Effect of BCG Revaccination on Occupationally Exposed Medical Personnel Vaccinated against SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Participants
2.2. Cytokine Immunoassays
2.3. Neutralizing Antibody Quantification
2.4. HLA Genotyping
2.5. Statistical Analysis
3. Results
3.1. Design and Participants
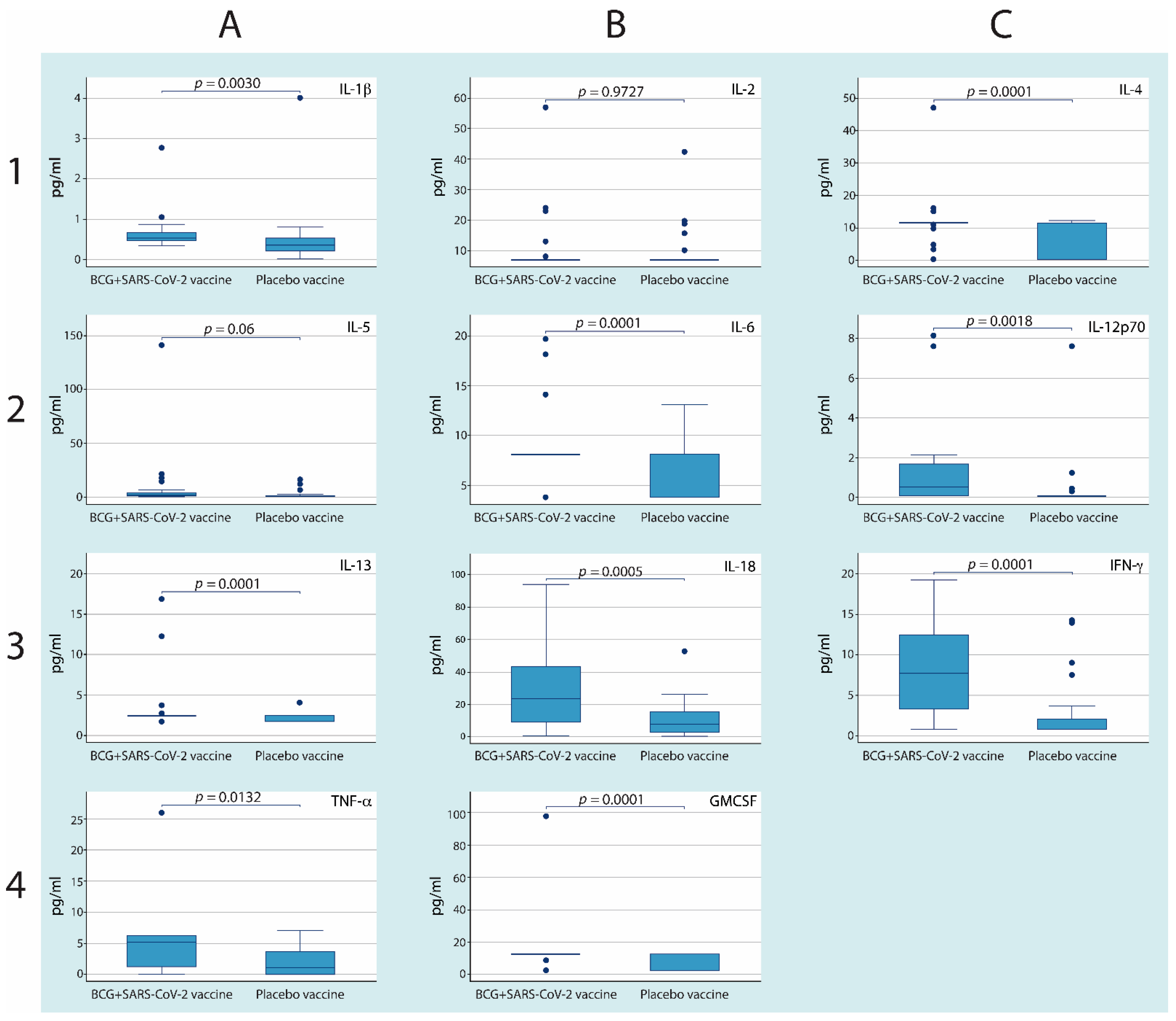
3.2. Revaccination with BCG with Subsequent Vaccination against SARS-CoV-2 Induces Higher Serum Cytokine Concentrations
3.3. Neutralizing Anti-SARS-CoV-2 Antibodies Were Higher in the Group of Participants with Revaccination with the BCG and Anti-SARS-CoV-2 Vaccines
3.4. HLA Genotyping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Short, R.K.; Kedzierska, K.; van de Sandt, C.E. Back to the Future: Lessons Learned From the 1918 Influenza Pandemic. Front. Cell. Infect. Microbiol. 2018, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Thone, M.N.; Kwon, Y.J. COVID-19 vaccines: The status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2021, 170, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Bala, A.; Sengupta, A.; Matsabisa, M.G.; Chabalala, H.P. COVID-19: Pathophysiology; Mechanism of Transmission and Possible Molecular Drug Target for Management. Curr. Mol. Pharmacol. 2020, 14, 4. [Google Scholar] [CrossRef]
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Simbana-Rivera, K.; Gomez-Barreno, L.; Rubio-Neira, M.; Guaman, L.P.; Kyriakidis, N.C.; Muslin, C.; Jaramillo, A.M.G.; Barba-Ostria, C.; Cevallos-Robalino, D.; et al. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn. Microbiol. Infect. Dis. 2020, 98, 115094. [Google Scholar] [CrossRef]
- Conceicao, C.; Thakur, N.; Human, S.; Kelly, J.T.; Logan, L.; Bialy, D.; Bhat, S.; Stevenson-Leggett, P.; Zagrajek, A.K.; Hollinghurst, P.; et al. The SARS-CoV-2 Spike protein has a broad tropism for mammalian ACE2 proteins. PLoS Biol. 2020, 18, e3001016. [Google Scholar] [CrossRef]
- He, Q.; Mao, Q.; Zhang, J.; Bian, L.; Gao, F.; Wang, J.; Xu, M.; Liang, Z. COVID-19 Vaccines: Current Understanding on Immunogenicity, Safety, and Further Considerations. Front. Immunol. 2021, 12, 669339. [Google Scholar] [CrossRef]
- Flanagan, K.L.; Best, E.; Crawford, N.W.; Giles, M.; Koirala, A.; Macartney, K.; Russell, F.; Teh, B.W.; Wen, S.C. Progress and Pitfalls in the Quest for Effective SARS-CoV-2 (COVID-19) Vaccines. Front. Immunol. 2020, 11, 579250. [Google Scholar] [CrossRef] [PubMed]
- Dye, C. Making wider use of the world’s most widely used vaccine: Bacille Calmette-Guerin revaccination reconsidered. J. R. Soc. Interface 2013, 10, 20130365. [Google Scholar] [CrossRef] [PubMed]
- Bhavanam, S.; Rayat, G.R.; Keelan, M.; Kunimoto, D.; Drews, S.J. Characterization of immune responses of human PBMCs infected with Mycobacterium tuberculosis H37Ra: Impact of donor declared BCG vaccination history on immune responses and M. tuberculosis growth. PLoS ONE 2018, 13, e0203822. [Google Scholar] [CrossRef] [PubMed]
- Buffen, K.; Oosting, M.; Quintin, J.; Ng, A.; Kleinnijenhuis, J.; Kumar, V.; van de Vosse, E.; Wijmenga, C.; van Crevel, R.; Oosterwijk, E.; et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog. 2014, 10, e1004485. [Google Scholar] [CrossRef]
- Stewart, J.H.T.; Levine, E.A. Role of bacillus Calmette-Guerin in the treatment of advanced melanoma. Expert Rev. Anticancer Ther. 2011, 11, 1671–1676. [Google Scholar] [CrossRef]
- Leentjens, J.; Kox, M.; Stokman, R.; Gerretsen, J.; Diavatopoulos, D.A.; van Crevel, R.; Rimmelzwaan, G.F.; Pickkers, P.; Netea, M.G. BCG Vaccination Enhances the Immunogenicity of Subsequent Influenza Vaccination in Healthy Volunteers: A Randomized, Placebo-Controlled Pilot Study. J. Infect. Dis. 2015, 212, 1930–1938. [Google Scholar] [CrossRef]
- Ocana-Guzman, R.; Tellez-Navarrete, N.A.; Preciado-Garcia, M.; Ponce-Gallegos, M.A.; Buendia-Roldan, I.; Falfan-Valencia, R.; Chavez-Galan, L. Multidrug-resistant tuberculosis patients expressing the HLA-DRB1*04 allele, and after treatment they show a low frequency of HLA-II+ monocytes and a chronic systemic inflammation. Microb. Pathog. 2021, 153, 104793. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.; Tiu, C.; Hu, Z.; Chen, V.C.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Morales-Nunez, J.J.; Munoz-Valle, J.F.; Meza-Lopez, C.; Wang, L.F.; Machado Sulbaran, A.C.; Torres-Hernandez, P.C.; Bedolla-Barajas, M.; la O-Gómez, D.; Balcazar-Felix, P.; Hernandez-Bello, J. Neutralizing Antibodies Titers and Side Effects in Response to BNT162b2 Vaccine in Healthcare Workers with and without Prior SARS-CoV-2 Infection. Vaccines 2021, 9, 742. [Google Scholar] [CrossRef]
- Dean, A.G.; Arner, A.T.; Sunki, G.G.; Friedman, R.; Lantinga, M.; Sangam, S.; Zubieta, J.C.; Sullivan, K.M.; Brendel, K.A.; Gao, Z.; et al. Epi Info, a Database and Statistics Program for Public Health Professionals; CDC: Atlanta, GA, USA, 2004. Available online: https://www.cdc.gov/epiinfo/index.html (accessed on 8 October 2021).
- Malik, Y.S.; Ansari, M.I.; Ganesh, B.; Sircar, S.; Bhat, S.; Pande, T.; Vinodhkumar, O.R.; Kumar, P.; Iqbal Yatoo, M.; Tiwari, R.; et al. BCG vaccine: A hope to control COVID-19 pandemic amid crisis. Hum. Vaccines Immunother. 2020, 16, 2954–2962. [Google Scholar] [CrossRef]
- Hensel, J.; McAndrews, K.M.; McGrail, D.J.; Dowlatshahi, D.P.; LeBleu, V.S.; Kalluri, R. Protection against SARS-CoV-2 by BCG vaccination is not supported by epidemiological analyses. Sci. Rep. 2020, 10, 18377. [Google Scholar] [CrossRef]
- Gong, W.; Aspatwar, A.; Wang, S.; Parkkila, S.; Wu, X. COVID-19 pandemic: SARS-CoV-2 specific vaccines and challenges, protection via BCG trained immunity, and clinical trials. Expert Rev. Vaccines 2021, 20, 857–880. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.K.; Yu, Q.; Salvador, C.E.; Melani, I.; Kitayama, S. Mandated Bacillus Calmette-Guerin (BCG) vaccination predicts flattened curves for the spread of COVID-19. Sci. Adv. 2020, 6, eabc1463. [Google Scholar] [CrossRef] [PubMed]
- Parlane, N.A.; Shu, D.; Subharat, S.; Wedlock, D.N.; Rehm, B.H.; de Lisle, G.W.; Buddle, B.M. Revaccination of cattle with bacille Calmette-Guerin two years after first vaccination when immunity has waned, boosted protection against challenge with Mycobacterium bovis. PLoS ONE 2014, 9, e106519. [Google Scholar] [CrossRef][Green Version]
- Angelidou, A.; Conti, M.G.; Diray-Arce, J.; Benn, C.S.; Shann, F.; Netea, M.G.; Liu, M.; Potluri, L.P.; Sanchez-Schmitz, G.; Husson, R.; et al. Licensed Bacille Calmette-Guerin (BCG) formulations differ markedly in bacterial viability, RNA content and innate immune activation. Vaccine 2020, 38, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, C.; Terpos, E.; Rosati, M.; Angel, M.; Bear, J.; Stellas, D.; Karaliota, S.; Apostolakou, F.; Bagratuni, T.; Patseas, D.; et al. Systemic IL-15, IFN-gamma, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients. Cell Rep. 2021, 36, 109504. [Google Scholar] [CrossRef]
- Rivas, M.N.; Ebinger, J.E.; Wu, M.; Sun, N.; Braun, J.; Sobhani, K.; Van Eyk, J.E.; Cheng, S.; Arditi, M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J. Clin. Investig. 2021, 131, e145157. [Google Scholar] [CrossRef]
- Pol, J.G.; Caudana, P.; Paillet, J.; Piaggio, E.; Kroemer, G. Effects of interleukin-2 in immunostimulation and immunosuppression. J. Exp. Med. 2020, 217, e20191247. [Google Scholar] [CrossRef]
- Thorne, L.G.; Reuschl, A.K.; Zuliani-Alvarez, L.; Whelan, M.V.X.; Turner, J.; Noursadeghi, M.; Jolly, C.; Towers, G.J. SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. EMBO J. 2021, 40, e107826. [Google Scholar] [CrossRef]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 509, 280–287. [Google Scholar] [CrossRef]
- Klinger, D.; Blass, I.; Rappoport, N.; Linial, M. Significantly Improved COVID-19 Outcomes in Countries with Higher BCG Vaccination Coverage: A Multivariable Analysis. Vaccines 2020, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Nuovo, G.; Tili, E.; Suster, D.; Matys, E.; Hupp, L.; Magro, C. Strong homology between SARS-CoV-2 envelope protein and a Mycobacterium sp. antigen allows rapid diagnosis of Mycobacterial infections and may provide specific anti-SARS-CoV-2 immunity via the BCG vaccine. Ann. Diagn. Pathol. 2020, 48, 151600. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Epidemiological transcriptomic data supports BCG protection in viral diseases including COVID-19. Gene 2021, 783, 145574. [Google Scholar] [CrossRef]
- Tomita, Y.; Sato, R.; Ikeda, T.; Sakagami, T. BCG vaccine may generate cross-reactive T cells against SARS-CoV-2: In silico analyses and a hypothesis. Vaccine 2020, 38, 6352–6356. [Google Scholar] [CrossRef]
- Muller, L.; Andree, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, ciab381. [Google Scholar] [CrossRef]
- Pawelec, G. Immunity and ageing in man. Exp. Gerontol. 2006, 41, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Accolla, R.S.; Ramia, E.; Tedeschi, A.; Forlani, G. CIITA-Driven MHC Class II Expressing Tumor Cells as Antigen Presenting Cell Performers: Toward the Construction of an Optimal Anti-tumor Vaccine. Front. Immunol. 2019, 10, 1806. [Google Scholar] [CrossRef]
- Fricke-Galindo, I.; Falfan-Valencia, R. Genetics Insight for COVID-19 Susceptibility and Severity: A Review. Front. Immunol. 2021, 12, 622176. [Google Scholar] [CrossRef]
- Teran-Escandon, D.; Teran-Ortiz, L.; Camarena-Olvera, A.; Gonzalez-Avila, G.; Vaca-Marin, M.A.; Granados, J.; Selman, M. Human leukocyte antigen-associated susceptibility to pulmonary tuberculosis: Molecular analysis of class II alleles by DNA amplification and oligonucleotide hybridization in Mexican patients. Chest 1999, 115, 428–433. [Google Scholar] [CrossRef]

| Variable | (n = 60) |
|---|---|
| Age, years | 41 (30–50) |
| Female, n (%) | 45 (75%) |
| Body mass index (BMI), kg/m2 | 27.15 (24.45–30.35) |
| BCG vaccine history, n (%) | 60 (100%) |
| Mantoux test (PPD), mm | 0 (0–5) |
| Time spent attending to patients with COVID-19, h/week | 25 |
| Variable | Placebo–anti-SARS-CoV-2 | BCG–anti-SARS-CoV-2 | p |
|---|---|---|---|
| Gender (n) F/M | 24/6 | 21/9 | 0.371 |
| Age (years) | 42 (33–49) | 38(28–54) | 0.51 |
| BMI (kg/m2) | 24.65 (22.83–28.47) | 25.23 (24.23–27.88) | 0.6465 |
| Mantoux test (PPD) (mm) | 0 (0–5) | 0 (0–6) | 0.812 |
| COVID-19 infection (without/with) (n) | 21/9 | 16/14 | 0.184 |
| Hospitalization (without/with) (n) | 30/0 | 28/2 | 0.313 |
| Allele | Placebo–anti-SARS-CoV-2 | BCG–anti-SARS-CoV-2 | |||
|---|---|---|---|---|---|
| n = 30 | (%) | n = 26 | (%) | p | |
| DRB1*01 | 2 | 3.33 | 6 | 11.54 | 0.141 |
| DRB1*03 | 1 | 1.67 | 0 | 0.00 | NA |
| DRB1*04 | 27 | 45.00 | 12 | 23.08 | 0.025 |
| DRB1*07 | 4 | 6.67 | 7 | 13.46 | 0.340 |
| DRB1*08 | 8 | 13.33 | 8 | 15.38 | 0.969 |
| DRB1*09 | 1 | 1.67 | 0 | 0.00 | NA |
| DRB1*10 | 2 | 3.33 | 0 | 0.00 | NA |
| DRB1*11 | 3 | 5.00 | 2 | 3.85 | 0.674 |
| DRB1*13 | 3 | 5.00 | 5 | 9.62 | 0.468 |
| DRB1*14:02 | 6 | 10.00 | 7 | 13.46 | 0.783 |
| DRB1*15 | 1 | 1.67 | 4 | 7.69 | 0.181 |
| DRB1*16 | 2 | 3.33 | 1 | 1.92 | 1.000 |
| Variable | Crude β (95% CI) | p | Adjusted β (95% CI) | p |
|---|---|---|---|---|
| BCG | 24.56 (10.73–38.39) | 0.001 | 23.80 (9.09–38.51) | 0.002 |
| HLA-DRB1*04 heterozygous | −1.12 (−19.16–16.91) | 0.9 | 0.08 (−17.42–17.6) | 0.99 |
| HLA-DR*04 homozygous | −11.30 (−30.14–7.54) | 0.25 | −3.83 (−22.9–15.23) | 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Martinez, E.; Falfán-Valencia, R.; Pérez-Rubio, G.; Andrade, W.A.; Rojas-Serrano, J.; Ambrocio-Ortiz, E.; Galicia-Álvarez, D.S.; Bárcenas-Montiel, I.; Velasco-Medina, A.; Velázquez-Sámano, G. Effect of BCG Revaccination on Occupationally Exposed Medical Personnel Vaccinated against SARS-CoV-2. Cells 2021, 10, 3179. https://doi.org/10.3390/cells10113179
Ramos-Martinez E, Falfán-Valencia R, Pérez-Rubio G, Andrade WA, Rojas-Serrano J, Ambrocio-Ortiz E, Galicia-Álvarez DS, Bárcenas-Montiel I, Velasco-Medina A, Velázquez-Sámano G. Effect of BCG Revaccination on Occupationally Exposed Medical Personnel Vaccinated against SARS-CoV-2. Cells. 2021; 10(11):3179. https://doi.org/10.3390/cells10113179
Chicago/Turabian StyleRamos-Martinez, Espiridión, Ramcés Falfán-Valencia, Gloria Pérez-Rubio, Warrison Athanasio Andrade, Jorge Rojas-Serrano, Enrique Ambrocio-Ortiz, Dennisse S. Galicia-Álvarez, Isaac Bárcenas-Montiel, Andrea Velasco-Medina, and Guillermo Velázquez-Sámano. 2021. "Effect of BCG Revaccination on Occupationally Exposed Medical Personnel Vaccinated against SARS-CoV-2" Cells 10, no. 11: 3179. https://doi.org/10.3390/cells10113179
APA StyleRamos-Martinez, E., Falfán-Valencia, R., Pérez-Rubio, G., Andrade, W. A., Rojas-Serrano, J., Ambrocio-Ortiz, E., Galicia-Álvarez, D. S., Bárcenas-Montiel, I., Velasco-Medina, A., & Velázquez-Sámano, G. (2021). Effect of BCG Revaccination on Occupationally Exposed Medical Personnel Vaccinated against SARS-CoV-2. Cells, 10(11), 3179. https://doi.org/10.3390/cells10113179