NK Cell Regulation in Cervical Cancer and Strategies for Immunotherapy
Abstract
:1. Introduction
Cervical Cancer
2. HPV
2.1. HPV Infection and Transformation
2.2. Activation of the Immune System in HPV Infection
3. NK Cells Populations
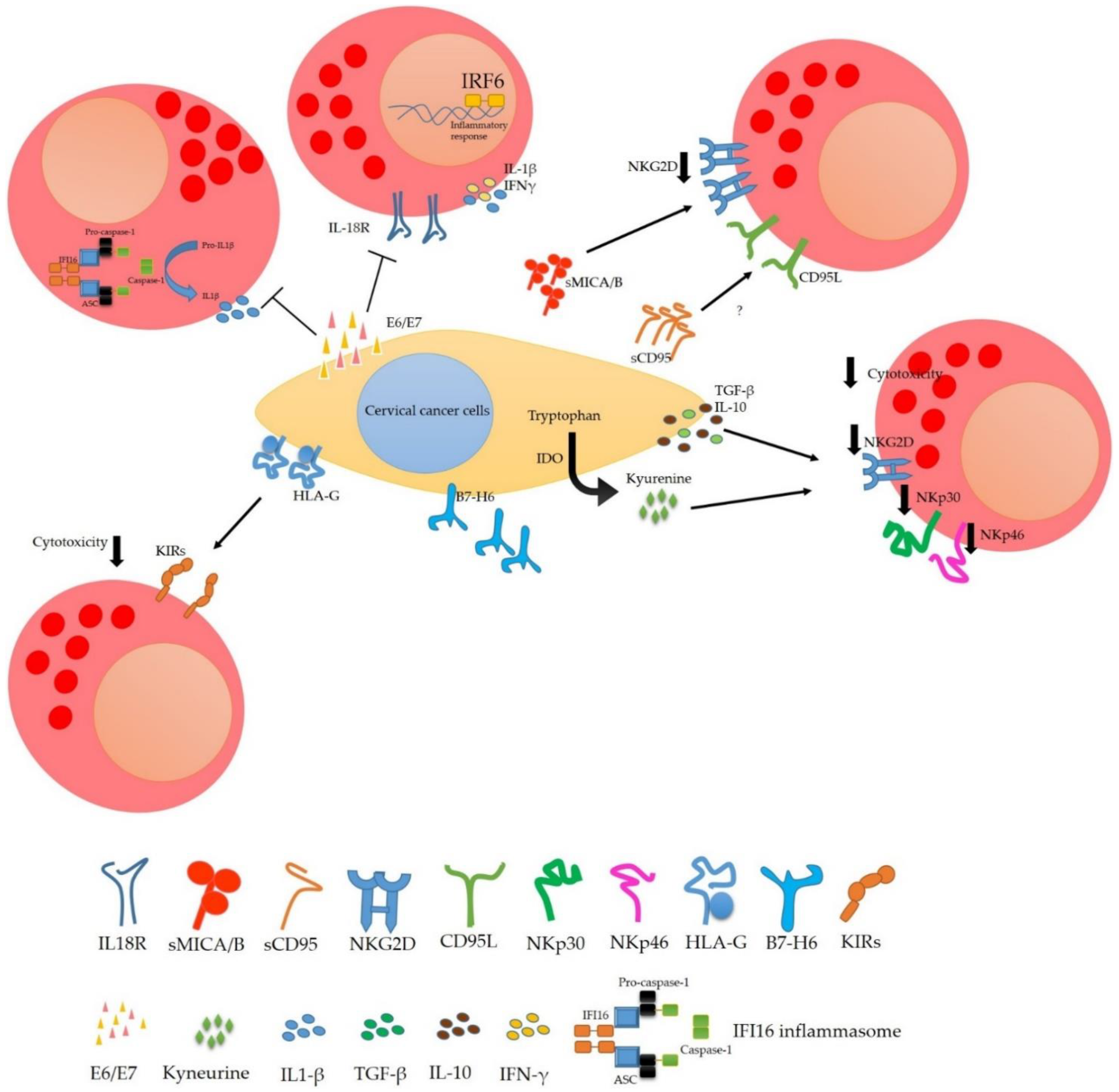
4. Modulation of the NK Cells Response in Cervical Cancer
5. NK Cells and Immunotherapy in Cervical Cancer
5.1. Treatments That Enhance NK Cell Activity
5.2. Therapies Based on the Infusion of NK Cells in Cervical Cancer
6. Novel Cellular Immunotherapies to Treat Cervical Cancer
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bansal, A.; Singh, M.P.; Rai, B. Human Papillomavirus-Associated Cancers: A Growing Global Problem. Int. J. Appl. Basic Med. Res. 2016, 6, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Huang, X.; Zhang, Y. Involvement of Human Papillomaviruses in Cervical Cancer. Front. Microbiol. 2018, 9, 2896. [Google Scholar] [CrossRef] [PubMed]
- Saraiya, M.; Unger, E.R.; Thompson, T.D.; Lynch, C.F.; Hernandez, B.Y.; Lyu, C.W.; Steinau, M.; Watson, M.; Wilkinson, E.J.; Hopenhayn, C.; et al. US Assessment of HPV Types in Cancers: Implications for Current and 9-Valent HPV Vaccines. JNCI J. Natl. Cancer Inst. 2015, 107, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wendland, E.; Villa, L.; Unger, E.R.; Domingues, C.; Benzaken, A.; POP-Brazil Study Group. Prevalence of HPV Infection among Sexually Active Adolescents and Young Adults in Brazil: The POP-Brazil Study. Sci. Rep. 2020, 10, 4920. [Google Scholar] [CrossRef] [Green Version]
- Dunne, E.F.; Unger, E.R.; Sternberg, M.; McQuillan, G.; Swan, D.C.; Patel, S.S.; Markowitz, L.E. Prevalence of HPV Infection Among Females in the United States. JAMA 2007, 297, 813–819. [Google Scholar] [CrossRef] [Green Version]
- Markowitz, L.E.; Hariri, S.; Lin, C.; Dunne, E.F.; Steinau, M.; McQuillan, G.; Unger, E.R. Reduction in Human Papillomavirus (HPV) Prevalence Among Young Women Following HPV Vaccine Introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J. Infect. Dis. 2013, 208, 385–393. [Google Scholar] [CrossRef] [Green Version]
- McQuillan, G.; Unger, E.R. Prevalence of HPV in Adults Aged 18–69: United States, 2011–2014; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2017; Volume 280, pp. 1–8.
- Shanmugasundaram, S.; You, J. Targeting Persistent Human Papillomavirus Infection. Viruses 2017, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Manzo-Merino, J.; del Toro-Arreola, S.; Rocha-Zavaleta, L.; Peralta-Zaragoza, Ó.; Jiménez-Lima, R.; Madrid-Marina, V. Immunology of Cervical Cancer. Rev. Investig. Clínica 2020, 72, 188–197. [Google Scholar] [CrossRef]
- Christensen, N.D. HPV Disease Transmission Protection and Control. Microb. Cell. 2016, 3, 476–490. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Tuong, Z.K.; Frazer, I.H. Papillomavirus Immune Evasion Strategies Target the Infected Cell and the Local Immune System. Front. Oncol. 2019, 9, 682. [Google Scholar] [CrossRef] [Green Version]
- Amador-Molina, A.; Hernández-Valencia, J.F.; Lamoyi, E.; Contreras-Paredes, A.; Lizano, M. Role of Innate Immunity against Human Papillomavirus (HPV) Infections and Effect of Adjuvants in Promoting Specific Immune Response. Viruses 2013, 5, 2624–2642. [Google Scholar] [CrossRef] [Green Version]
- Sasagawa, T.; Takagi, H.; Makinoda, S. Immune Responses against Human Papillomavirus (HPV) Infection and Evasion of Host Defense in Cervical Cancer. J. Infect. Chemother. 2012, 18, 807–815. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Y.; Shi, C. Targeting Natural Killer Cells for Tumor Immunotherapy. Front. Immunol. 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK Cell-Based Cancer Immunotherapy: From Basic Biology to Clinical Development. J. Hematol. Oncol. 2021, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Regalado Porras, G.O.; Chávez Nogueda, J.; Poitevin Chacón, A. Chemotherapy and Molecular Therapy in Cervical Cancer. Rep. Pract. Oncol. Radiother. 2018, 23, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Peiretti, M.; Zapardiel, I.; Zanagnolo, V.; Landoni, F.; Morrow, C.P.; Maggioni, A. Management of Recurrent Cervical Cancer: A Review of the Literature. Surg. Oncol. 2012, 21, e59–e66. [Google Scholar] [CrossRef]
- Hung, M.-C.; Liu, M.-T.; Cheng, Y.; Wang, J.-D. Estimation of Savings of Life-Years and Cost from Early Detection of Cervical Cancer: A Follow-up Study Using Nationwide Databases for the Period 2002–2009. BMC Cancer 2014, 14, 505. [Google Scholar] [CrossRef] [Green Version]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef] [Green Version]
- Graham, S.V. The Human Papillomavirus Replication Cycle, and Its Links to Cancer Progression: A Comprehensive Review. Clin. Sci. 2017, 131, 2201–2221. [Google Scholar] [CrossRef] [Green Version]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic Human Papillomavirus Infection. Nat. Rev. Dis. Primers 2016, 2, 1–20. [Google Scholar] [CrossRef]
- Spriggs, C.; Laimins, L. Human Papillomavirus and the DNA Damage Response: Exploiting Host Repair Pathways for Viral Replication. Viruses 2017, 9, 232. [Google Scholar] [CrossRef] [Green Version]
- Syrjänen, S. Oral Manifestations of Human Papillomavirus Infections. Eur. J. Oral. Sci. 2018, 126, 49–66. [Google Scholar] [CrossRef]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front. Microbiol. 2020, 10, 3116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, K.; Peh, W.; Southern, S.; Griffin, H.; Sotlar, K.; Nakahara, T.; El-Sherif, A.; Morris, L.; Seth, R.; Hibma, M.; et al. Organization of Human Papillomavirus Productive Cycle during Neoplastic Progression Provides a Basis for Selection of Diagnostic Markers. J. Virol. 2003, 77, 10186–10201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trimble, C.L. Spontaneous Regression of High-Grade Cervical Dysplasia: Effects of Human Papillomavirus Type and HLA Phenotype. Clin. Cancer Res. 2005, 11, 4717–4723. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.W.; So, K.A.; Lee, J.K.; Hong, J.H. Type-Specific Persistence or Regression of Human Papillomavirus Genotypes in Women with Cervical Intraepithelial Neoplasia 1: A Prospective Cohort Study. Obs. Gynecol. Sci. 2015, 58, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Lebre, M.C.; van der Aar, A.M.G.; van Baarsen, L.; van Capel, T.M.M.; Schuitemaker, J.H.N.; Kapsenberg, M.L.; de Jong, E.C. Human Keratinocytes Express Functional Toll-Like Receptor 3, 4, 5, and 9. J. Investig. Dermatol. 2007, 127, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Nasu, K.; Narahara, H. Pattern Recognition via the Toll-Like Receptor System in the Human Female Genital Tract. Mediat. Inflamm. 2010, 2010, 976024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamoutounour, S.; Han, S.-J.; Deckers, J.; Constantinides, M.G.; Hurabielle, C.; Harrison, O.J.; Bouladoux, N.; Linehan, J.L.; Link, V.M.; Vujkovic-Cvijin, I.; et al. Keratinocyte-Intrinsic MHCII Expression Controls Microbiota-Induced Th1 Cell Responses. Proc. Natl. Acad. Sci. USA 2019, 116, 23643–23652. [Google Scholar] [CrossRef]
- Gonçalves, M.A.G.; Donadi, E.A. Immune Cellular Response to HPV: Current Concepts. Braz. J. Infect. Dis. 2004, 8, 1–9. [Google Scholar] [CrossRef]
- Hibma, M.H. The Immune Response to Papillomavirus During Infection Persistence and Regression. Open Virol. J. 2012, 6, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Mah, A.Y.; Cooper, M.A. Metabolic Regulation of Natural Killer Cell IFN-γ Production. Crit. Rev. Immunol. 2016, 36, 131–147. [Google Scholar] [CrossRef]
- Ferns, D.M.; Heeren, A.M.; Samuels, S.; Bleeker, M.C.G.; de Gruijl, T.D.; Kenter, G.G.; Jordanova, E.S. Classical and Non-Classical HLA Class I Aberrations in Primary Cervical Squamous- and Adenocarcinomas and Paired Lymph Node Metastases. J. Immunother. Cancer 2016, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Chung, J.-Y.; Kim, S.; Braunschweig, T.; Kang, T.H.; Kim, J.; Chung, E.J.; Hewitt, S.M.; Kim, J.-H. MICA/B and ULBP1 NKG2D Ligands Are Independent Predictors of Good Prognosis in Cervical Cancer. BMC Cancer 2014, 14, 957. [Google Scholar] [CrossRef] [PubMed]
- Utami, T. NK-Cell Count and Its Function in Producing Interferon Gamma Associated with the Cervical Cancer Natural History. Glob. J. Reprod. Med. 2018, 8, 1–4. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [Green Version]
- Miyazato, K.; Hayakawa, Y. Pharmacological Targeting of Natural Killer Cells for Cancer Immunotherapy. Cancer Sci. 2020, 111, 1869–1875. [Google Scholar] [CrossRef]
- Long, E.O. Negative Signalling by Inhibitory Receptors: The NK Cell Paradigm. Immunol. Rev. 2008, 224, 70–84. [Google Scholar] [CrossRef] [Green Version]
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Ghayur, T.; Carson, W.E.; Caligiuri, M.A. Human Natural Killer Cells: A Unique Innate Immunoregulatory Role for the CD56bright Subset. Blood 2001, 97, 3146–3151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The Biology of Human Natural Killer-Cell Subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Jenkins, D.; Tay, S.K.; Singer, A.; Tay, S.K. Natural Killer Cells in Cervical Intraepithelial Neoplasia and Human Papillomavirus Infection. BJOG Int. J. Obstet. Gynaecol. 1987, 94, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.B.; Tozetti, I.A.; Gatto, F.A.; Cassandri, F.; Ferreira, A.M.T.; Carlos Eurico dos Santos, F.; Falcão, G.R.; Scapulatempo, I.D.L.; Padovani, C.T.J.; Abdo, M.A.G.S. Linfócitos CD4, CD8 e células NK no estroma da cérvice uterina de mulheres infectadas pelo papilomavírus humano. Rev. Soc. Bras. Med. Trop. 2010, 43, 425–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Jin, S.; Li, X.; Liu, L.; Xi, L.; Wang, F.; Zhang, S. Human Papillomavirus Type 16 Disables the Increased Natural Killer Cells in Early Lesions of the Cervix. J. Immunol. Res. 2019, 2019, e9182979. [Google Scholar] [CrossRef] [PubMed]
- Textor, S.; Dürst, M.; Jansen, L.; Accardi, R.; Tommasino, M.; Trunk, M.J.; Porgador, A.; Watzl, C.; Gissmann, L.; Cerwenka, A. Activating NK Cell Receptor Ligands Are Differentially Expressed during Progression to Cervical Cancer. Int. J. Cancer 2008, 123, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Vaquer, S.; Jordá, J.; de la Osa, E.L.; de los Heros, J.A.; López-García, N.; de Mon, M.A. Clinical Implications of Natural Killer (NK) Cytotoxicity in Patients with Squamous Cell Carcinoma of the Uterine Cervix. Gynecol. Oncol. 1990, 36, 90–92. [Google Scholar] [CrossRef]
- Senju, H.; Kumagai, A.; Nakamura, Y.; Yamaguchi, H.; Nakatomi, K.; Fukami, S.; Shiraishi, K.; Harada, Y.; Nakamura, M.; Okamura, H.; et al. Effect of IL-18 on the Expansion and Phenotype of Human Natural Killer Cells: Application to Cancer Immunotherapy. Int. J. Biol. Sci. 2018, 14, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-J.; Cho, Y.; Cho, M.-C.; Shim, J.-H.; Lee, K.-S.; Ko, K.-K.; Choe, Y.; Park, S.-N.; Hoshino, T.; Kim, S.; et al. Both E6 and E7 Oncoproteins of Human Papillomavirus 16 Inhibit IL-18-Induced IFN-γ Production in Human Peripheral Blood Mononuclear and NK Cells. J. Immunol. 2001, 167, 497–504. [Google Scholar] [CrossRef]
- Song, Y.; Wu, X.; Xu, Y.; Zhu, J.; Li, J.; Zou, Z.; Chen, L.; Zhang, B.; Hua, C.; Rui, H.; et al. HPV E7 Inhibits Cell Pyroptosis by Promoting TRIM21-Mediated Degradation and Ubiquitination of the IFI16 Inflammasome. Int. J. Biol. Sci. 2020, 16, 2924–2937. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Ponnappan, A.; Mehta, V.; Wewers, M.D.; Caligiuri, M.A. Interleukin-1β Costimulates Interferon-γ Production by Human Natural Killer Cells. Eur. J. Immunol. 2001, 31, 792–801. [Google Scholar] [CrossRef]
- Ainouze, M.; Rochefort, P.; Parroche, P.; Roblot, G.; Tout, I.; Briat, F.; Zannetti, C.; Marotel, M.; Goutagny, N.; Auron, P.; et al. Human Papillomavirus Type 16 Antagonizes IRF6 Regulation of IL-1β. PLOS Pathog. 2018, 14, e1007158. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Iglesias, T.; del Toro-Arreola, A.; Albarran-Somoza, B.; del Toro-Arreola, S.; Sanchez-Hernandez, P.E.; Ramirez-Dueñas, M.G.; Balderas-Peña, L.M.A.; Bravo-Cuellar, A.; Ortiz-Lazareno, P.C.; Daneri-Navarro, A. Low NKp30, NKp46 and NKG2D Expression and Reduced Cytotoxic Activity on NK Cells in Cervical Cancer and Precursor Lesions. BMC Cancer 2009, 9, 186. [Google Scholar] [CrossRef] [Green Version]
- Weiss-Steider, B.; Soto-Cruz, I.; Martinez-Campos, C.A.; Mendoza-Rincon, J.F. Expression of MICA, MICB and NKG2D in Human Leukemic Myelomonocytic and Cervical Cancer Cells. J. Exp. Clin. Cancer Res. 2011, 30, 37. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Hoya, A.; Zerecero-Carreón, O.; Valle-Mendiola, A.; Moreno-Lafont, M.; López-Santiago, R.; Weiss-Steider, B.; Soto-Cruz, I. Cervical Cancer Cells Express Markers Associated with Immunosurveillance. J. Immunol. Res. 2019, 2019, 1242979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arreygue-Garcia, N.A.; Daneri-Navarro, A.; del Toro-Arreola, A.; Cid-Arregui, A.; Gonzalez-Ramella, O.; Jave-Suarez, L.F.; Aguilar-Lemarroy, A.; Troyo-Sanroman, R.; Bravo-Cuellar, A.; Delgado-Rizo, V.; et al. Augmented Serum Level of Major Histocompatibility Complex Class I-Related Chain A (MICA) Protein and Reduced NKG2D Expression on NK and T Cells in Patients with Cervical Cancer and Precursor Lesions. BMC Cancer 2007, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Lazarova, M.; Steinle, A. Impairment of NKG2D-Mediated Tumor Immunity by TGF-β. Front. Immunol. 2019, 10, 2689. [Google Scholar] [CrossRef] [Green Version]
- Regis, S.; Dondero, A.; Caliendo, F.; Bottino, C.; Castriconi, R. NK Cell Function Regulation by TGF-β-Induced Epigenetic Mechanisms. Front. Immunol. 2020, 11, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Wei, M.; Becknell, B.; Trotta, R.; Liu, S.; Boyd, Z.; Jaung, M.S.; Blaser, B.W.; Sun, J.; Benson, D.M.; et al. Pro- and Antiinflammatory Cytokine Signaling: Reciprocal Antagonism Regulates Interferon-Gamma Production by Human Natural Killer Cells. Immunity 2006, 24, 575–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.-H.; Yan, W.-H.; Lin, A. The Role of HLA-G in Human Papillomavirus Infections and Cervical Carcinogenesis. Front. Immunol. 2020, 11, 1349. [Google Scholar] [CrossRef]
- Shukla, S.; Mahata, S.; Shishodia, G.; Pandey, A.; Tyagi, A.; Vishnoi, K.; Basir, S.F.; Das, B.C.; Bharti, A.C. Functional Regulatory Role of STAT3 in HPV16-Mediated Cervical Carcinogenesis. PLoS ONE 2013, 8, e67849. [Google Scholar] [CrossRef] [Green Version]
- Cacalano, N.A. Regulation of Natural Killer Cell Function by STAT3. Front. Immunol. 2016, 7, 128. [Google Scholar] [CrossRef]
- Venancio, P.A.; Consolaro, M.E.L.; Derchain, S.F.; Boccardo, E.; Villa, L.L.; Maria-Engler, S.S.; Campa, A.; Discacciati, M.G. Indoleamine 2,3-Dioxygenase and Tryptophan 2,3-Dioxygenase Expression in HPV Infection, SILs, and Cervical Cancer. Cancer Cytopathol. 2019, 127, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Ferns, D.M.; Kema, I.P.; Buist, M.R.; Nijman, H.W.; Kenter, G.G.; Jordanova, E.S. Indoleamine-2,3-Dioxygenase (IDO) Metabolic Activity is Detrimental for Cervical Cancer Patient Survival. OncoImmunology 2015, 4, e981457. [Google Scholar] [CrossRef] [PubMed]
- Inaba, T.; Ino, K.; Kajiyama, H.; Shibata, K.; Yamamoto, E.; Kondo, S.; Umezu, T.; Nawa, A.; Takikawa, O.; Kikkawa, F. Indoleamine 2,3-Dioxygenase Expression Predicts Impaired Survival of Invasive Cervical Cancer Patients Treated with Radical Hysterectomy. Gynecol. Oncol. 2010, 117, 423–428. [Google Scholar] [CrossRef]
- Song, H.; Park, H.; Kim, J.; Park, G.; Kim, Y.-S.; Kim, S.M.; Kim, D.; Seo, S.K.; Lee, H.-K.; Cho, D.; et al. IDO Metabolite Produced by EBV-Transformed B Cells Inhibits Surface Expression of NKG2D in NK Cells via the c-Jun N-Terminal Kinase (JNK) Pathway. Immunol. Lett. 2011, 136, 187–193. [Google Scholar] [CrossRef]
- Song, H.; Park, H.; Kim, Y.-S.; Kim, K.D.; Lee, H.-K.; Cho, D.-H.; Yang, J.-W.; Hur, D.Y. L-Kynurenine-Induced Apoptosis in Human NK Cells is Mediated by Reactive Oxygen Species. Int. Immunopharmacol. 2011, 11, 932–938. [Google Scholar] [CrossRef]
- Chiesa, M.D.; Carlomagno, S.; Frumento, G.; Balsamo, M.; Cantoni, C.; Conte, R.; Moretta, L.; Moretta, A.; Vitale, M. The Tryptophan Catabolite L-Kynurenine Inhibits the Surface Expression of NKp46- and NKG2D-Activating Receptors and Regulates NK-Cell Function. Blood 2006, 108, 4118–4125. [Google Scholar] [CrossRef] [PubMed]
- Cooley, S.; He, F.; Bachanova, V.; Vercellotti, G.M.; DeFor, T.E.; Curtsinger, J.M.; Robertson, P.; Grzywacz, B.; Conlon, K.C.; Waldmann, T.A.; et al. First-in-Human Trial of RhIL-15 and Haploidentical Natural Killer Cell Therapy for Advanced Acute Myeloid Leukemia. Blood Adv. 2019, 3, 1970–1980. [Google Scholar] [CrossRef]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful Adoptive Transfer and in Vivo Expansion of Human Haploidentical NK Cells in Patients with Cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [Green Version]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-Induced Memory-like Natural Killer Cells Exhibit Enhanced Responses against Myeloid Leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, N.; Ishikawa, T.; Kokura, S.; Okayama, T.; Oka, K.; Ideno, M.; Sakai, F.; Kato, A.; Tanabe, M.; Enoki, T.; et al. Phase I Clinical Trial of Autologous NK Cell Therapy Using Novel Expansion Method in Patients with Advanced Digestive Cancer. J. Transl. Med. 2015, 13, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Khatua, S.; Cooper, L.J.N.; Sandberg, D.I.; Ketonen, L.; Johnson, J.M.; Rytting, M.E.; Liu, D.D.; Meador, H.; Trikha, P.; Nakkula, R.J.; et al. Phase I Study of Intraventricular Infusions of Autologous Ex Vivo Expanded NK Cells in Children with Recurrent Medulloblastoma and Ependymoma. Neuro-Oncol. 2020, 22, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lim, O.; Kim, T.M.; Ahn, Y.-O.; Choi, H.; Chung, H.; Min, B.; Her, J.H.; Cho, S.Y.; Keam, B.; et al. Phase I Study of Random Healthy Donor–Derived Allogeneic Natural Killer Cell Therapy in Patients with Malignant Lymphoma or Advanced Solid Tumors. Cancer Immunol. Res. 2016, 4, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Shimasaki, N.; Jain, A.; Campana, D. NK Cells for Cancer Immunotherapy. Nat. Rev. Drug. Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Xia, C.; He, Z.; Cai, Y.; Liang, S. Vorinostat Upregulates MICA via the PI3K/Akt Pathway to Enhance the Ability of Natural Killer Cells to Kill Tumor Cells. Eur. J. Pharmacol. 2020, 875, 173057. [Google Scholar] [CrossRef]
- Sato, N.; Saga, Y.; Mizukami, H.; Wang, D.; Takahashi, S.; Nonaka, H.; Fujiwara, H.; Takei, Y.; Machida, S.; Takikawa, O.; et al. Downregulation of Indoleamine-2,3-Dioxygenase in Cervical Cancer Cells Suppresses Tumor Growth by Promoting Natural Killer Cell Accumulation. Oncol. Rep. 2012, 28, 1574–1578. [Google Scholar] [CrossRef] [Green Version]
- Tang, K.; Wu, Y.-H.; Song, Y.; Yu, B. Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitors in Clinical Trials for Cancer Immunotherapy. J Hematol. Oncol. 2021, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- News in Brief. Blocking IDO1 Helps Shrink Bladder, Cervical Tumors. Cancer Discov. 2018, 8, OF3. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Lomelí, P.; Bravo-Cuellar, A.; Hernández-Flores, G.; Jave-Suárez, L.F.; Aguilar-Lemarroy, A.; Lerma-Díaz, J.M.; Domínguez-Rodríguez, J.R.; Sánchez-Reyes, K.; Ortiz-Lazareno, P.C. Increase of IFN-γ and TNF-γ Production in CD107a + NK-92 Cells Co-Cultured with Cervical Cancer Cell Lines Pre-Treated with the HO-1 Inhibitor. Cancer Cell Int. 2014, 14, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, A.; Zenclussen, A.C. Effects of Heme Oxygenase-1 on Innate and Adaptive Immune Responses Promoting Pregnancy Success and Allograft Tolerance. Front. Pharm. 2015, 5, 288. [Google Scholar] [CrossRef] [Green Version]
- García, D.A.; Pérez, P.; García, L.; Cid-Arregui, A.; Aristizabal, F. Expresión génica de ligandos mica, micb y ulbp (1–6) del receptor NKG2D de células natural killer y metaloproteinasas adam10, adam17 y mmp14 en lineas celulares de cancer de cervical. Rev. Colomb. Biotecnol. 2019, 21, 29–38. [Google Scholar] [CrossRef]
- Isa, S.A.M.; Salleh, M.S.M.; Ismail, M.P.; Hairon, S.M. ADAM9 Expression in Uterine Cervical Cancer and Its Associated Factors. Asian Pac. J. Cancer Prev. 2019, 20, 1081–1087. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Ying, M.; Chen, G.; Lin, A.; Xie, Y.; Ohara, N.; Zhou, D. ADAM17 is Associated with EMMPRIN and Predicts Poor Prognosis in Patients with Uterine Cervical Carcinoma. Tumor. Biol. 2014, 35, 7575–7586. [Google Scholar] [CrossRef]
- Zubel, A.; Flechtenmacher, C.; Edler, L.; Alonso, A. Expression of ADAM9 in CIN3 Lesions and Squamous Cell Carcinomas of the Cervix. Gynecol. Oncol. 2009, 114, 332–336. [Google Scholar] [CrossRef]
- Schlecker, E.; Fiegler, N.; Arnold, A.; Altevogt, P.; Rose-John, S.; Moldenhauer, G.; Sucker, A.; Paschen, A.; von Strandmann, E.P.; Textor, S.; et al. Metalloprotease-Mediated Tumor Cell Shedding of B7-H6, the Ligand of the Natural Killer Cell–Activating Receptor NKp30. Cancer Res. 2014, 74, 3429–3440. [Google Scholar] [CrossRef] [Green Version]
- Waldhauer, I.; Goehlsdorf, D.; Gieseke, F.; Weinschenk, T.; Wittenbrink, M.; Ludwig, A.; Stevanovic, S.; Rammensee, H.-G.; Steinle, A. Tumor-Associated MICA Is Shed by ADAM Proteases. Cancer Res. 2008, 68, 6368–6376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Liu, S.-L.; Wang, H.; Shi, G.; Yang, P.; Chen, X.-L. MiR-126 Suppresses the Proliferation of Cervical Cancer Cells and Alters Cell Sensitivity to the Chemotherapeutic Drug Bleomycin. Asian Pac. J. Cancer Prev. 2013, 14, 6569–6572. [Google Scholar] [CrossRef] [Green Version]
- Mishra, H.K.; Dixon, K.J.; Pore, N.; Felices, M.; Miller, J.S.; Walcheck, B. Activation of ADAM17 by IL-15 Limits Human NK Cell Proliferation. Front. Immunol. 2021, 12, 2958. [Google Scholar] [CrossRef]
- Pham, D.; Kim, J.-S.; Kim, S.-K.; Shin, D.; Uong, N.-T.-T.; Hyun, H.; Yoon, M.; Kang, S.J.; Ryu, Y.; Cho, J.; et al. Effects of ADAM10 and ADAM17 Inhibitors on Natural Killer Cell Expansion and Antibody-Dependent Cellular Cytotoxicity Against Breast Cancer Cells In Vitro. Anticancer Res. 2017, 37, 5507–5513. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Nie, J.; Huang, J.-P.; Zheng, G.-J.; Feng, B. Targeting STAT3 Inhibition to Reverse Cisplatin Resistance. Biomed. Pharmacother. 2019, 117, 109135. [Google Scholar] [CrossRef]
- Gotthardt, D.; Putz, E.M.; Straka, E.; Kudweis, P.; Biaggio, M.; Poli, V.; Strobl, B.; Müller, M.; Sexl, V. Loss of STAT3 in Murine NK Cells Enhances NK Cell–Dependent Tumor Surveillance. Blood 2014, 124, 2370–2379. [Google Scholar] [CrossRef]
- Saraswati, W.; Dahlan, E.G.; Saputra, K.; Sutrisno, T.C. Effect of Electroacupuncture on Natural-Killer Cells and Tumor Size in Patients with Cervical Squamous-Cell Carcinoma: A Randomized Controlled Trial. Med. Acupunct. 2019, 31, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, Q.; Zhang, X.; Wang, X.; Su, Y.; He, W.; Li, J.; Wan, H.; Jing, X. Electroacupuncture Regulates Inflammatory Cytokines by Activating the Vagus Nerve to Enhance Antitumor Immunity in Mice with Breast Tumors. Life Sci. 2021, 272, 119259. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.F.; Ortiz Sánchez, E.; Vujanovic, N.L.; Li, W. Acupuncture May Stimulate Anticancer Immunity via Activation of Natural Killer Cells. Evid.-Based Complement. Altern. Med. 2011, 2011, enep236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M.H.; Kim, J.; Lim, S.A.; Kim, J.; Kim, S.-J.; Lee, K.-M. NK Cell-Based Immunotherapies in Cancer. Immune Netw. 2020, 20, e14. [Google Scholar] [CrossRef]
- Veluchamy, J.P.; Heeren, A.M.; Spanholtz, J.; van Eendenburg, J.D.H.; Heideman, D.A.M.; Kenter, G.G.; Verheul, H.M.; van der Vliet, H.J.; Jordanova, E.S.; de Gruijl, T.D. High-Efficiency Lysis of Cervical Cancer by Allogeneic NK Cells Derived from Umbilical Cord Progenitors is Independent of HLA Status. Cancer Immunol. Immunother. 2017, 66, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.; Xu, K.; Liang, S.; Wang, X.; Liang, Y.; Zhang, M.; Chen, J.; Niu, L. Prospective Study of Percutaneous Cryoablation Combined with Allogenic NK Cell Immunotherapy for Advanced Renal Cell Cancer. Immunol. Lett. 2017, 184, 98–104. [Google Scholar] [CrossRef]
- Marofi, F.; Al-Awad, A.S.; Sulaiman Rahman, H.; Markov, A.; Abdelbasset, W.K.; Ivanovna Enina, Y.; Mahmoodi, M.; Hassanzadeh, A.; Yazdanifar, M.; Stanley Chartrand, M.; et al. CAR-NK Cell: A New Paradigm in Tumor Immunotherapy. Front. Oncol. 2021, 11, 2078. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Zhang, F.; Li, J.; Lu, X.; Yuan, N.; Hao, X.; Zhang, Z. Antitumor activity of chimeric antigen receptor NK-92 cells targeting PSCA against cervical cancer. Chin. J. Cancer Biother. 2021, 27, 1345–1350. [Google Scholar] [CrossRef]
- Huang, R.-S.; Shih, H.-A.; Lai, M.-C.; Chang, Y.-J.; Lin, S. Enhanced NK-92 Cytotoxicity by CRISPR Genome Engineering Using Cas9 Ribonucleoproteins. Front. Immunol. 2020, 11, 1008. [Google Scholar] [CrossRef]


| Receptor | Ligand | |
|---|---|---|
| Activating Receptors | ||
| NKp30 | B7-H6, BAG6, Galetin-3, heparan sulfate proteoglycan (HSPG) | |
| NKp44 | Viral hemagglutinin (HA), haemagglutinin-neuraminidase (HN), glycoproteins and proteoglycans, nuclear proteins that can be exposed outside the cell | |
| NKp46 | HA, HN, heparan sulfate (HS), glucosaminoglycans (GAGs) | |
| NKp80 | activation-induced C-type lectin (AICL) | |
| KIR-S | HLA-C, HLA-B | |
| NKG2C | HLA-E | |
| NKG2D | MICA/B, UBLP1-6 | |
| NKG2E | HLA-E | |
| CD2 | CD48 | |
| CD16 | Fc IgG | |
| CD95L | CD95 | |
| CD96 | CD155 | |
| CD226 (DNAM-1) | CD112, CD155 | |
| Inhibiting Receptors | ||
| KIR-L | HLA-A, B, C | |
| NKG2A | HLA-E | |
| NKG2B | HLA-E | |
| TIGIT | Nectin 4, CD112, CD155 | |
| PD-1 | PDL1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Hoya, A.; Soto-Cruz, I. NK Cell Regulation in Cervical Cancer and Strategies for Immunotherapy. Cells 2021, 10, 3104. https://doi.org/10.3390/cells10113104
Gutiérrez-Hoya A, Soto-Cruz I. NK Cell Regulation in Cervical Cancer and Strategies for Immunotherapy. Cells. 2021; 10(11):3104. https://doi.org/10.3390/cells10113104
Chicago/Turabian StyleGutiérrez-Hoya, Adriana, and Isabel Soto-Cruz. 2021. "NK Cell Regulation in Cervical Cancer and Strategies for Immunotherapy" Cells 10, no. 11: 3104. https://doi.org/10.3390/cells10113104
APA StyleGutiérrez-Hoya, A., & Soto-Cruz, I. (2021). NK Cell Regulation in Cervical Cancer and Strategies for Immunotherapy. Cells, 10(11), 3104. https://doi.org/10.3390/cells10113104






