The Combined Effect of ZnO and CeO2 Nanoparticles on Pisum sativum L.: A Photosynthesis and Nutrients Uptake Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles Characteristic
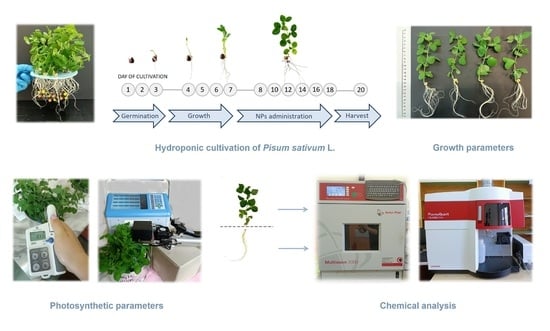
2.2. Plants Growth Conditions and Treatments
2.3. Growth Parameters
2.4. Elements Content
2.5. Tolerance Index (TI) and Translocation Factor (TF)
2.6. Photosynthetic Pigments
2.7. Photosynthetic Parameters
2.8. Statistical Analysis
3. Results and Discussion
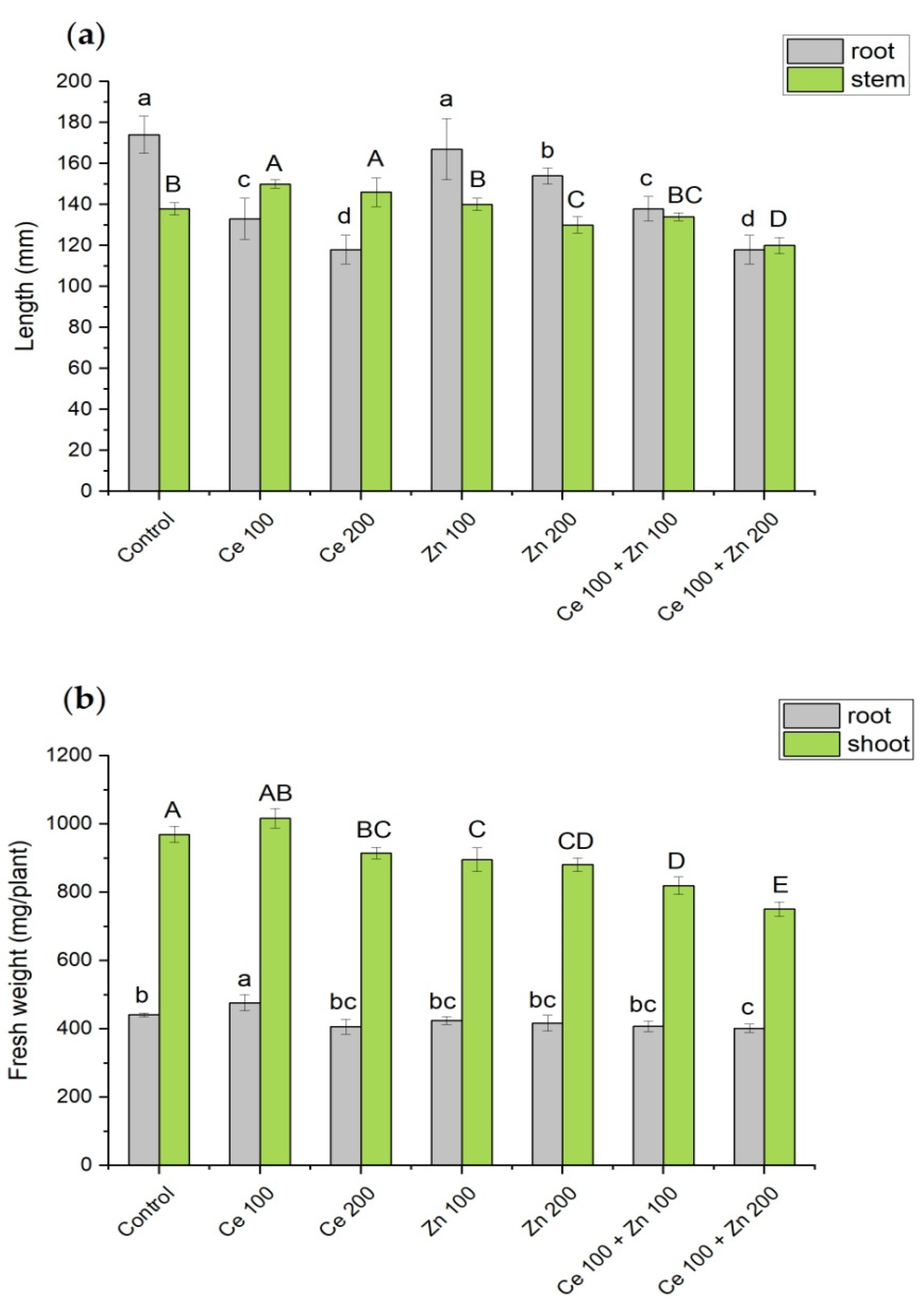
3.1. Growth Parameters
3.2. Cerium and Zinc Concentrations
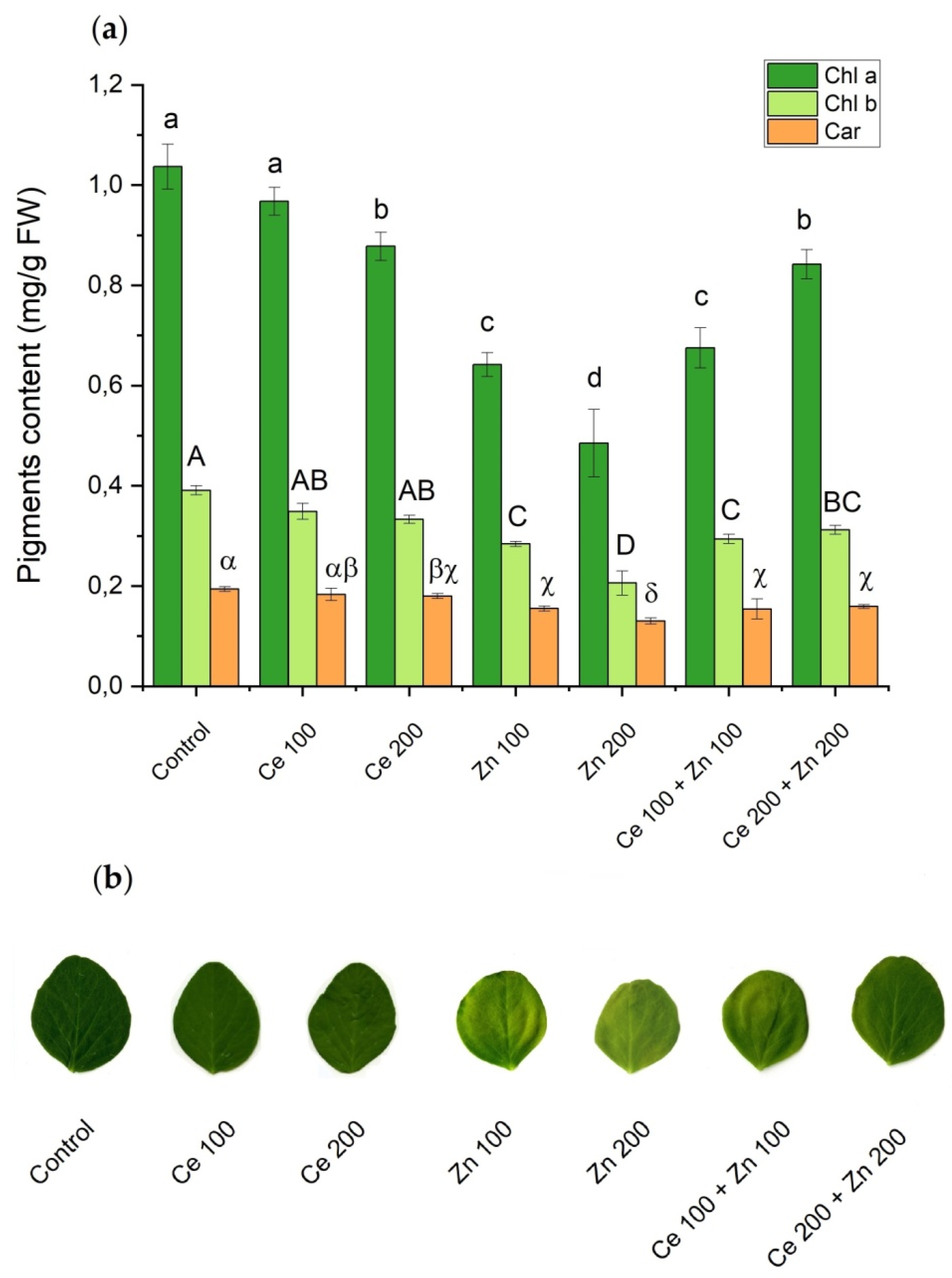
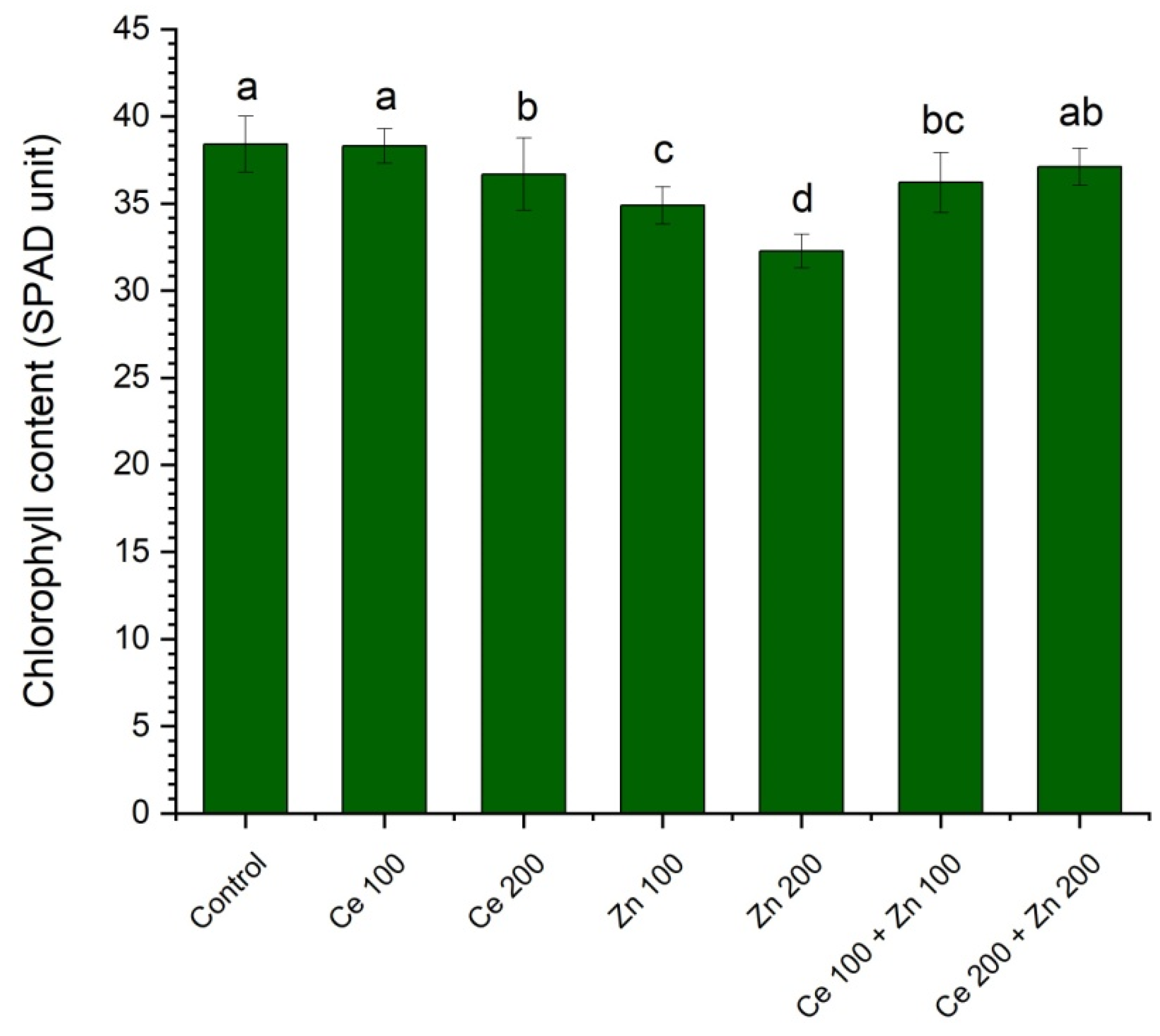
3.3. Photosynthetic Pigments
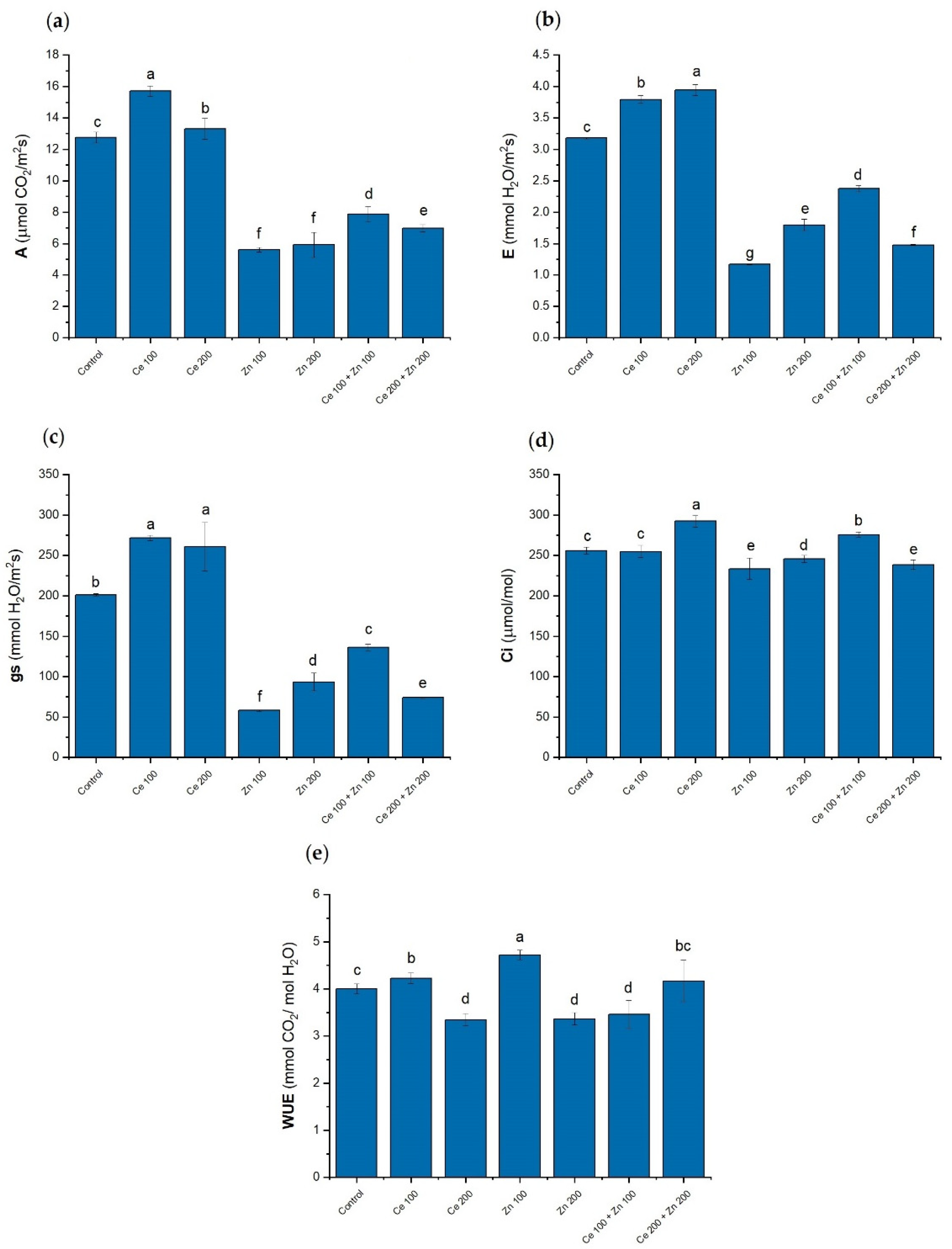
3.4. Photosynthesis Parameters
3.5. Elements Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, A.; Yadav, M. Application of nanomaterials in architecture—An overview. Mater. Today Proc. 2021, 43, 2921–2925. [Google Scholar] [CrossRef]
- Hoang, A.T. Combustion behavior, performance and emission characteristics of diesel engine fuelled with biodiesel containing cerium oxide nanoparticles: A review. Fuel Process. Technol. 2021, 218, 106840. [Google Scholar] [CrossRef]
- Yu, J.; Luo, M.; Lv, Z.; Huang, S.; Hsu, H.H.; Kuo, C.C.; Han, S.T.; Zhou, Y. Recent advances in optical and optoelectronic data storage based on luminescent nanomaterials. Nanoscale 2020, 12, 23391–23423. [Google Scholar] [CrossRef]
- Caro, C.; Gámez, F.; Quaresma, P.; Páez-Muñoz, J.M.; Domínguez, A.; Pearson, J.R.; Pernía Leal, M.; Beltrán, A.M.; Fernandez-Afonso, Y.; De La Fuente, J.M.; et al. Fe3O4-Au core-shell nanoparticles as a multimodal platform for in vivo imaging and focused photothermal therapy. Pharmaceutics 2021, 13, 416. [Google Scholar] [CrossRef]
- Jandt, K.D.; Watts, D.C. Nanotechnology in dentistry: Present and future perspectives on dental nanomaterials. Dent. Mater. 2020, 36, 1365–1378. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Z.; Guo, Z.; Huang, X.J. Regulation of intrinsic physicochemical properties of metal oxide nanomaterials for energy conversion and environmental detection applications. J. Mater. Chem. A 2020, 8, 17326–17359. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chia, W.Y.; Tang, D.Y.Y.; Show, P.L.; Chew, K.W.; Chen, W.H. Nanomaterials utilization in biomass for biofuel and bioenergy production. Energies 2020, 13, 892. [Google Scholar] [CrossRef] [Green Version]
- Sengwa, R.J.; Dhatarwal, P.; Choudhary, S. A comparative study of different metal oxide nanoparticles dispersed PVDF/PEO blend matrix-based advanced multifunctional nanodielectrics for flexible electronic devices. Mater. Today Commun. 2020, 25, 101380. [Google Scholar] [CrossRef]
- Degfie, T.A.; Mamo, T.T.; Mekonnen, Y.S. Optimized biodiesel production from waste cooking oil (WCO) using calcium oxide (CaO) nano-catalyst. Sci. Rep. 2019, 9, 18982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, X.; Downing, J.R.; Hersam, M.C.; Chen, J. Additive manufacturing and applications of nanomaterial-based sensors. Mater. Today 2021. [Google Scholar] [CrossRef]
- Hou, Q.; Qi, X.; Zhen, M.; Qian, H.; Nie, Y.; Bai, C.; Zhang, S.; Bai, X.; Ju, M. Biorefinery roadmap based on catalytic production and upgrading 5-hydroxymethylfurfural. Green Chem. 2021, 23, 119–231. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, H.; Liu, T.; Zeb, S.; Nie, Y.; Zhao, Y.; Qin, C.; Jiang, X. Advanced development of metal oxide nanomaterials for H2 gas sensing applications. Mater. Adv. 2021, 2, 1530–1569. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Singh, H.; Sharma, A.; Bhardwaj, S.K.; Arya, S.K.; Bhardwaj, N.; Khatri, M. Recent advances in the applications of nano-agrochemicals for sustainable agricultural development. Environ. Sci. Process. Impacts 2021, 23, 213–239. [Google Scholar] [CrossRef]
- Tarrahi, R.; Mahjouri, S.; Khataee, A. A review on in vivo and in vitro nanotoxicological studies in plants: A headlight for future targets. Ecotoxicol. Environ. Saf. 2021, 208, 111697. [Google Scholar] [CrossRef]
- Wang, M.; Li, S.; Chen, Z.; Zhu, J.; Hao, W.; Jia, G.; Chen, W.; Zheng, Y.; Qu, W.; Liu, Y. Safety assessment of nanoparticles in food: Current status and prospective. Nano Today 2021, 39, 101169. [Google Scholar] [CrossRef]
- Ameen, F.; Alsamhary, K.; Alabdullatif, J.A.; ALNadhari, S. A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol. Environ. Saf. 2021, 213, 112027. [Google Scholar] [CrossRef] [PubMed]
- Metal Oxide Nanoparticles Market—Growth, Trends, COVID-19 Impact, and Forecasts (2021–2026). Available online: https://www.mordorintelligence.com/industry-reports/metal-oxide-nanoparticles-market (accessed on 5 May 2021).
- Choi, S.J.; Choy, J.H. Effect of physico-chemical parameters on the toxicity of inorganic nanoparticles. J. Mater. Chem. 2011, 21, 5547–5554. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.F.; Qu, Y.; Chai, Z.; Zhao, Y. Advanced nuclear analytical and related techniques for the growing challenges in nanotoxicology. Chem. Soc. Rev. 2013, 42, 8266–8303. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Stowers, C.; Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of soybean (Glycine max (L.) Merr.). Environ. Sci. Nano 2017, 4, 1086–1094. [Google Scholar] [CrossRef]
- Bindra, H.S.; Singh, B. Nanofertilizers and nanopesticides: Future of plant protection. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture, 1st ed.; Jogaiah, S., Singh, H.B., Fraceto, L.F., de Lima, R., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 57–84. [Google Scholar]
- Verma, S.K.; Das, A.K.; Patel, M.K.; Shah, A.; Kumar, V.; Gantait, S. Engineered nanomaterials for plant growth and development: A perspective analysis. Sci. Total Environ. 2018, 630, 1413–1435. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Ali, S.; Rizwan, M.; Zia ur Rehman, M.; Javed, M.R.; Imran, M.; Chatha, S.A.S.; Nazir, R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018, 242, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef]
- Marchiol, L.; Filippi, A.; Adamiano, A.; Esposti, L.D.; Iafisco, M.; Mattiello, A.; Petrussa, E.; Braidot, E. Influence of hydroxyapatite nanoparticles on germination and plant metabolism of tomato (Solanum lycopersicum L.): Preliminary evidence. Agronomy 2019, 9, 161. [Google Scholar] [CrossRef] [Green Version]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhiman, S.; Bakshi, P.; Kapoor, N.; Sharma, P.; Kohli, S.K.; Mir, B.A.; Bhardwaj, R. Nanoparticle-induced oxidative stress. In Plant Responses to Nanomaterials. Recent Interventions and Physiological and Biochemical Responses; Singh, V.P., Singh, S., Prasad, S.M., Chauhan, D.K., Tripathi, D.K., Eds.; Springer: New York, NY, USA, 2021; pp. 269–313. [Google Scholar]
- Kalaji, M.H.; Goltsev, V.N.; Żuk-Golaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence: Understanding Crop Performance—Basics and Applications; CRC Press Taylor & Francis Group: New York, NY, USA, 2017. [Google Scholar]
- Rodea-Palomares, I.; Gonzalo, S.; Santiago-Morales, J.; Leganés, F.; García-Calvo, E.; Rosal, R.; Fernández-Piñas, F. An insight into the mechanisms of nanoceria toxicity in aquatic photosynthetic organisms. Aquat. Toxicol. 2012, 122–123, 133–143. [Google Scholar] [CrossRef]
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano 2017, 11, 11283–11297. [Google Scholar] [CrossRef]
- Wang, X.P.; Li, Q.Q.; Pei, Z.M.; Wang, S.C. Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol. Plant. 2018, 62, 801–808. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Hayat, S. Effective use of zinc oxide nanoparticles through root dipping on the performance of growth, quality, photosynthesis and antioxidant system in tomato. J. Plant Biochem. Biotechnol. 2020, 29, 553–567. [Google Scholar] [CrossRef]
- Tighe-Neira, R.; Carmora, E.; Recio, G.; Nunes-Nesi, A.; Reyes-Diaz, M.; Alberdi, M.; Rengel, Z.; Inostroza-Blancheteau, C. Metallic nanoparticles influence the structure and function of the photosynthetic apparatus in plants. Plant Physiol. Biochem. 2018, 130, 408–417. [Google Scholar] [CrossRef]
- Zhao, L.; Peralta-Videa, J.R.; Rico, C.M.; Hernandez-Viezcas, J.A.; Sun, Y.; Niu, G.; Servin, A.; Nunez, J.E.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. CeO2 and ZnO nanoparticles change the nutritional qualities of cucumber (Cucumis sativus). J. Agric. Food Chem. 2014, 62, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Guo, H. Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant Physiol. Biochem. 2017, 110, 210–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.; Peralta-Videa, J.R.; Bandyopadhyay, S.; Rico, C.M.; Zhao, L.; Gardea-Torresdey, J.L. Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 2014, 6, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Reddy Pullagurala, V.L.; Adisa, I.O.; Rawat, S.; Kalagara, S.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. ZnO nanoparticles increase photosynthetic pigments and decrease lipid peroxidation in soil grown cilantro (Coriandrum sativum). Plant Physiol. Biochem. 2018, 132, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mu, Q.; Du, Y.; Luo, J.; Liu, Y.; Li, T. Growth and photosynthetic inhibition of cerium oxide nanoparticles on soybean (Glycine max). Bull. Environ. Contam. Toxicol. 2020, 105, 119–126. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, J.; Tang, L.; Li, H.; Zhu, Y.; Jiang, D.; Song, B.; Chen, M.; Zeng, G. Nano-pesticides: A great challenge for biodiversity? Nano Today 2019, 28, 100757. [Google Scholar] [CrossRef]
- Silva, L.P.; Bonatto, C.C. Green nanotechnology for sustained release of eco-friendly agrochemicals. In Sustainable Agrochemistry. A Compendium of Technologies; Vaz, S., Jr., Ed.; Springer: New York, NY, USA, 2019; pp. 113–129. [Google Scholar]
- Mohammadi, M.H.Z.; Panahirad, S.; Navai, A.; Bahrami, M.K.; Kulak, M.; Gohari, G. Cerium oxide nanoparticles (CeO2-NPs) improve growth parameters and antioxidant defense system in Moldavian Balm (Dracocephalummoldavica, L.) under salinity stress. Plant Stress 2021, 1, 100006. [Google Scholar] [CrossRef]
- Jahani, S.; Saadatmand, S.; Mahmoodzadeh, H.; Khavari-Nejad, R.A. Effect of foliar application of cerium oxide nanoparticles on growth, photosynthetic pigments, electrolyte leakage, compatible osmolytes and antioxidant enzymes activities of Calendula officinalis L. Biologia 2019, 74, 1063–1075. [Google Scholar] [CrossRef]
- Jurkow, R.; Pokluda, R.; Sȩkara, A.; Kalisz, A. Impact of foliar application of some metal nanoparticles on antioxidant system in oakleaf lettuce seedlings. BMC Plant Biol. 2020, 20, 290. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yue, L.; Wang, C.; Zhu, X.; Wang, Z.; Xing, B. Photosynthetic response mechanisms in typical C3 and C4 plants upon La2O3 nanoparticle exposure. Environ. Sci. Nano 2020, 7, 81–92. [Google Scholar] [CrossRef]
- Naasz, S.; Altenburger, R.; Kühnel, D. Environmental mixtures of nanomaterials and chemicals: The Trojan-horse phenomenon and its relevance for ecotoxicity. Sci. Total Environ. 2018, 635, 1170–1181. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, A. Binary mixture of nanoparticles in sewage sludge: Impact on spinach growth. Chemosphere 2020, 254, 126794. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kumar, A. Quantification of metal uptake in Spinacia oleracea irrigated with water containing a mixture of CuO and ZnO nanoparticles. Chemosphere 2020, 243, 125239. [Google Scholar] [CrossRef]
- Skiba, E.; Michlewska, S.; Pietrzak, M.; Wolf, W.M. Additive interactions of nanoparticulate ZnO with copper, manganese and iron in Pisum sativum L., a hydroponic study. Sci. Rep. 2020, 10, 13574. [Google Scholar] [CrossRef]
- Skiba, E.; Pietrzak, M.; Gapińska, M.; Wolf, W.M. Metal homeostasis and gas exchange dynamics in Pisum sativum L. exposed to cerium oxide nanoparticles. Int. J. Mol. Sci. 2020, 21, 8497. [Google Scholar] [CrossRef]
- Skiba, E.; Wolf, W.M. Cerium oxide nanoparticles affect heavy metals uptake by pea in a divergent way than their ionic and bulk counterparts. Water Air Soil Pollut. 2019, 230, 248. [Google Scholar] [CrossRef] [Green Version]
- Kreplak, J.; Madoui, M.A.; Cápal, P.; Novák, P.; Labadie, K.; Aubert, G.; Bayer, P.E.; Gali, K.K.; Syme, R.A.; Main, D.; et al. A reference genome for pea provides insight into legume genome evolution. Nat. Genet. 2019, 51, 1411–1422. [Google Scholar] [CrossRef]
- Burstin, J.; Kreplak, J.; Macas, J.; Lichtenzveig, J. Pisum sativum (Pea). Trends Genet. 2020, 36, 312–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wan, S.; Hao, J.; Hu, J.; Yang, T.; Zong, X. Large-scale evaluation of pea (Pisum sativum L.) germplasm for cold tolerance in the field during winter in Qingdao. Crop. J. 2016, 4, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Blackwell Wissenschafts: Berlin, Germany, 1997. [Google Scholar]
- Samczyński, Z.; Dybczyński, R.S.; Polkowska-Motrenko, H.; Chajduk, E.; Pyszynska, M.; Danko, B.; Czerska, E.; Kulisa, K.; Doner, K.; Kalbarczyk, P. Two new reference materials based on tobacco leaves: Certification for over a dozen of toxic and essential elements. Sci. World J. 2012, 2012, 216380. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.G.; Marshal, C. Accumulation of zinc by subcellular root of Agrostis tannis Sibth. in relation of zinc tolerance. New Phytol. 1972, 71, 671–676. [Google Scholar] [CrossRef]
- KaramiMehrian, S.; Heidari, R.; Rahmani, F.; Najafi, S. Effect of chemical synthesis silver nanoparticles on germination indices and seedlings growth in seven varieties of Lycopersiconesculentum Mill (tomato) plants. J. Clust. Sci. 2016, 27, 327–340. [Google Scholar] [CrossRef]
- Liu, Z.; He, X.; Chen, W.; Yuan, F.; Yan, K.; Tao, D. Accumulation and tolerance characteristics of cadmium in a potential hyperaccumulator-Lonicera japonica Thunb. J. Hazard. Mater. 2009, 169, 170–175. [Google Scholar] [CrossRef]
- Mayerová, M.; Petrová, Š.; Madaras, M.; Lipavský, J.; Šimon, T.; Vaněk, T. Non-enhanced phytoextraction of cadmium, zinc, and lead by high-yielding crops. Environ. Sci. Pollut. Res. 2017, 24, 14706–14716. [Google Scholar] [CrossRef]
- Oren, R.; Werk, K.S.; Buchmann, N.; Zimmermann, R. Chlorophyll-nutrient relationships identify nutritionally caused decline in Piceaabies stands. Can. J. For. Res. 1993, 23, 1187–1195. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org (accessed on 10 January 2021).
- Duursma, R.A. Plantecophys—An R package for analysing and modelling leaf gas exchange data. PLoS ONE 2015, 10, e0143346. [Google Scholar] [CrossRef]
- Marchiol, L.; Mattiello, A.; Pošćić, F.; Fellet, G.; Zavalloni, C.; Carlino, E.; Musetti, R. Changes in physiological and agronomical parameters of barley (Hordeum vulgare) exposed to cerium and titanium dioxide nanoparticles. Int. J. Environ. Res. Public Health 2016, 13, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herber, R.F.M.; Sallé, H.J.A. Statistics and data evaluation. Tech. Instrum. Anal. Chem. 1994, 15, 257–271. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; pp. 191–248. [Google Scholar]
- Hu, X.; Tanaka, A.; Tanaka, R. Simple extraction methods that prevent the artifactual conversion of chlorophyll to chlorophyllide during pigment isolation from leaf samples. Plant Methods 2013, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Genter, R.B. Ecotoxicology of inorganic chemical stress to algae. In Algal Ecology Freshwater Benthic Ecosystems, 1st ed.; Stevenson, R.J., Bothwell, M.L., Lowe, R.L., Thorp, J., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 403–468. [Google Scholar]
- Hu, C.; Liu, X.; Li, X.; Zhao, Y. Evaluation of growth and biochemical indicators of Salvinia natans exposed to zinc oxide nanoparticles and zinc accumulation in plants. Environ. Sci. Pollut. Res. 2014, 21, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Hou, J.; Zhang, W.J.; Li, W.; Lin, Z.P. The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 2009, 75, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Ebbs, S.; Uchil, S. Cadmium and zinc induced chlorosis in Indian mustard [Brassica juncea (L.) Czern] involves preferential loss of chlorophyll b. Photosynthetica 2008, 46, 49–55. [Google Scholar] [CrossRef]
- Andrejić, G.; Gajić, G.; Prica, M.; Dželetović, Ž.; Rakić, T. Zinc accumulation, photosynthetic gas exchange, and chlorophyll a fluorescence in Zn-stressed Miscanthus x giganteus plants. Photosynthetica 2018, 56, 1249–1258. [Google Scholar] [CrossRef]
- Zoufan, P.; Baroonian, M.; Zargar, B. ZnO nanoparticles-induced oxidative stress in Chenopodium murale L., Zn uptake, and accumulation under hydroponic culture. Environ. Sci. Pollut. Res. 2020, 27, 11066–11078. [Google Scholar] [CrossRef]
- Salehi, H.; De Diego, N.; Chehregani Rad, A.; Benjamin, J.J.; Trevisan, M.; Lucini, L. Exogenous application of ZnO nanoparticles and ZnSO4 distinctly influence the metabolic response in Phaseolus vulgaris L. Sci. Total Environ. 2021, 778, 146331. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, 15. [Google Scholar] [CrossRef] [Green Version]
- Kim, C. ROS-driven oxidative modification: Its impact on chloroplasts-nucleus communication. Front. Plant Sci. 2020, 10, 1729. [Google Scholar] [CrossRef] [PubMed]
- Küpper, H.; Küpper, F.; Spiller, M. Environmental relevance of heavy metal-substituted chlorophylls using the example of water plants. J. Exp. Bot. 1996, 47, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Sharkey, T.D. What gas exchange data can tell us about photosynthesis. Plant Cell Environ. 2016, 39, 1161–1163. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Bernacchi, C.J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haworth, M.; Marino, G.; Centritto, M. An introductory guide to gas exchange analysis of photosynthesis and its application to plant phenotyping and precision irrigation to enhance water use efficiency. J. Water Clim. Chang. 2018, 9, 786–808. [Google Scholar] [CrossRef] [Green Version]
- Vassilev, A.; Nikolova, A.; Koleva, L.; Lidon, F. Effects of excess Zn on growth and photosynthetic performance of young bean plants. J. Phytol. 2011, 3, 58–62. [Google Scholar]
- Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc hyperaccumulation in plants: A review. Plants 2020, 9, 562. [Google Scholar] [CrossRef]
- Anwaar, S.A.; Ali, S.; Ali, S.; Ishaque, W.; Farid, M.; Farooq, M.A.; Najeeb, U.; Abbas, F.; Sharif, M. Silicon (Si) alleviates cotton (Gossypium hirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environ. Sci. Pollut. Res. 2015, 22, 3441–3450. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Z.; Chen, F.; Yue, L.; Zou, H.; Lyu, J.; Wang, Z. Metallic oxide nanomaterials act as antioxidant nanozymes in higher plants: Trends, meta-analysis, and prospect. Sci. Total Environ. 2021, 780, 146578. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Nair, R.; Giraldo, J.P.; Prasad, P.V.V. Cerium oxide nanoparticles decrease drought-induced oxidative damage in Sorghum Leading to higher photosynthesis and grain yield. ACS Omega 2018, 3, 14406–14416. [Google Scholar] [CrossRef]
- Baldim, V.; Bedioui, F.; Mignet, N.; Margaill, I.; Berret, J.F. The enzyme-like catalytic activity of cerium oxide nanoparticles and its dependency on Ce3+ surface area concentration. Nanoscale 2018, 10, 6971–6980. [Google Scholar] [CrossRef] [Green Version]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shabala, L.; Shabala, S.; Giraldo, J.P. Hydroxyl radical scavenging by cerium oxide nanoparticles improves Arabidopsis salinity tolerance by enhancing leaf mesophyll potassium retention. Environ. Sci. Nano 2018, 5, 1567–1583. [Google Scholar] [CrossRef]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of nanoparticles alleviates heavy metals stress and promotes plant growth: An overview. Nanomaterials 2021, 11, 26. [Google Scholar] [CrossRef]
- Xue, Y.; Luan, Q.; Yang, D.; Yao, X.; Zhou, K. Direct evidence for hydroxyl radical scavenging activity of cerium oxide nanoparticles. J. Phys. Chem. C 2011, 115, 4433–4438. [Google Scholar] [CrossRef]
- Cao, Z.; Rossi, L.; Stowers, C.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the physiology of soybean (Glycine max (L.) Merr.) under different soil moisture conditions. Environ. Sci. Pollut. Res. 2018, 25, 930–939. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Jahan, B.; Alajmi, M.F.; Rehman, M.T.; Khan, N.A. Exogenously-sourced ethylene modulates defense mechanisms and promotes tolerance to zinc stress in mustard (Brassica juncea L.). Plants 2019, 8, 540. [Google Scholar] [CrossRef] [Green Version]
- Feigl, G.; Lehotai, N.; Molnár, Á.; Ördög, A.; Rodríguez-Ruiz, M.; Palma, J.M.; Corpas, F.J.; Erdei, L.; Kolbert, Z. Zinc induces distinct changes in the metabolism of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Ann. Bot. 2015, 116, 613–625. [Google Scholar] [CrossRef] [Green Version]
- Deans, R.M.; Farquhar, G.D.; Busch, F.A. Estimating stomatal and biochemical limitations during photosynthetic induction. Plant Cell Environ. 2019, 42, 3227–3240. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Yanik, F.; Vardar, F. Mechanism and interaction of nanoparticle-induced programmed cel death in plants. In Phytotoxicity of Nanoparticles, 1st ed.; Faisal, M., Saquib, Q., Alator, A.A., Al-Khedhairy, A.A., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 175–196. [Google Scholar]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
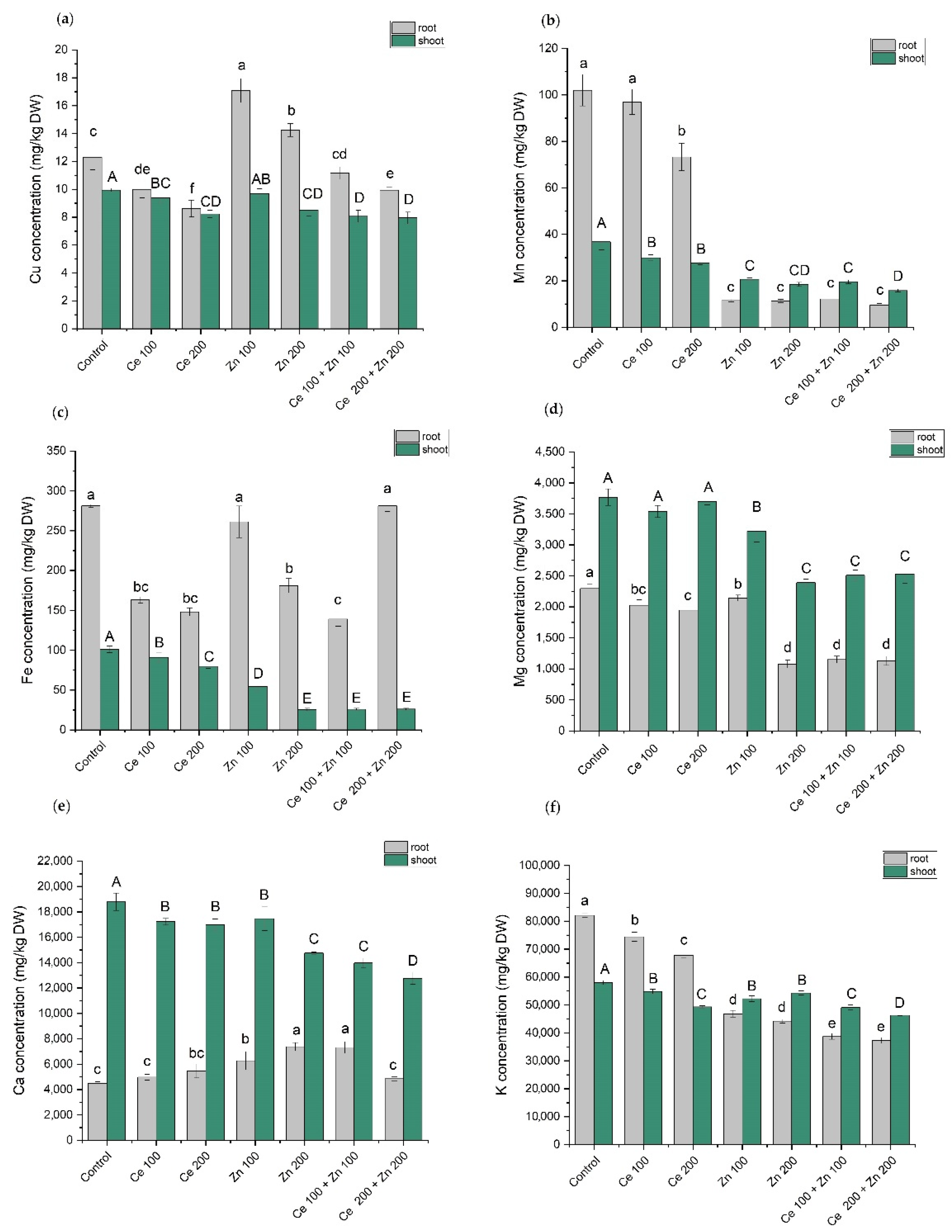
| Treatment | Zinc Concentration (mg/kg DW) | TF | Cerium Concentrations (mg/kg DW) | TF | ||
|---|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | |||
| Control | 97.8 ± 4.5 d | 61.4 ± 1.1 d | 0.63 | nd | nd | nd |
| Ce 100 | 74.3 ± 5.5 d | 52.4 ± 2.3 d | 0.71 | 14,874 ± 495 b | 110 ± 11 c | 0.01 |
| Ce 200 | 68.3 ± 6.2 d | 51.5 ± 2.2 d | 0.75 | 16,977 ± 1067 a | 206 ± 14 a | 0.01 |
| Zn 100 | 25,921 ± 2307 b | 1160 ± 61 bc | 0.04 | nd | nd | nd |
| Zn 200 | 30,522 ± 2140 a | 1455 ± 54 a | 0.05 | nd | nd | nd |
| Ce 100 + Zn 100 | 18,192 ± 703 c | 1079 ± 113 c | 0.06 | 6058 ± 824 d | 120 ± 7 bc | 0.02 |
| Ce 200 + Zn 200 | 28,097 ± 3773 ab | 1236 ± 88 b | 0.04 | 10,301 ± 1484 c | 142 ± 10 b | 0.01 |
| Treatment | Vcmax (µmol/m2 s) | Jmax (µmol/m2 s) | CO2 Compensation Point (µmol/mol) |
|---|---|---|---|
| Control | 62.22 ± 1.22 c | 80.31 ± 0.93 b | 57.40 ± 1.12 b |
| Ce 100 | 71.07 ± 1.30 a | 87.84 ± 0.69 a | 43.00 ± 0.62 c |
| Ce 200 | 66.39 ± 0.58 b | 85.02 ± 1.10 a | 58.84 ± 1.00 b |
| Zn 100 | 50.29 ± 1.07 d | 73.96 ± 1.27 c | 97.51 ± 0.91 a |
| Zn 200 | 50.21 ± 0.82 d | 67.82 ± 1.00 c | 97.95 ± 1.28 a |
| Ce 100 + Zn 100 | 60.75 ± 1.41 c | 79.09 ± 1.01 b | 96.91 ± 1.34 a |
| Ce 200 + Zn 200 | 53.81 ± 2.58 d | 72.78 ± 1.80 c | 57.39 ± 3.27 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skiba, E.; Pietrzak, M.; Glińska, S.; Wolf, W.M. The Combined Effect of ZnO and CeO2 Nanoparticles on Pisum sativum L.: A Photosynthesis and Nutrients Uptake Study. Cells 2021, 10, 3105. https://doi.org/10.3390/cells10113105
Skiba E, Pietrzak M, Glińska S, Wolf WM. The Combined Effect of ZnO and CeO2 Nanoparticles on Pisum sativum L.: A Photosynthesis and Nutrients Uptake Study. Cells. 2021; 10(11):3105. https://doi.org/10.3390/cells10113105
Chicago/Turabian StyleSkiba, Elżbieta, Monika Pietrzak, Sława Glińska, and Wojciech M. Wolf. 2021. "The Combined Effect of ZnO and CeO2 Nanoparticles on Pisum sativum L.: A Photosynthesis and Nutrients Uptake Study" Cells 10, no. 11: 3105. https://doi.org/10.3390/cells10113105
APA StyleSkiba, E., Pietrzak, M., Glińska, S., & Wolf, W. M. (2021). The Combined Effect of ZnO and CeO2 Nanoparticles on Pisum sativum L.: A Photosynthesis and Nutrients Uptake Study. Cells, 10(11), 3105. https://doi.org/10.3390/cells10113105