Regenerating Skeletal Muscle Compensates for the Impaired Macrophage Functions Leading to Normal Muscle Repair in Retinol Saturase Null Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Animals
2.3. Cardiotoxin-Induced Muscle Injury Model
2.4. Hematoxylin/Eosin and Immunofluorescent Staining of the Regenerating Muscle
2.5. Quantification of Necrotic Areas
2.6. Isolation of Muscle-Derived CD45+ Leukocytes
2.7. Generation of Bone Marrow-Derived Macrophages (BMDMs) for NEO Cassette Expression Analysis
2.8. Gene Expression Analysis
2.9. Quantification of Satellite and Fibrocyte-Adipocyte Progenitor Cells in the TA Muscle Following CTX-Induced Injury
2.10. Quantification of Intramuscular Immune Cells by Flow Cytometry
2.11. In Vitro Phagocytosis Assay by F4/80+ Cells
2.12. Statistical Analysis
3. Results and Discussion
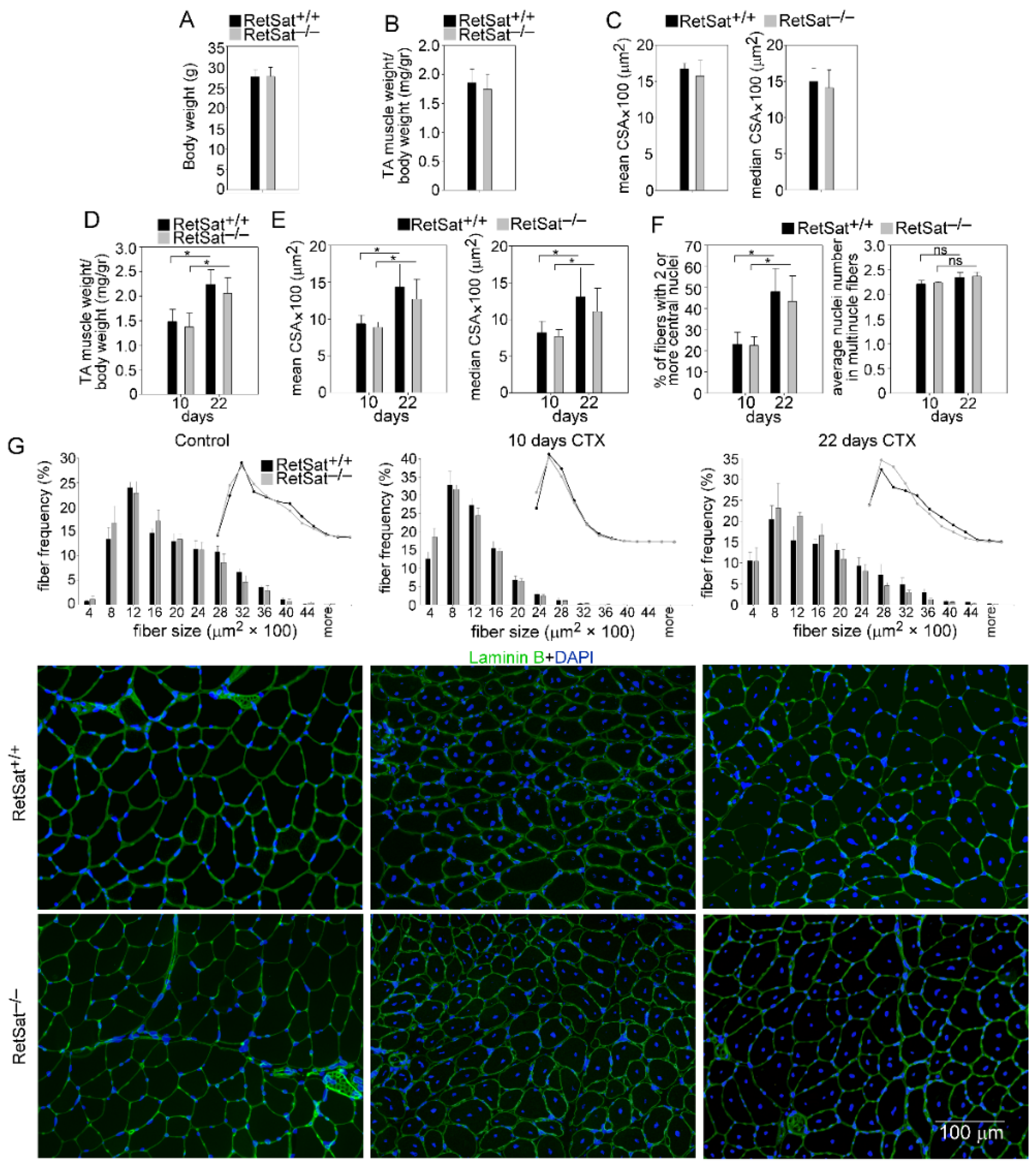
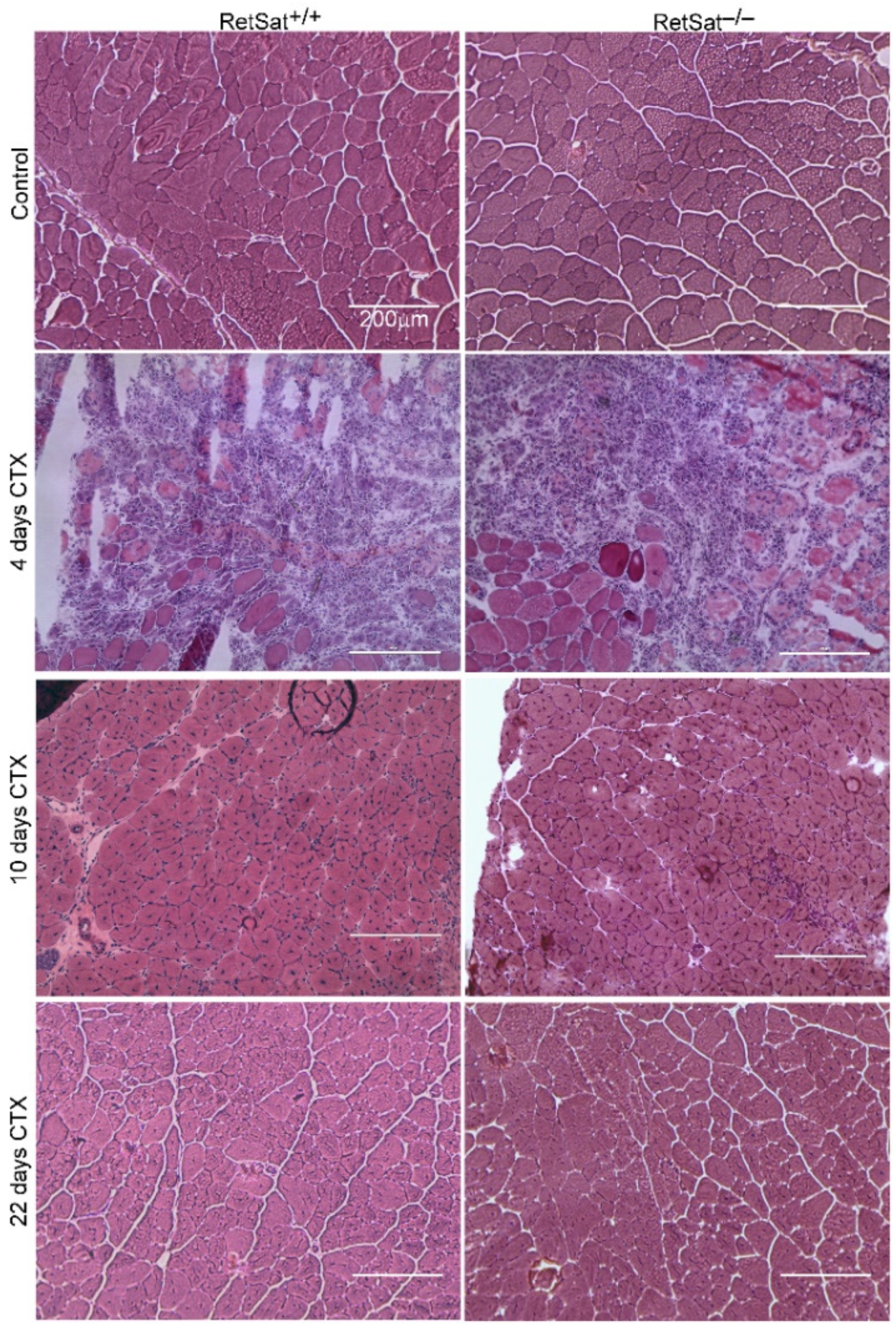
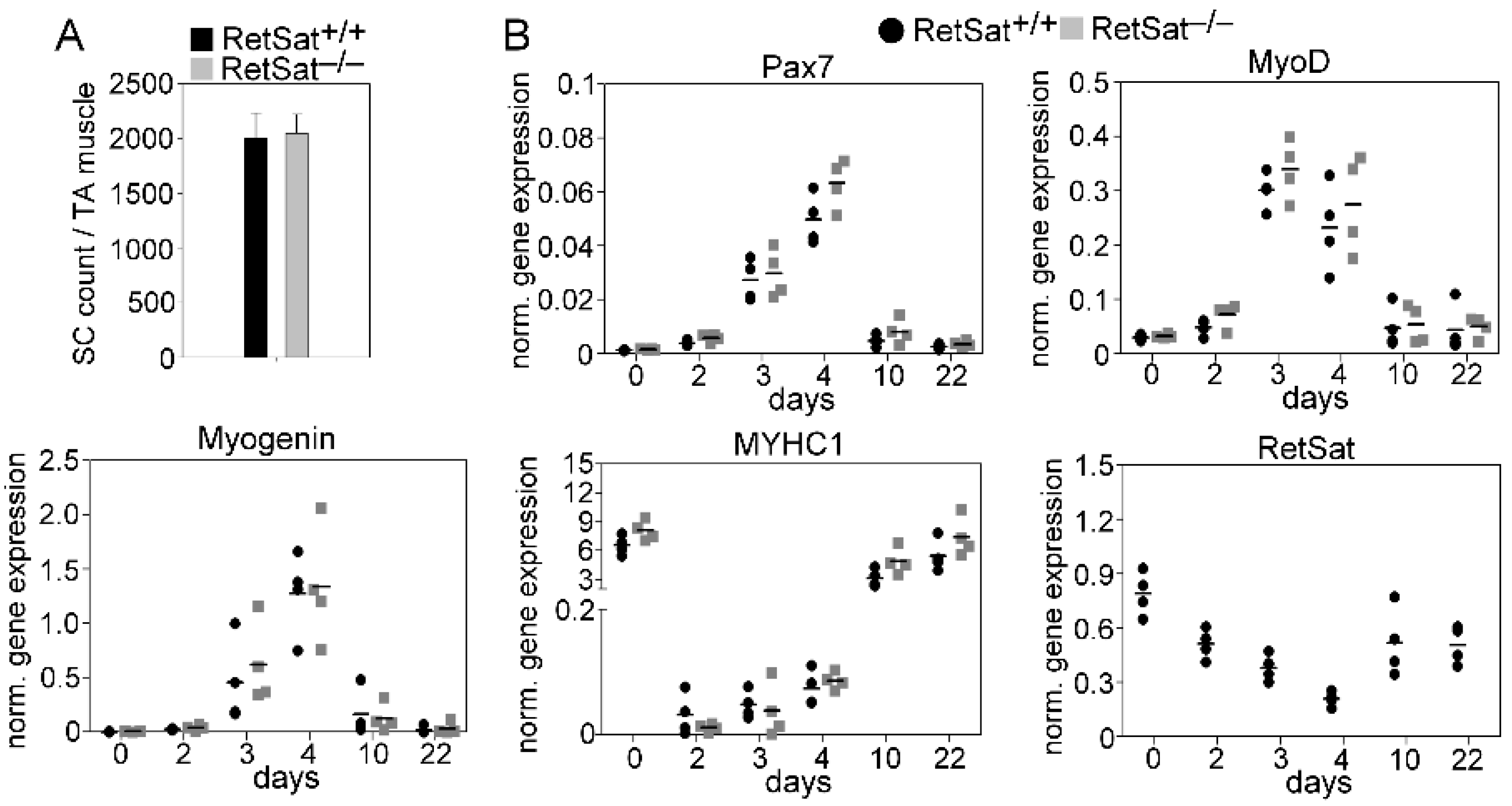
3.1. Loss of RetSat Does Not Alter the Regeneration Program in the Tibialis Anterior Muscle Following Cardiotoxin Injury
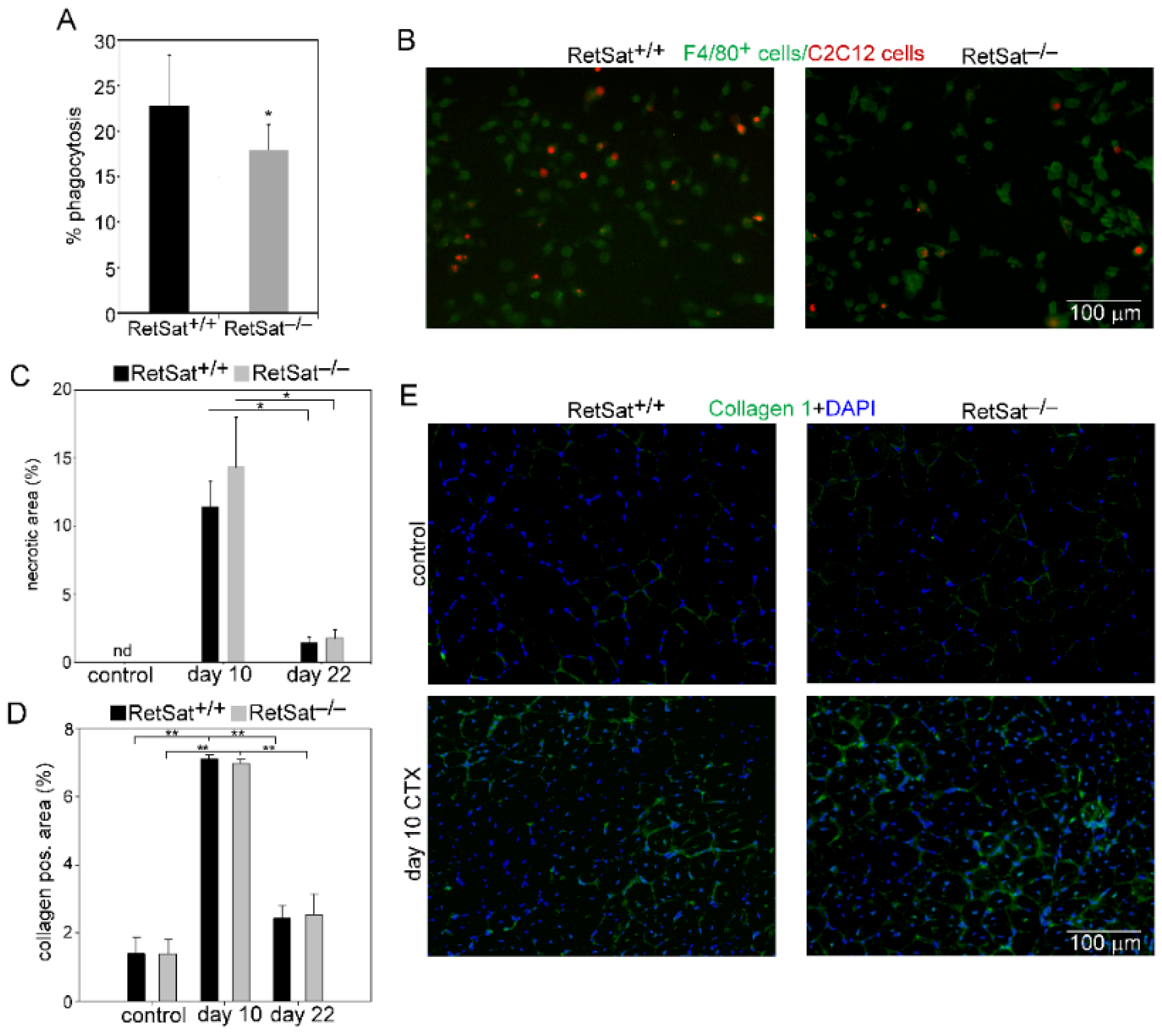
3.2. Decreased Recruitment of Mϕs and Neutrophils after Injury in the Absence of RetSat
3.3. Myoblasts Compensate for Attenuated MFG-E8 Levels of Macrophages in RetSat-Null Mice
3.4. Altered NPY Levels Both in Mϕs and in the Skeletal Muscle of RetSat-Null Mice
3.5. A Transient Delay in the M1/M2 Phenotypic Switch in Mϕs of RetSat-Null Mice during the Muscle Regeneration Process
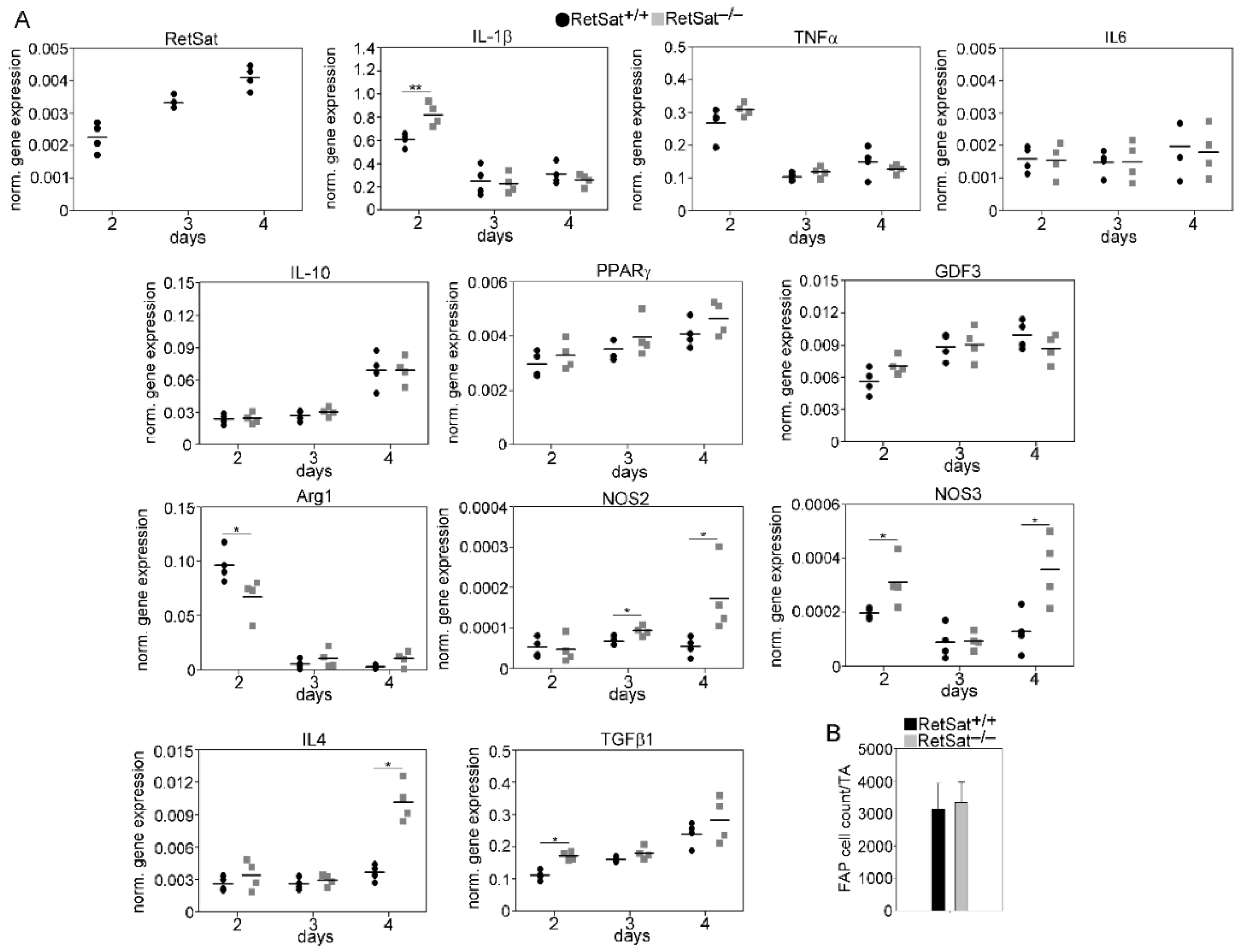
3.6. mRNA Expression of RetSat, Various Cytokines, and Growth Factors in the CD45+ Macrophages Derived from the Regenerating TA Muscles of RetSat+/+ and RetSat−/− Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tedesco, F.S.; Dellavalle, A.; Diaz-Manera, J.; Messina, G.; Cossu, G. Repairing skeletal muscle: Regenerative potential of skeletal muscle stem cells. J. Clin. Investig. 2010, 120, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, M.B.; Tajbakhsh, S. Regulation and phylogeny of skeletal muscle regeneration. Dev. Biol. 2018, 433, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hu, P. Skeletal muscle regeneration is modulated by inflammation. J. Orthop. Translat. 2018, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Bazgir, B.; Fathi, R.; Valojerdi, M.R.; Mozdziak, P.; Asgari, A. Satellite Cells Contribution to Exercise Mediated Muscle Hypertrophy and Repair. Cell J. 2017, 18, 473–484. [Google Scholar] [PubMed]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1071–1081. [Google Scholar] [CrossRef]
- Giannakis, N.; Sansbury, B.E.; Patsalos, A.; Hays, T.T.; Riley, C.O.; Han, X.; Spite, M.; Nagy, L. Dynamic changes to lipid mediators support transitions among macrophage subtypes during muscle regeneration. Nat. Immunol. 2019, 20, 626–636. [Google Scholar] [CrossRef]
- Strle, K.; McCusker, R.H.; Johnson, R.W.; Zunich, S.M.; Dantzer, R.; Kelley, K.W. Prototypical anti-inflammatory cytokine IL-10 prevents loss of IGF-I-induced myogenin protein expression caused by IL-1beta. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E709–E718. [Google Scholar] [CrossRef][Green Version]
- Kim, J.; Lee, J. Role of transforming growth factor-β in muscle damage and regeneration: Focused on eccentric muscle contraction. J. Excerc. Rehabil. 2017, 13, 621–626. [Google Scholar] [CrossRef]
- Varga, T.; Mounier, R.; Patsalos, A.; Gogolák, P.; Peloquin, M.; Horvath, A.; Pap, A.; Daniel, B.; Nagy, G.; Pintye, E.; et al. Macrophage PPARγ, a Lipid Activated Transcription Factor Controls the Growth Factor GDF3 and Skeletal Muscle Regeneration. Immunity 2016, 49, 615–626. [Google Scholar] [CrossRef]
- Chazaud, B. Inflammation and skeletal muscle regeneration: Leave it to the macrophages. Trends. Immunol. 2020, 6, 481–492. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, C.; Li, T.; Cui, W.; Wang, X.; Du, J. Phagocytosis mediated by scavenger receptor class BI promotes macrophage transition during skeletal muscle regeneration. J. Biol. Chem. 2019, 294, 15672–15685. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaeed, N.; Budai, Z.; Szondy, Z.; Sarang, Z. TAM kinase signaling is indispensable for the proper skeletal muscle regeneration process. Cell. Death. Dis. 2021, 12, 611. [Google Scholar] [CrossRef] [PubMed]
- Budai, Z.; Al-Zaeed, N.; Szentesi, P.; Halász, H.; Csernoch, L.; Szondy, Z.; Sarang, Z. Impaired Skeletal Muscle Development and Regeneration in Transglutaminase 2 Knockout Mice. Cells 2021, 10, 3089. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 48, 2207–2216. [Google Scholar]
- Jeong, J.; Conboy, I.M. Phosphatidylserine directly and positively regulates fusion of myoblasts into myotubes. Biochem. Biophys. Res. Commun. 2011, 414, 9–13. [Google Scholar] [CrossRef]
- Hochreiter-Hufford, A.E.; Lee, C.S.; Kinchen, J.M.; Sokolowski, J.D.; Arandjelovic, S.; Call, J.A.; Klibanov, A.L.; Yan, Z.; Mandell, J.W.; Ravichandran, K.S. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature 2011, 497, 263–267. [Google Scholar] [CrossRef]
- Park, S.Y.; Yun, Y.; Lim, J.S.; Kim, M.J.; Kim, S.Y.; Kim, J.E.; Kim, I.S. Stabilin-2 modulates the efficiency of myoblast fusion during myogenic differentiation and muscle regeneration. Nat. Commun. 2016, 7, 10871. [Google Scholar] [CrossRef]
- Blaschuk, K.L.; Guérin, C.; Holland, P.C. Myoblast alpha v beta3 integrin levels are controlled by transcriptional regulation of expression of the beta3 subunit and down-regulation of beta3 subunit expression is required for skeletal muscle cell differentiation. Dev. Biol. 1997, 184, 266–277. [Google Scholar] [CrossRef][Green Version]
- Hanayama, R.; Miyasaka, K.; Nakaya, M.; Nagata, S. MFG-E8-dependent clearance of apoptotic cells, and autoimmunity caused by its failure. Curr. Dir. Autoimmun. 2006, 9, 162–172. [Google Scholar]
- Chikazawa, M.; Shimizu, M.; Yamauchi, Y.; Sato, R. Bridging molecules are secreted from the skeletal muscle and potentially regulate muscle differentiation. Biochem. Biophys. Res. Commun. 2020, 522, 113–120. [Google Scholar] [CrossRef]
- Hanayama, R.; Tanak, M.; Miwa, K.; Shinohara, A.; Iwamatsu, A.; Nagata, S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002, 417, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Moise, A.R.; Domínguez, M.; Alvarez, S.; Alvarez, R.; Schupp, M.; Cristancho, A.G.; Kiser, P.D.; de Lera, A.R.; Lazar, M.A.; Palczewski, K. Stereospecificity of retinol saturase: Absolute configuration, synthesis, and biological evaluation of dihydroretinoids. J. Am. Chem. Soc. 2008, 130, 1154–1155. [Google Scholar] [CrossRef] [PubMed]
- Moise, A.R.; Alvarez, S.; Domínguez, M.; Alvarez, R.; Golczak, M.; Lobo, G.P.; von Lintig, J.; de Lera, A.R.; Palczewski, K. Activation of retinoic acid receptors by dihydroretinoids. Mol. Pharmacol. 2009, 76, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Rühl, R.; Krzyżosiak, A.; Niewiadomska-Cimicka, A.; Rochel, N.; Szeles, L.; Vaz, B.; Wietrzych-Schindler, M.; Álvarez, S.; Szklenar, M.; Nagy, L.; et al. 9-Cis-13,14-dihydroretinoic acid is an endogenous retinoid acting as RXR ligand in mice. PLoS Genet. 2015, 11, e1005213. [Google Scholar] [CrossRef]
- Sarang, Z.; Sághy, T.; Budai, Z.; Ujlaky-Nagy, L.; Bedekovics, J.; Beke, L.; Méhes, G.; Nagy, G.; Rühl, R.; Moise, A.R.; et al. Retinol Saturase Knock-Out Mice are Characterized by Impaired Clearance of Apoptotic Cells and Develop Mild Autoimmunity. Biomolecules 2019, 9, 737. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Stanojević, S.; Mitić, K.; Kustrimović, N.; Vujić, V.; Miletić, T.; Kovacević-Jovanović, V. The anti-inflammatory effect of neuropeptide Y (NPY) in rats is dependent on dipeptidyl peptidase 4 (DP4) activity and age. Peptides 2008, 29, 2179–2187. [Google Scholar] [CrossRef]
- Singer, K.; Morris, D.L.; Oatmen, K.E.; Wang, T.; DelProposto, J.; Mergian, T.; Cho, K.W.; Lumeng, C.N. Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PLoS ONE 2013, 8, e57929. [Google Scholar] [CrossRef]
- Tanaka, M.; Miyake, Y. Apoptotic cell clearance and autoimmune disorder. Curr. Med. Chem. 2007, 14, 2892–2897. [Google Scholar] [CrossRef]
- Moise, A.R.; Lobo, G.P.; Erokwu, B.; Wilson, D.L.; Peck, D.; Alvarez, S.; Domínguez, M.; Alvarez, R.; Flask, C.A.; de Lera, A.R.; et al. Increased adiposity in the retinol saturase-knockout mouse. FASEB J. 2010, 24, 1261–1270. [Google Scholar] [CrossRef]
- Budai, Z.; Újlaki-Nagy, L.; Kis, N.G.; Antal, M.; Bankó, C.; Szondy, Z.; Sarang, Z. Macrophages engulf apoptotic and primary necrotic thymocytes through similar phosphatidylserine-dependent mechanisms. FEBS Open Bio. 2019, 9, 446–456. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, H. Extracellular matrix: An important regulator of cell functions and skeletal muscle development. Cell Biosci. 2021, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.W.; Michalkiewicz, M.; Kitlinska, J.; Kalezic, I.; Switalska, H.; Yoo, P.; Sangkharat, A.; Ji, H.; Li, L.; Michalkiewicz, T.; et al. Neuropeptide Y induces ischemic angiogenesis and restores function of ischemic skeletal muscles. J. Clin. Investig. 2003, 111, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.proteinatlas.org/ENSG00000122585-NPY/tissue (accessed on 5 January 2022).
- Lundberg, J.M.; Rudehill, A.; Sollevi, A.; Fried, G.; Wallin, G. Co-release of neuropeptide Y and noradrenaline from pig spleen in vivo: Importance of subcellular storage, nerve impulse frequency and pattern, feedback regulation and resupply by axonal transport. Neuroscience 1989, 28, 475–486. [Google Scholar] [CrossRef]
- Farzi, A.; Reichmann, F.; Holzer, P. The homeostatic role of neuropeptide Y in immune function and its impact on mood and behaviour. Acta. Physiol. 2015, 213, 603–627. [Google Scholar] [CrossRef] [PubMed]
- Beitzel, F.; Sillence, M.N.; Lynch, G.S. Beta-Adrenoceptor signaling in regenerating skeletal muscle after beta-agonist administration. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E932–E940. [Google Scholar] [CrossRef] [PubMed]
- Laplante, P.; Brillant-Marquis, F.; Brissette, M.J.; Joannette-Pilon, B.; Cayrol, R.; Kokta, V.; Cailhier, J.F. MFG-E8 Reprogramming of Macrophages Promotes Wound Healing by Increased bFGF Production and Fibroblast Functions. J. Investig. Dermatol. 2017, 137, 2005–2013. [Google Scholar] [CrossRef]
- Sarang, Z.; Joós, G.; Garabuczi, E.; Rühl, R.; Gregory, C.D.; Szondy, Z. Macrophages Engulfing Apoptotic Cells Produce Non-classical Retinoids to Enhance Their Phagocytic Capacity. J. Immunol. 2014, 192, 5730–5738. [Google Scholar] [CrossRef]
- Chaweewannakorn, C.; Tsuchiya, M.; Koide, M.; Hatakeyama, H.; Tanaka, Y.; Yoshida, S.; Sugawara, S.; Hagiwara, Y.; Sasaki, K.; Kanzaki, M. Roles of IL-1α/β in regeneration of cardiotoxin-injured muscle and satellite cell function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R90–R103. [Google Scholar] [CrossRef]
- Anderson, J.E. Role for Nitric Oxide in Muscle Repair: Nitric Oxide–mediated Activation of Muscle Satellite Cells. Mol. Biol. Cell 2000, 11, 1859–1874. [Google Scholar] [CrossRef]
- Morbidelli, L.; Donnini, S.; Ziche, M. Role of nitric oxide in the modulation of angiogenesis. Curr. Pharm. Des. 2003, 9, 521–530. [Google Scholar] [CrossRef]
- Tyurina, Y.Y.; Basova, L.V.; Konduru, N.V.; Tyurin, V.A.; Potapovich, A.I.; Cai, P.; Bayir, H.; Stoyanovsky, D.; Pitt, B.R.; Shvedova, A.A.; et al. Nitrosative stress inhibits the aminophospholipid translocase resulting inphosphatidylserine externalization and macrophage en-gulfment: Implications for the resolution of inflammation. J. Biol. Chem. 2007, 282, 8498–8509. [Google Scholar] [CrossRef] [PubMed]
- Heredia, J.E.; Mukundan, L.; Chen, F.M.; Mueller, A.A.; Deo, R.C.; Locksley, R.M.; Rando, T.A.; Chawla, A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 2013, 153, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Theret, M.; Rossi, F.M.V.; Contreras, O. Evolving Roles of Muscle-Resident Fibro-Adipogenic Progenitors in Health, Regeneration, Neuromuscular Disorders, and Aging. Front. Physiol. 2021, 12, 673404. [Google Scholar] [CrossRef] [PubMed]
- Contreras, O.; Cruz-Soca, M.; Theret, M.; Soliman, H.; Tung, L.W.; Groppa, E.; Rossi, F.M.; Brandan, E. Cross-talk between TGF-β and PDGFRα signaling pathways regulates the fate of stromal fibro-adipogenic progenitors. J. Cell Sci. 2019, 132, jcs232157. [Google Scholar] [CrossRef]
- Wosczyna, M.N.; Rando, T.A. A Muscle Stem Cell Support Group: Coordinated Cellular Responses in Muscle Regeneration. Dev. Cell 2018, 46, 135–143. [Google Scholar] [CrossRef]
- Biferali, B.; Proietti, D.; Mozzetta, C.; Madaro, L. Fibro-Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front. Physiol. 2019, 10, 1074. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarban, N.; Halász, H.; Gogolák, P.; Garabuczi, É.; Moise, A.R.; Palczewski, K.; Sarang, Z.; Szondy, Z. Regenerating Skeletal Muscle Compensates for the Impaired Macrophage Functions Leading to Normal Muscle Repair in Retinol Saturase Null Mice. Cells 2022, 11, 1333. https://doi.org/10.3390/cells11081333
Tarban N, Halász H, Gogolák P, Garabuczi É, Moise AR, Palczewski K, Sarang Z, Szondy Z. Regenerating Skeletal Muscle Compensates for the Impaired Macrophage Functions Leading to Normal Muscle Repair in Retinol Saturase Null Mice. Cells. 2022; 11(8):1333. https://doi.org/10.3390/cells11081333
Chicago/Turabian StyleTarban, Nastaran, Hajnalka Halász, Péter Gogolák, Éva Garabuczi, Alexander R. Moise, Krzysztof Palczewski, Zsolt Sarang, and Zsuzsa Szondy. 2022. "Regenerating Skeletal Muscle Compensates for the Impaired Macrophage Functions Leading to Normal Muscle Repair in Retinol Saturase Null Mice" Cells 11, no. 8: 1333. https://doi.org/10.3390/cells11081333
APA StyleTarban, N., Halász, H., Gogolák, P., Garabuczi, É., Moise, A. R., Palczewski, K., Sarang, Z., & Szondy, Z. (2022). Regenerating Skeletal Muscle Compensates for the Impaired Macrophage Functions Leading to Normal Muscle Repair in Retinol Saturase Null Mice. Cells, 11(8), 1333. https://doi.org/10.3390/cells11081333







