. [Google Scholar] [CrossRef] [Green Version]
- Caratti, G.; Desgeorges, T.; Juban, G.; Koenen, M.; Kozak, B.; Théret, M.; Chazaud, B.; Tuckermann, J.P.; Mounier, R. Ampkα1 is essential for glucocorticoid receptor triggered anti-inflammatory macrophage activation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Maderna, P.; Yona, S.; Perretti, M.; Godson, C. Modulation of phagocytosis of apoptotic neutrophils by supernatant from dexamethasone-treated macrophages and annexin-derived peptide ac(2-26). J. Immunol. 2005, 174, 3727–3733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perretti, M.; D’Acquisto, F. Annexin a1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009, 9, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, B. Inflammation and skeletal muscle regeneration: Leave it to the macrophages! Trends Immunol. 2020, 41, 481–492. [Google Scholar] [CrossRef]
- Markworth, J.F.; Brown, L.A.; Lim, E.; Floyd, C.; Larouche, J.; Castor-Macias, J.A.; Sugg, K.B.; Sarver, D.C.; Macpherson, P.C.D.; Davis, C.S.; et al. Resolvin d1 supports skeletal myofiber regeneration via actions on myeloid and muscle stem cells. JCI Insight 2020, 5, e137713. [Google Scholar] [CrossRef] [PubMed]
- Rothlin, C.V.; Hille, T.D.; Ghosh, S. Determining the effector response to cell death. Nat. Rev. Immunol. 2020, 21, 292–304. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juban, G.; Chazaud, B. Efferocytosis during Skeletal Muscle Regeneration. Cells 2021, 10, 3267. https://doi.org/10.3390/cells10123267
Juban G, Chazaud B. Efferocytosis during Skeletal Muscle Regeneration. Cells. 2021; 10(12):3267. https://doi.org/10.3390/cells10123267
Chicago/Turabian StyleJuban, Gaëtan, and Bénédicte Chazaud. 2021. "Efferocytosis during Skeletal Muscle Regeneration" Cells 10, no. 12: 3267. https://doi.org/10.3390/cells10123267
APA StyleJuban, G., & Chazaud, B. (2021). Efferocytosis during Skeletal Muscle Regeneration. Cells, 10(12), 3267. https://doi.org/10.3390/cells10123267





