The Challenging Melanoma Landscape: From Early Drug Discovery to Clinical Approval
Abstract
1. Introduction
2. Melanoma—Therapeutic Management
3. Drug Discovery and Development (Preclinical Research)
3.1. In Silico Models
3.1.1. Structure-Based Approaches
3.1.2. Ligand-Based Approaches
3.2. In Vitro Models
3.2.1. 2D Models
3.2.2. 3D Models
Spheroid Model
Tumorosphere Model
Organoid Model
Skin Reconstruct Model and Bioprinting
Melanoma-on-Chip Model
Neoangiogenesis Model
3.3. In Vivo Models
3.3.1. Murine Models of Melanoma
Syngeneic Model
| Experimental Model | Advantages | Disadvantages | References |
|---|---|---|---|
| Syngeneic | Functional immune system. Fast and easy to establish Tumor interaction with the microenvironment Metastasis formation Both tumor cells and mouse with the same genetic background | Less predictive for clinical translation Different anatomy, physiology and biochemistry compared to human (e.g., adhesion proteins and growth factors) Not properly reproducing the interactions between cancer cells and the immune system Limited availability of cell lines Rapid and uncontrolled cell growth | [73,90,109,186] |
| Xenograft | Use of human tumor samples Heterogeneity Metastasis formation Simple to accomplish Possibilities for “co-clinical trials” Study of drug resistance Large number of available human cell lines Tumors are easily and precisely measured | Time-consuming Expensive (compared with immunocompetent mice) Lack of immune system Poorly predictive of clinical outcomes Lack of standardized and reproducible protocols and inadequacy to study the early phases of tumor growth (PDX models) Different tumor evolution as compared to parental lesion | [90,109,181,186] |
| Genetically Engineered | Specific gene mutation Combination of multiple gene mutations Functional immune system Stepwise tumor progression Phenotypic, histological, and genetic similarities to human counterparts Modulation of human cancer under physiological conditions Tumors develop in the tissue of origin | Inability to replicate the characteristics of the advanced melanoma Expensive, time-consuming and labor intensive Different anatomy, physiology, and biochemistry (mouse versus human) Lack of different genetic background and tissue-specific promoters Asynchronous development of tumors. Heterogeneity Restricted use due to intellectual property rights and patents | [90,109,181,186,203,204] |
| Radiation-induced | Useful for studying the risk factors, pathogenesis and development of human melanoma | Long time for tumor development High costs in animal maintenance/care Lack of responsiveness by mice Histologically and anatomically different from human melanoma | [186] |
| Carcinogen-induced | Simple to accomplish The tumors are easily visualized, not requiring invasive processes for tumor monitoring Recapitulate the time-dependent and multi-stage progression of tumor pathogenesis Functional immune system Can be used in combination with other models | Repeated use of carcinogenic agents Outbred mice with non-uniform genetic backgrounds and varied sensitivity to carcinogens Nonpigmented lesions when melanoma is induced by certain carcinogenic agents Not clinically relevant for human melanoma | [185,186,203,205] |
Xenograft Model
| Mice Identification | Main Features | Melanoma Research Applications | References |
|---|---|---|---|
| Nude (nu/nu) | Athymic Homozygous for mutation Foxn1nu T cell deficient Hairless Cell line engraftment | Pathophysiological mechanisms Novel therapies/therapy resistance Nano-based therapeutic approaches Prognostic biomarkers and molecular imaging | [213,214,215,216,217,218,219] |
| SCID | Homozygous for the spontaneous mutation Prkdcscid T and B cell deficient Cell line/tumor engraftment | Pathophysiological mechanisms Biodistribution studies | [220,221,222,223] |
| NOD/SCID | Homozygous for the spontaneous mutation Prkdcscid T and B cell deficient Impaired function of macrophages, DC and NK cells Cell line/tumor engraftment | Pathophysiological mechanisms Gene therapy Adjuvant therapy for brain metastasis Discovery of novel therapeutic targets | [224,225,226,227] |
| NSG | NOD/SCID IL2rgnull T and B cell deficient Impaired function of macrophages and DC Lack of NK cells Enhanced tumor engraftment | Pathophysiological mechanisms Therapy resistance Novel therapies/combination therapy Chemoprevention Development of imaging probes Biodistribution studies Identification of cell subpopulations | [217,226,228,229,230,231,232,233,234,235] |
| hu-NSG | NSG with humanized immune system induced by CD34+ HSC or PBMC | Prediction of patients’ response to immunotherapy Imaging of therapeutic targets Therapy resistance | [236,237,238,239] |
Genetically Engineered Model
Radiation-Induced Model
Carcinogen-Induced Model
3.3.2. Zebrafish Model
3.3.3. Canine Models
3.3.4. Other Animal Models
4. Ongoing Clinical Trials
5. Approval for Marketing by Regulatory Agencies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eddy, K.; Chen, S. Overcoming immune evasion in melanoma. Int. J. Mol. Sci. 2020, 21, 8984. [Google Scholar] [CrossRef]
- Boussios, S.; Rassy, E.; Samartzis, E.; Moschetta, M.; Sheriff, M.; Pérez-Fidalgo, J.A.; Pavlidis, N. Melanoma of unknown primary: New perspectives for an old story. Crit. Rev. Oncol. Hematol. 2020, 158, 103208. [Google Scholar] [CrossRef]
- Pinho, J.O.; Matias, M.; Gaspar, M.M. Emergent nanotechnological strategies for systemic chemotherapy against melanoma. Nanomaterials 2019, 9, 1455. [Google Scholar] [CrossRef]
- Rahmati, M.; Ebrahim, S.; Hashemi, S.; Motamedi, M.; Moosavi, M.A. New insights on the role of autophagy in the pathogenesis and treatment of melanoma. Mol. Biol. Rep. 2020, 47, 9021–9032. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, N.; Yin, C.; Zhu, B.; Li, X. Ultraviolet radiation and melanomagenesis: From mechanism to immunotherapy. Front. Oncol. 2020, 10, 951. [Google Scholar] [CrossRef]
- Mo, R.; Chen, C.; Mi, L.; Ma, Z.; Tan, Q. Skin melanoma survival is not superior in females in the new stage IIID of the 8th edition of the staging system: An analysis of data from the Surveillance, Epidemiology, and End Results (SEER) database. Ann. Transl. Med. 2020, 8, 1381. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.; Smith, C.; Wernberg, J. Epidemiology and Risk Factors of Melanoma. Surg. Clin. N. Am. 2020, 100, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of Skin Cancer: Update 2019. Adv. Exp. Med. Biol. 2020, 1268, 123–139. [Google Scholar] [PubMed]
- Ombra, M.N.; Paliogiannis, P.; Stucci, L.S.; Colombino, M.; Casula, M.; Sini, M.C.; Manca, A.; Palomba, G.; Stanganelli, I.; Mandalà, M.; et al. Dietary compounds and cutaneous malignant melanoma: Recent advances from a biological perspective. Nutr. Metab. 2019, 16, 33. [Google Scholar] [CrossRef]
- Sawada, Y.; Nakamura, M. Daily lifestyle and cutaneous malignancies. Int. J. Mol. Sci. 2021, 22, 5227. [Google Scholar] [CrossRef]
- GLOBOCAN. Available online: https://gco.iarc.fr/ (accessed on 18 May 2021).
- Efimenko, M.; Ignatev, A.; Koshechkin, K. Review of medical image recognition technologies to detect melanomas using neural networks. BMC Bioinform. 2020, 21, 270. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.; Olazagasti, J.M.; Cordoro, K.M.; Brewer, J.D.; Taylor, S.C.; Bordeaux, J.S.; Chren, M.M.; Sober, A.J.; Tegeler, C.; Bhushan, R.; et al. Early detection of melanoma: Reviewing the ABCDEs. J. Am. Acad. Dermatol. 2015, 72, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Martos-Cabrera, L.; Sampedro-Ruiz, R.; Delgado-Jimenez, Y.; Gallo, E.; Navarro, R.; Aragües, M.; Llamas-Velasco, M.; Chicharro, P.; Rodríguez-Jiménez, P. Geometric border as a marker for melanoma diagnosis. Study of 200 consecutive melanocytic lesions. Dermatol. Ther. 2020, 34, e14617. [Google Scholar]
- Scolyer, R.A.; Rawson, R.V.; Gershenwald, J.E.; Ferguson, P.M.; Prieto, V.G. Melanoma pathology reporting and staging. Mod. Pathol. 2020, 33, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Hessler, M.; Jalilian, E.; Xu, Q.; Reddy, S.; Horton, L.; Elkin, K.; Manwar, R.; Tsoukas, M.; Mehregan, D.; Avanaki, K. Melanoma biomarkers and their potential application for in vivo diagnostic imaging modalities. Int. J. Mol. Sci. 2020, 21, 9583. [Google Scholar] [CrossRef]
- Suresh, R.; Ziemys, A.; Holder, A.M. Dissecting the lymphatic system to predict melanoma metastasis. Front. Oncol. 2020, 10, 576190. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo 2014, 28, 1005–1012. [Google Scholar]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Leilabadi, S.; Chen, A.; Tsai, S.; Soundararajan, V.; Silberman, H.; Wong, A. Update and review on the surgical management of primary cutaneous melanoma. Healthcare 2014, 2, 234–249. [Google Scholar] [CrossRef]
- Clark, W.H.; Elder, D.E.; Guerry, D.; Epstein, M.N.; Greene, M.H.; Van Horn, M. A study of tumor progression: The precursor lesions of superficial spreading and nodular melanoma. Hum. Pathol. 1984, 15, 1147–1165. [Google Scholar] [CrossRef]
- Mervic, L. Prognostic factors in patients with localized primary cutaneous melanoma. Acta Dermatovenerol. Alp. Pannonica Adriat. 2012, 21, 27–31. [Google Scholar] [PubMed]
- Ward, W.H.; Lambreton, F.; Goel, N.; Yu, J.Q.; Farma, J.M. Clinical Presentation and Staging of Melanoma. In Cutaneous Melanoma: Etiology and Therapy; Codon Publications: Brisbane, Australia, 2017; pp. 79–89. [Google Scholar]
- Moore, M.R.; Friesner, I.D.; Rizk, E.M.; Fullerton, B.T.; Mondal, M.; Trager, M.H.; Mendelson, K.; Chikeka, I.; Kurc, T.; Gupta, R.; et al. Automated digital TIL analysis (ADTA) adds prognostic value to standard assessment of depth and ulceration in primary melanoma. Sci. Rep. 2021, 11, 2809. [Google Scholar] [CrossRef]
- Coit, D.G.; Thompson, J.A.; Albertini, M.R.; Barker, C.; Carson, W.E.; Contreras, C.; Daniels, G.A.; DiMaio, D.; Fields, R.C.; Fleming, M.D.; et al. Cutaneous melanoma, version 2.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 367–402. [Google Scholar] [CrossRef]
- Trinidad, C.M.; Torres-Cabala, C.A.; Curry, J.L.; Prieto, V.G.; Aung, P.P. Update on eighth edition American Joint Committee on Cancer classification for cutaneous melanoma and overview of potential pitfalls in histological examination of staging parameters. J. Clin. Pathol. 2019, 72, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Hauschild, A.; Lindenblatt, N.; Pentheroudakis, G.; Keilholz, U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v126–v132. [Google Scholar] [CrossRef]
- Scala, J.; Vojvodic, A.; Vojvodic, P.; Vlaskovic-Jovicevic, T.; Peric-Hajzler, Z.; Matovic, D.; Dimitrijevic, S.; Vojvodic, J.; Sijan, G.; Stepic, N.; et al. New trends in cutaneous melanoma surgery. Open Access Maced. J. Med. Sci. 2019, 7, 3090–3092. [Google Scholar] [CrossRef] [PubMed]
- Falk Delgado, A.; Zommorodi, S.; Falk Delgado, A. Sentinel lymph node biopsy and complete lymph node dissection for melanoma. Curr. Oncol. Rep. 2019, 21, 54. [Google Scholar] [CrossRef]
- Facciolà, A.; Venanzi Rullo, E.; Ceccarelli, M.; D’Andrea, F.; Coco, M.; Micali, C.; Cacopardo, B.; Marino, A.; Cannavò, S.P.; Di Rosa, M.; et al. Malignant melanoma in HIV: Epidemiology, pathogenesis, and management. Dermatol. Ther. 2020, 33, e13180. [Google Scholar] [CrossRef]
- Pavri, S.N.; Clune, J.; Ariyan, S.; Narayan, D. Malignant melanoma: Beyond the basics. Plast. Reconstr. Surg. 2016, 138, 330e–340e. [Google Scholar] [CrossRef]
- Enomoto, L.M.; Levine, E.A.; Shen, P.; Votanopoulos, K.I. Role of surgery for metastatic melanoma. Surg. Clin. N. Am. 2020, 100, 127–139. [Google Scholar] [CrossRef]
- Kuryk, L.; Bertinato, L.; Staniszewska, M.; Pancer, K.; Wieczorek, M.; Salmaso, S.; Caliceti, P.; Garofalo, M. From conventional therapies to immunotherapy: Melanoma treatment in review. Cancers 2020, 12, 3057. [Google Scholar] [CrossRef] [PubMed]
- Mishra, H.; Mishra, P.K.; Ekielski, A.; Jaggi, M.; Iqbal, Z.; Talegaonkar, S. Melanoma treatment: From conventional to nanotechnology. J. Cancer Res. Clin. Oncol. 2018, 144, 2283–2302. [Google Scholar] [CrossRef] [PubMed]
- Swayden, M.; Chhouri, H.; Anouar, Y.; Grumolato, L. Tolerant/persister cancer cells and the path to resistance to targeted therapy. Cells 2020, 9, 2601. [Google Scholar] [CrossRef] [PubMed]
- Falcone, I.; Conciatori, F.; Bazzichetto, C.; Ferretti, G.; Cognetti, F.; Ciuffreda, L.; Milella, M. Tumor microenvironment: Implications in melanoma resistance to targeted therapy and immunotherapy. Cancers 2020, 12, 2870. [Google Scholar] [CrossRef] [PubMed]
- Unterrainer, M.; Ruzicka, M.; Fabritius, M.P.; Mittlmeier, L.M.; Winkelmann, M.; Rübenthaler, J.; Brendel, M.; Subklewe, M.; von Bergwelt-Baildon, M.; Ricke, J.; et al. PET/CT imaging for tumour response assessment to immunotherapy: Current status and future directions. Eur. Radiol. Exp. 2020, 4, 63. [Google Scholar] [CrossRef]
- Toor, K.; Middleton, M.R.; Chan, K.; Amadi, A.; Moshyk, A.; Kotapati, S. Comparative efficacy and safety of adjuvant nivolumab versus other treatments in adults with resected melanoma: A systematic literature review and network meta-analysis. BMC Cancer 2021, 21, 3. [Google Scholar] [CrossRef]
- Zhang, Q.; Huo, G.; Zhang, H.; Song, Y. Efficacy of pembrolizumab for advanced/metastatic melanoma: A meta-analysis. Open Med. 2020, 15, 447–456. [Google Scholar] [CrossRef]
- Khunger, A.; Piazza, E.; Warren, S.; Smith, T.H.; Ren, X.; White, A.; Elliott, N.; Cesano, A.; Beechem, J.M.; Kirkwood, J.M.; et al. CTLA-4 blockade and interferon-α induce proinflammatory transcriptional changes in the tumor immune landscape that correlate with pathologic response in melanoma. PLoS ONE 2021, 16, e0245287. [Google Scholar] [CrossRef]
- Mohs, R.C.; Greig, N.H. Drug discovery and development: Role of basic biological research. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 651–657. [Google Scholar] [CrossRef]
- Deore, A.B.; Dhumane, J.R.; Wagh, H.V.; Sonawane, R.B. The stages of drug discovery and development process. Asian J. Pharm. Res. Dev. 2019, 7, 62–67. [Google Scholar] [CrossRef]
- FDA. Available online: https://www.fda.gov/ (accessed on 18 May 2021).
- Matias, M.; Fortuna, A.; Bicker, J.; Silvestre, S.; Falcão, A.; Alves, G. Screening of pharmacokinetic properties of fifty dihydropyrimidin(thi)one derivatives using a combo of in vitro and in silico assays. Eur. J. Pharm. Sci. 2017, 109, 334–346. [Google Scholar] [CrossRef]
- Canário, C.; Matias, M.; Brito, V.; Santos, A.O.; Silvestre, S.; Alves, G. New estrone oxime derivatives: Synthesis, cytotoxic evaluation and docking studies. Molecules 2021, 26, 2687. [Google Scholar] [CrossRef]
- Couto, G.K.; Segatto, N.V.; Oliveira, T.L.; Seixas, F.K.; Schachtschneider, K.M.; Collares, T. The Melding of Drug Screening Platforms for Melanoma. Front. Oncol. 2019, 9, 512. [Google Scholar] [CrossRef]
- Powley, I.R.; Patel, M.; Miles, G.; Pringle, H.; Howells, L.; Thomas, A.; Kettleborough, C.; Bryans, J.; Hammonds, T.; MacFarlane, M.; et al. Patient-derived explants (PDEs) as a powerful preclinical platform for anti-cancer drug and biomarker discovery. Br. J. Cancer 2020, 122, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Basith, S.; Cui, M.; Macalino, S.J.Y.; Choi, S. Expediting the design, discovery and development of anticancer drugs using computational approaches. Curr. Med. Chem. 2017, 24, 4753–4778. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Lucarelli, P.; Kulms, D.; Sauter, T. Computational models of melanoma. Theor. Biol. Med. Model. 2020, 17, 8. [Google Scholar] [CrossRef]
- Pennisi, M.; Russo, G.; Di Salvatore, V.; Candido, S.; Libra, M.; Pappalardo, F. Computational modeling in melanoma for novel drug discovery. Expert Opin. Drug Discov. 2016, 11, 609–621. [Google Scholar] [CrossRef]
- Cui, W.; Aouidate, A.; Wang, S.; Yu, Q.; Li, Y.; Yuan, S. Discovering anti-cancer drugs via computational methods. Front. Pharmacol. 2020, 11, 733. [Google Scholar] [CrossRef]
- Prada-Gracia, D.; Huerta-Yépez, S.; Moreno-Vargas, L.M. Application of computational methods for anticancer drug discovery, design, and optimization. Bol. Med. Hosp. Infant. Mex. 2016, 73, 411–423. [Google Scholar]
- Umar, A.B.; Uzairu, A.; Shallangwa, G.A.; Uba, S. QSAR modelling and molecular docking studies for anti-cancer compounds against melanoma cell line SK-MEL-2. Heliyon 2020, 6, e03640. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Yang, B.; Zou, Y.; Li, G.; Zhu, H.L. Design, biological evaluation and 3D QSAR studies of novel dioxin-containing triaryl pyrazoline derivatives as potential B-Raf inhibitors. Bioorg. Med. Chem. 2016, 24, 3052–3061. [Google Scholar] [CrossRef] [PubMed]
- Couto, G.K.; Pacheco, B.S.; Borba, V.M.; Junior, J.C.R.; Oliveira, T.L.; Segatto, N.V.; Seixas, F.K.; Acunha, T.V.; Iglesias, B.A.; Collares, T. Tetra-cationic platinum(II) porphyrins like a candidate photosensitizers to bind, selective and drug delivery for metastatic melanoma. J. Photochem. Photobiol. B Biol. 2020, 202, 111725. [Google Scholar] [CrossRef]
- Kalal, B.S.; Pai, V.R.; Behera, S.K.; Somashekarappa, H.M. HDAC2 inhibitor valproic acid increases radiation sensitivity of drug-resistant melanoma cells. Med. Sci. 2019, 7, 51. [Google Scholar] [CrossRef]
- Wagner, T.; Brand, P.; Heinzel, T.; Krämer, O.H. Histone deacetylase 2 controls p53 and is a critical factor in tumorigenesis. Biochim. Biophys. Acta 2014, 1846, 524–538. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Choromańska, A.; Abel, R.; Preissner, R.; Saczko, J.; Matkowski, A.; Hadzik, J. Cytotoxic effect of vanicosides A and B from Reynoutria sachalinensis against melanotic and amelanotic melanoma cell lines and in silico evaluation for inhibition of BRAFV600E and MEK1. Int. J. Mol. Sci. 2020, 21, 4611. [Google Scholar] [CrossRef] [PubMed]
- Thiriveedhi, A.; Nadh, R.V.; Srinivasu, N.; Bobde, Y.; Ghosh, B.; Sekhar, K.V.G.C. Design, synthesis and anti-tumour activity of new pyrimidine-pyrrole appended triazoles. Toxicol. Vitr. 2019, 60, 87–96. [Google Scholar] [CrossRef]
- Nazir, Y.; Saeed, A.; Rafiq, M.; Afzal, S.; Ali, A.; Latif, M.; Zuegg, J.; Hussein, W.M.; Fercher, C.; Barnard, R.T.; et al. Hydroxyl substituted benzoic acid/cinnamic acid derivatives: Tyrosinase inhibitory kinetics, anti-melanogenic activity and molecular docking studies. Bioorg. Med. Chem. Lett. 2020, 30, 126722. [Google Scholar] [CrossRef]
- Santi, M.D.; Peralta, M.A.; Puiatti, M.; Cabrera, J.L.; Ortega, M.G. Melanogenic inhibitory effects of Triangularin in B16F0 melanoma cells, in vitro and molecular docking studies. Bioorg. Med. Chem. 2019, 27, 3722–3728. [Google Scholar] [CrossRef]
- Jha, V.; Biagi, M.; Spinelli, V.; Di Stefano, M.; Macchia, M.; Minutolo, F.; Granchi, C.; Poli, G.; Tuccinardi, T. Discovery of monoacylglycerol lipase (MAGL) inhibitors based on a pharmacophore-guided virtual screening study. Molecules 2021, 26, 78. [Google Scholar] [CrossRef]
- Baba, Y.; Funakoshi, T.; Mori, M.; Emoto, K.; Masugi, Y.; Ekmekcioglu, S.; Amagai, M.; Tanese, K. Expression of monoacylglycerol lipase as a marker of tumour invasion and progression in malignant melanoma. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, X.; Lin, X. A review on applications of computational methods in drug screening and design. Molecules 2020, 25, 1375. [Google Scholar] [CrossRef]
- Madden, J.C.; Enoch, S.J.; Paini, A.; Cronin, M.T.D. A review of in silico tools as alternatives to animal testing: Principles, resources and applications. Altern. Lab. Anim. 2020, 48, 146–172. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.K.; Moura, A.S.; Cordeiro, M.N.D.S. QSAR modelling: A therapeutic patent review 2010-present. Expert Opin. Ther. Pat. 2018, 28, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Ancuceanu, R.; Dinu, M.; Neaga, I.; Laszlo, F.G.; Boda, D. Development of QSAR machine learning-based models to forecast the effect of substances on malignant melanoma cells. Oncol. Lett. 2019, 17, 4188–4196. [Google Scholar] [CrossRef]
- Anbar, H.S.; El-Gamal, M.I.; Tarazi, H.; Lee, B.S.; Jeon, H.R.; Kwon, D.; Oh, C.H. Imidazothiazole-based potent inhibitors of V600E-B-RAF kinase with promising anti-melanoma activity: Biological and computational studies. J. Enzym. Inhib. Med. Chem. 2020, 35, 1712–1726. [Google Scholar] [CrossRef]
- Girgis, A.S.; Panda, S.S.; Srour, A.M.; Farag, H.; Ismail, N.S.M.; Elgendy, M.; Abdel-Aziz, A.K.; Katritzky, A.R. Rational design, synthesis and molecular modeling studies of novel anti-oncological alkaloids against melanoma. Org. Biomol. Chem. 2015, 13, 6619–6633. [Google Scholar] [CrossRef]
- Medeiros Turra, K.; Pineda Rivelli, D.; Berlanga de Moraes Barros, S.; Mesquita Pasqualoto, K.F. Constructing and validating 3D-pharmacophore models to a set of MMP-9 inhibitors for designing novel anti-melanoma agents. Mol. Inform. 2016, 35, 238–252. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. In vitro assays and techniques utilized in anticancer drug discovery. J. Appl. Toxicol. 2019, 39, 38–71. [Google Scholar] [CrossRef] [PubMed]
- Balis, F.M. Evolution of anticancer drug discovery and the role of cell-based screening. J. Natl. Cancer Inst. 2002, 94, 78–79. [Google Scholar] [CrossRef]
- Liu, Z.; Delavan, B.; Roberts, R.; Tong, W. Lessons learned from two decades of anticancer drugs. Trends Pharmacol. Sci. 2017, 38, 852–872. [Google Scholar] [CrossRef]
- Marconi, A.; Quadri, M.; Saltari, A.; Pincelli, C. Progress in melanoma modelling in vitro. Exp. Dermatol. 2018, 27, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, V.W.; Somasundaram, R.; Herlyn, M. Pre-clinical modeling of cutaneous melanoma. Nat. Commun. 2020, 11, 2858. [Google Scholar] [CrossRef] [PubMed]
- Su, D.M.; Zhang, Q.; Wang, X.; He, P.; Zhu, Y.J.; Zhao, J.; Rennert, O.M.; Su, Y.A. Two types of human malignant melanoma cell lines revealed by expression patterns of mitochondrial and survival-apoptosis genes: Implications for malignant melanoma therapy. Mol. Cancer Ther. 2009, 8, 1292–1304. [Google Scholar] [CrossRef]
- Herlyn, M.; Fukunaga-Kalabis, M. What is a good model for melanoma? J. Investig. Dermatol. 2010, 130, 911–912. [Google Scholar] [CrossRef]
- Caputo, E.; Maiorana, L.; Vasta, V.; Pezzino, F.M.; Sunkara, S.; Wynne, K.; Elia, G.; Marincola, F.M.; McCubrey, J.A.; Libra, M.; et al. Characterization of human melanoma cell lines and melanocytes by proteome analysis. Cell Cycle 2011, 10, 2924–2936. [Google Scholar] [CrossRef]
- Beberok, A.; Rzepka, Z.; Rok, J.; Banach, K.; Wrześniok, D. UVA radiation enhances lomefloxacin-mediated cytotoxic, growth-inhibitory and pro-apoptotic effect in human melanoma cells through excessive reactive oxygen species generation. Int. J. Mol. Sci. 2020, 21, 8937. [Google Scholar] [CrossRef]
- Yin, S.J.; Lee, J.R.; Hahn, M.J.; Yang, J.M.; Qian, G.Y.; Park, Y.D. Tyrosinase-mediated melanogenesis in melanoma cells: Array comparative genome hybridization integrating proteomics and bioinformatics studies. Int. J. Biol. Macromol. 2021, 170, 150–163. [Google Scholar] [CrossRef]
- Ietta, F.; Valacchi, G.; Benincasa, L.; Pecorelli, A.; Cresti, L.; Maioli, E. Multiple mechanisms of Rottlerin toxicity in A375 melanoma cells. BioFactors 2019, 45, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Rebecca, V.W.; Herlyn, M. The melanoma patient-derived xenograft (PDX) Model. J. Vis. Exp. 2019, 147, 59508. [Google Scholar] [CrossRef]
- Harris, A.L.; Joseph, R.W.; Copland, J.A. Patient-derived tumor xenograft models for melanoma drug discovery. Expert Opin. Drug Discov. 2016, 11, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Coricovac, D.; Dehelean, C.; Moaca, E.A.; Pinzaru, I.; Bratu, T.; Navolan, D.; Boruga, O. Cutaneous melanoma—A long road from experimental models to clinical outcome: A review. Int. J. Mol. Sci. 2018, 19, 1566. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J.; Nicolson, G.L. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J. Natl. Cancer Inst. 1976, 57, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. Selection of successive tumour lines for metastasis. Nat. New Biol. 1973, 242, 148–149. [Google Scholar] [CrossRef]
- Predoi, M.-C.; Mîndrilă, I.; Buteică, S.A.; Mărginean, O.M.; Mîndrilă, B.; Niculescu, M. Pigmented melanoma cell migration study on murine syngeneic B16F10 melanoma cells or tissue transplantation models. J. Mind Med. Sci. 2019, 6, 327–333. [Google Scholar] [CrossRef]
- Carminati, L.; Pinessi, D.; Borsotti, P.; Minoli, L.; Giavazzi, R.; D’Incalci, M.; Belotti, D.; Taraboletti, G. Antimetastatic and antiangiogenic activity of trabectedin in cutaneous melanoma. Carcinogenesis 2019, 40, 303–312. [Google Scholar] [CrossRef]
- Meeth, K.; Wang, J.; Micevic, G.; Damsky, W.; Bosenberg, M.W. The YUMM lines: A series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment Cell Melanoma Res. 2016, 29, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, K.; Mohana-Kumaran, N.; Haass, N. Modeling Melanoma In Vitro and In Vivo. Healthcare 2014, 2, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yu, P.; Du, G.; Wang, W.; Zhu, H.; Li, N.; Zhao, H.; Dong, Z.; Ye, L.; Tian, J. PCC0208025 (BMS202), a small molecule inhibitor of PD-L1, produces an antitumor effect in B16-F10 melanoma-bearing mice. PLoS ONE 2020, 15, e0228339. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.; Pinho, J.O.; Soveral, G.; Ascensão, L.; Matela, N.; Reis, C.; Gaspar, M.M. A novel hybrid nanosystem integrating cytotoxic and magnetic properties as a tool to potentiate melanoma therapy. Nanomaterials 2020, 10, 693. [Google Scholar] [CrossRef]
- Lopes, J.; Coelho, J.M.P.; Vieira, P.M.C.; Viana, A.S.; Gaspar, M.M.; Reis, C. Preliminary assays towards melanoma cells using phototherapy with gold-based nanomaterials. Nanomaterials 2020, 10, 1536. [Google Scholar] [CrossRef]
- Lopes, J.; Ferreira-Gonçalves, T.; Figueiredo, I.V.; Rodrigues, C.M.P.; Ferreira, H.; Ferreira, D.; Viana, A.S.; Faísca, P.; Gaspar, M.M.; Coelho, J.M.P.; et al. Proof-of-concept study of multifunctional hybrid nanoparticle system combined with nir laser irradiation for the treatment of melanoma. Biomolecules 2021, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Pinho, J.O.; Amaral, J.D.; Castro, R.E.; Rodrigues, C.M.P.; Casini, A.; Soveral, G.; Gaspar, M.M. Copper complex nanoformulations featuring highly promising therapeutic potential in murine melanoma models. Nanomedicine 2019, 14, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Costantini, F.; Di Sano, C.; Barbieri, G. The hydroxytyrosol induces the death for apoptosis of human melanoma cells. Int. J. Mol. Sci. 2020, 21, 8074. [Google Scholar] [CrossRef]
- Calado, S.; Eleutério, C.; Mendes, E.; de Jesus Rocha, M.; Francisco, A.P.; Gaspar, M.M. Nanoformulations of a Triazene Analogue with Specific Affinity to Human Melanoma. J. Nanosci. Adv. Technol. 2016, 1, 9. [Google Scholar]
- Nave, M.; Castro, R.E.; Rodrigues, C.M.P.; Casini, A.; Soveral, G.; Gaspar, M.M. Nanoformulations of a potent copper-based aquaporin inhibitor with cytotoxic effect against cancer cells. Nanomedicine 2016, 11, 1817–1830. [Google Scholar] [CrossRef]
- Sousa, A.; Santos, F.; Gaspar, M.M.; Calado, S.; Pereira, J.D.; Mendes, E.; Francisco, A.P.; Perry, M.J. The selective cytotoxicity of new triazene compounds to human melanoma cells. Bioorg. Med. Chem. 2017, 25, 3900–3910. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Pan, C.; Fan, J.; Zhong, X.; Tang, S. Glaucocalyxin A induces cell cycle arrest and apoptosis via inhibiting NF-κB/p65 signaling pathway in melanoma cells. Life Sci. 2021, 271, 119185. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, S.H.; Kim, Y.M.; Kim, S.H.; Yoo, E.S.; Woo, J.S.; Jung, G.H.; Jung, S.H.; Kim, B.S.; Jung, J.Y. Shikonin inhibits proliferation of melanoma cells by MAPK pathway-mediated induction of apoptosis. Biosci. Rep. 2021, 41, BSR20203834. [Google Scholar] [CrossRef]
- Guo, L.; Kang, J.S.; Kang, N.J.; Choi, Y.W. S-petasin induces apoptosis and inhibits cell migration through activation of p53 pathway signaling in melanoma B16F10 cells and A375 cells. Arch. Biochem. Biophys. 2020, 692, 108519. [Google Scholar] [CrossRef]
- Hida, T.; Kamiya, T.; Kawakami, A.; Ogino, J.; Sohma, H.; Uhara, H.; Jimbow, K. Elucidation of melanogenesis cascade for identifying pathophysiology and therapeutic approach of pigmentary disorders and melanoma. Int. J. Mol. Sci. 2020, 21, 6129. [Google Scholar] [CrossRef] [PubMed]
- Roulier, B.; Pérès, B.; Haudecoeur, R. Advances in the design of genuine human tyrosinase inhibitors for targeting melanogenesis and related pigmentations. J. Med. Chem. 2020, 63, 13428–13443. [Google Scholar] [CrossRef]
- Choi, I.; Park, Y.; Ryu, I.Y.; Jung, H.J.; Ullah, S.; Choi, H.; Park, C.; Kang, D.; Lee, S.; Chun, P.; et al. In silico and in vitro insights into tyrosinase inhibitors with a 2-thioxooxazoline-4-one template. Comput. Struct. Biotechnol. J. 2021, 19, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.S.; Almeida, J.; Cabral, G.; Severino, P.; Videira, P.A.; Sousa, A.; Nunes, R.; Pereira, J.D.; Francisco, A.P.; Perry, M.J.; et al. Synthesis and evaluation of N-acylamino acids derivatives of triazenes. Activation by tyrosinase in human melanoma cell lines. Eur. J. Med. Chem. 2013, 70, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.L.; Bodmer, W.F. Cancer cell lines for drug discovery and development. Cancer Res. 2014, 74, 2377–2384. [Google Scholar] [CrossRef]
- Barutello, G.; Rolih, V.; Arigoni, M.; Tarone, L.; Conti, L.; Quaglino, E.; Buracco, P.; Cavallo, F.; Riccardo, F. Strengths and weaknesses of pre-clinical models for human melanoma treatment: Dawn of dogs’ revolution for immunotherapy. Int. J. Mol. Sci. 2018, 19, 799. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Eglen, R.M. Three-dimensional cell cultures in drug discovery and development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [PubMed]
- Boucherit, N.; Gorvel, L.; Olive, D. 3D tumor models and their use for the testing of immunotherapies. Front. Immunol. 2020, 11, 603640. [Google Scholar] [CrossRef]
- Smalley, K.S.M.; Lioni, M.; Noma, K.; Haass, N.K.; Herlyn, M. In vitro three-dimensional tumor microenvironment models for anticancer drug discovery. Expert Opin. Drug Discov. 2008, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, M.; Maroufi, N.F.; Tazehkand, A.P.; Akbarzadeh, M.; Bastani, S.; Safdari, R.; Farzane, A.; Fattahi, A.; Nejabati, H.R.; Nouri, M.; et al. Current approaches in identification and isolation of cancer stem cells. J. Cell. Physiol. 2019, 234, 14759–14772. [Google Scholar] [CrossRef]
- Lee, C.H.; Yu, C.C.; Wang, B.Y.; Chang, W.W. Tumorsphere as an effective in vitro platform for screening anticancer stem cell drugs. Oncotarget 2016, 7, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Levesque, M.P.; Cheng, P.F.; Raaijmakers, M.I.G.; Saltari, A.; Dummer, R. Metastatic melanoma moves on: Translational science in the era of personalized medicine. Cancer Metastasis Rev. 2017, 36, 7–21. [Google Scholar] [CrossRef]
- Velazquez, O.C.; Snyder, R.; Liu, Z.J.; Fairman, R.M.; Herlyn, M. Fibroblast-dependent differentiation of human microvascular endothelial cells into capillary-like 3-dimensional networks. FASEB J. 2002, 16, 1316–1318. [Google Scholar] [CrossRef]
- Brassard-Jollive, N.; Monnot, C.; Muller, L.; Germain, S. In vitro 3D systems to model tumor angiogenesis and interactions with stromal cells. Front. Cell Dev. Biol. 2020, 8, 594903. [Google Scholar] [CrossRef]
- Sutherland, R.M.; McCredie, J.A.; Inch, W.R. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J. Natl. Cancer Inst. 1971, 46, 113–120. [Google Scholar] [PubMed]
- Srisongkram, T.; Weerapreeyakul, N.; Thumanu, K. Evaluation of melanoma (SK-MEL-2) cell growth between three-dimensional (3D) and two-dimensional (2D) cell cultures with fourier transform infrared (FTIR) microspectroscopy. Int. J. Mol. Sci. 2020, 21, 4141. [Google Scholar] [CrossRef] [PubMed]
- Müller, I.; Kulms, D. A 3D organotypic melanoma spheroid skin model. J. Vis. Exp. 2018, 135, e57500. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.K.; Yeow, C.H. Proton NMR characterization of intact primary and metastatic melanoma cells in 2D & 3D cultures. Biol. Res. 2017, 50, 12. [Google Scholar]
- Novotný, J.; Strnadová, K.; Dvoranková, B.; Kocourková, S.; Jaksa, R.; Dundr, P.; Paces, V.; Smetana Jr, K.; Kolár, M.; Lacina, L. Single-cell RNA sequencing unravels heterogeneity of the stromal niche in cutaneous melanoma heterogeneous spheroids. Cancers 2020, 12, 3324. [Google Scholar] [CrossRef]
- Avagliano, A.; Ruocco, M.R.; Nasso, R.; Aliotta, F.; Sanità, G.; Iaccarino, A.; Bellevicine, C.; Calì, G.; Fiume, G.; Masone, S.; et al. Development of a stromal microenvironment experimental model containing proto-myofibroblast like cells and analysis of its crosstalk with melanoma cells: A new tool to potentiate and stabilize tumor suppressor phenotype of dermal myofibroblasts. Cells 2019, 8, 1435. [Google Scholar] [CrossRef]
- Klicks, J.; Maßlo, C.; Kluth, A.; Rudolf, R.; Hafner, M. A novel spheroid-based co-culture model mimics loss of keratinocyte differentiation, melanoma cell invasion, and drug-induced selection of ABCB5-expressing cells. BMC Cancer 2019, 19, 402. [Google Scholar] [CrossRef] [PubMed]
- Murali, V.S.; Chang, B.J.; Fiolka, R.; Danuser, G.; Cobanoglu, M.C.; Welf, E.S. An image-based assay to quantify changes in proliferation and viability upon drug treatment in 3D microenvironments. BMC Cancer 2019, 19, 502. [Google Scholar] [CrossRef]
- Shah, M.K.; Leary, E.A.; Darling, E.M. Integration of hyper-compliant microparticles into a 3D melanoma tumor model. J. Biomech. 2019, 82, 46–53. [Google Scholar] [CrossRef]
- Hofschroër, V.; Koch, K.A.; Ludwig, F.T.; Friedl, P.; Oberleithner, H.; Stock, C.; Schwab, A. Extracellular protonation modulates cell-cell interaction mechanics and tissue invasion in human melanoma cells. Sci. Rep. 2017, 7, 42369. [Google Scholar] [CrossRef] [PubMed]
- Sandri, S.; Faião-Flores, F.; Tiago, M.; Pennacchi, P.C.; Massaro, R.R.; Alves-Fernandes, D.K.; Berardinelli, G.N.; Evangelista, A.F.; de Lima Vazquez, V.; Reis, R.M.; et al. Vemurafenib resistance increases melanoma invasiveness and modulates the tumor microenvironment by MMP-2 upregulation. Pharmacol. Res. 2016, 111, 523–533. [Google Scholar] [CrossRef]
- Tevis, K.M.; Colson, Y.L.; Grinstaff, M.W. Embedded spheroids as models of the cancer microenvironment. Adv. Biosyst. 2017, 1, 1700083. [Google Scholar] [CrossRef] [PubMed]
- Ryabaya, O.; Prokofieva, A.; Akasov, R.; Khochenkov, D.; Emelyanova, M.; Burov, S.; Markvicheva, E.; Inshakov, A.; Stepanova, E. Metformin increases antitumor activity of MEK inhibitor binimetinib in 2D and 3D models of human metastatic melanoma cells. Biomed. Pharmacother. 2019, 109, 2548–2560. [Google Scholar] [CrossRef]
- Wessels, D.; Lusche, D.F.; Voss, E.; Kuhl, S.; Buchele, E.C.; Klemme, M.R.; Russell, K.B.; Ambrose, J.; Soll, B.A.; Bossler, A.; et al. Melanoma cells undergo aggressive coalescence in a 3D Matrigel model that is repressed byanti-CD44. PLoS ONE 2017, 12, e0173400. [Google Scholar] [CrossRef] [PubMed]
- Ganga Reddy, V.; Srinivasa Reddy, T.; Privér, S.H.; Bai, Y.; Mishra, S.; Wlodkowic, D.; Mirzadeh, N.; Bhargava, S. Synthesis of gold(I) complexes containing cinnamide: In vitro evaluation of anticancer activity in 2D and 3D spheroidal models of melanoma and in vivo angiogenesis. Inorg. Chem. 2019, 58, 5988–5999. [Google Scholar] [CrossRef]
- Cruz Rodríguez, N.; Lineros, J.; Rodríguez, C.S.; Martínez, L.M.; Rodríguez, J.A. Establishment of two dimensional (2D) and three-dimensional (3D) melanoma primary cultures as a tool for in vitro drug resistance studies. Methods Mol. Biol. 2019, 1913, 119–131. [Google Scholar]
- Guo, D.; Lui, G.Y.L.; Lai, S.L.; Wilmott, J.S.; Tikoo, S.; Jackett, L.A.; Quek, C.; Brown, D.L.; Sharp, D.M.; Kwan, R.Y.Q.; et al. RAB27A promotes melanoma cell invasion and metastasis via regulation of pro-invasive exosomes. Int. J. Cancer 2019, 144, 3070–3085. [Google Scholar] [CrossRef]
- De Luca, A.; Carpanese, D.; Rapanotti, M.C.; Suarez Viguria, T.M.; Forgione, M.A.; Rotili, D.; Fulci, C.; Iorio, E.; Quintieri, L.; Chimenti, S.; et al. The nitrobenzoxadiazole derivative MC3181 blocks melanoma invasion and metastasis. Oncotarget 2017, 8, 15520–15538. [Google Scholar] [CrossRef][Green Version]
- Hundsberger, H.; Stierschneider, A.; Sarne, V.; Ripper, D.; Schimon, J.; Weitzenböck, H.P.; Schild, D.; Jacobi, N.; Eger, A.; Atzler, J.; et al. Concentration-dependent pro- and antitumor activities of quercetin in human melanoma spheroids: Comparative analysis of 2D and 3D cell culture models. Molecules 2021, 26, 717. [Google Scholar] [CrossRef] [PubMed]
- Olbryt, M.; Rusin, A.; Fokt, I.; Habryka, A.; Tudrej, P.; Student, S.; Sochanik, A.; Zieliński, R.; Priebe, W. Bis-anthracycline WP760 abrogates melanoma cell growth by transcription inhibition, p53 activation and IGF1R downregulation. Investig. New Drugs 2017, 35, 545–555. [Google Scholar] [CrossRef]
- Grissenberger, S.; Riedl, S.; Rinner, B.; Leber, R.; Zweytick, D. Design of human lactoferricin derived antitumor peptides-activity and specificity against malignant melanoma in 2D and 3D model studies. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183264. [Google Scholar] [CrossRef]
- Rittler, D.; Baranyi, M.; Molnár, E.; Garay, T.; Jalsovszky, I.; Varga, I.K.; Hegedűs, L.; Aigner, C.; Tóvári, J.; Tímár, J.; et al. The antitumor effect of lipophilic bisphosphonate BPH1222 in melanoma models: The role of the PI3K/Akt pathway and the small G protein rheb. Int. J. Mol. Sci. 2019, 20, 4917. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Wilhelm, M.; Höiom, V.; Franco Marquez, R.; Costa Svedman, F.; Hansson, J.; Tuominen, R.; Egyhàzi Brage, S. Combining ERBB family and MET inhibitors is an effective therapeutic strategy in cutaneous malignant melanoma independent of BRAF/NRAS mutation status. Cell Death Dis. 2019, 10, 663. [Google Scholar] [CrossRef] [PubMed]
- Sastry, K.S.R.; Al-Muftah, M.A.; Li, P.; Al-Kowari, M.K.; Wang, E.; Ismail Chouchane, A.; Kizhakayil, D.; Kulik, G.; Marincola, F.M.; Haoudi, A.; et al. Targeting proapoptotic protein BAD inhibits survival and self-renewal of cancer stem cells. Cell Death Differ. 2014, 21, 1936–1949. [Google Scholar] [CrossRef]
- Singh, S.K.; Clarke, I.D.; Hide, T.; Dirks, P.B. Cancer stem cells in nervous system tumors. Oncogene 2004, 23, 7267–7273. [Google Scholar] [CrossRef]
- Lin, X.; Sun, B.; Zhu, D.; Zhao, X.; Sun, R.; Zhang, Y.; Zhang, D.; Dong, X.; Gu, Q.; Li, Y.; et al. Notch4+ cancer stem-like cells promote the metastatic and invasive ability of melanoma. Cancer Sci. 2016, 107, 1079–1091. [Google Scholar] [CrossRef]
- Stecca, B.; Santini, R.; Pandolfi, S.; Penachioni, J.Y. Culture and isolation of melanoma-initiating cells. Curr. Protoc. Stem Cell Biol. 2013, 24, 3–6. [Google Scholar] [CrossRef]
- Hu, J.; Guo, X.; Yang, L. Morin inhibits proliferation and self-renewal of CD133+ melanoma cells by upregulating miR-216a. J. Pharmacol. Sci. 2018, 136, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wang, J.; Yao, Z.; Hu, Y.; Ma, S.; Fan, Q.; Gao, F.; Sun, Y.; Sun, J. Fascin induces melanoma tumorigenesis and stemness through regulating the Hippo pathway. Cell Commun. Signal. 2018, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.Y.; Cheng, Y.C.; Hsieh, C.H.; Tseng, T.T.; Jiang, S.S.; Lee, S.C. Drug-selected population in melanoma A2058 cells as melanoma stem-like cells retained angiogenic features—The potential roles of heparan-sulfate binding ANGPTL4 protein. Aging 2020, 12, 22700–22717. [Google Scholar] [CrossRef] [PubMed]
- Marzagalli, M.; Moretti, R.M.; Messi, E.; Marelli, M.M.; Fontana, F.; Anastasia, A.; Bani, M.R.; Beretta, G.; Limonta, P. Targeting melanoma stem cells with the Vitamin E derivative δ-tocotrienol. Sci. Rep. 2018, 8, 587. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Almeida, A.; Partyka, K.A.; Lu, Y.; Schwan, J.V.; Lambert, K.; Rogers, M.; Robinson, W.A.; Robinson, S.E.; Applegate, A.J.; et al. Combining a GSI and BCL-2 inhibitor to overcome melanoma’s resistance to current treatments. Oncotarget 2016, 7, 84594–84607. [Google Scholar] [CrossRef] [PubMed]
- Vilgelm, A.E.; Bergdorf, K.; Wolf, M.; Bharti, V.; Shattuck-Brandt, R.; Blevins, A.; Jones, C.; Phifer, C.; Lee, M.; Lowe, C.; et al. Fine-needle aspiration-based patient-derived cancer organoids. iScience 2020, 23, 101408. [Google Scholar] [CrossRef] [PubMed]
- Votanopoulos, K.I.; Forsythe, S.; Sivakumar, H.; Mazzocchi, A.; Aleman, J.; Miller, L.; Levine, E.; Triozzi, P.; Skardal, A. Model of patient-specific immune-enhanced organoids for immunotherapy screening: Feasibility study. Ann. Surg. Oncol. 2020, 27, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Troiani, T.; Giunta, E.F.; Tufano, M.; Vigorito, V.; D’Arrigo, P.; Argenziano, G.; Ciardiello, F.; Romano, M.F.; Romano, S. Alternative macrophage polarisation associated with resistance to anti-PD1 blockade is possibly supported by the splicing of FKBP51 immunophilin in melanoma patients. Br. J. Cancer 2020, 122, 1782–1790. [Google Scholar] [CrossRef]
- Brohem, C.A.; Beatriz Da, L.; Cardeal, S.; Tiago, M.; Soengas, M.S.; Berlanga De Moraes Barros, S.; Maria-Engler, S.S. Artificial skin in perspective: Concepts and applications. Pigment Cell Melanoma Res. 2011, 24, 35–50. [Google Scholar] [CrossRef]
- Li, L.; Fukunaga-Kalabis, M.; Herlyn, M. Establishing human skin grafts in mice as model for melanoma progression. In Methods in Molecular Biology; Humana Press: Tortowa, NJ, USA, 2015; pp. 1–11. [Google Scholar]
- Li, L.; Fukunaga-Kalabis, M.; Herlyn, M. The three-dimensional human skin reconstruct model: A tool to study normal skin and melanoma progression. J. Vis. Exp. 2011, 54, e2937. [Google Scholar] [CrossRef]
- Gibot, L.; Galbraith, T.; Huot, J.; Auger, F.A. Development of a tridimensional microvascularized human skin substitute to study melanoma biology. Clin. Exp. Metastasis 2013, 30, 83–90. [Google Scholar] [CrossRef]
- Kaur, A.; Ecker, B.L.; Douglass, S.M.; Iii, C.H.K.; Webster, M.R.; Almeida, F.V.; Somasundaram, R.; Hayden, J.; Ban, E.; Ahmadzadeh, H.; et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 2019, 9, 64–81. [Google Scholar] [CrossRef]
- Zabierowski, S.E.; Fukunaga-Kalabis, M.; Li, L.; Herlyn, M. Dermis-derived stem cells: A source of epidermal melanocytes and melanoma? Pigment Cell Melanoma Res. 2011, 24, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zheng, Y.; Li, L.; Liu, S.; Burrows, M.; Wei, Z.; Nace, A.; Herlyn, M.; Cui, R.; Guo, W.; et al. Direct conversion of mouse and human fibroblasts to functional melanocytes by defined factors. Nat. Commun. 2014, 5, 5807. [Google Scholar] [CrossRef]
- Michielon, E.; López González, M.; Burm, J.L.A.; Waaijman, T.; Jordanova, E.S.; de Gruijl, T.D.; Gibbs, S. Micro-environmental cross-talk in an organotypic human melanoma-in-skin model directs M2-like monocyte differentiation via IL-10. Cancer Immunol. Immunother. 2020, 69, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Aranha, E.S.P.; de Sousa Portilho, A.J.; Bentes de Sousa, L.; da Silva, E.L.; Mesquita, F.P.; Rocha, W.C.; Araújo da Silva, F.M.; Lima, E.S.; Alves, A.P.N.N.; Koolen, H.H.F.; et al. 22β-hydroxytingenone induces apoptosis and suppresses invasiveness of melanoma cells by inhibiting MMP-9 activity and MAPK signaling. J. Ethnopharmacol. 2021, 267, 113605. [Google Scholar] [CrossRef] [PubMed]
- Massaro, R.R.; Faião-Flores, F.; Rebecca, V.W.; Sandri, S.; Alves-Fernandes, D.K.; Pennacchi, P.C.; Smalley, K.S.M.; Maria-Engler, S.S. Inhibition of proliferation and invasion in 2D and 3D models by 2-methoxyestradiol in human melanoma cells. Pharmacol. Res. 2017, 119, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Caputo, E.; Miceli, R.; Motti, M.L.; Taté, R.; Fratangelo, F.; Botti, G.; Mozzillo, N.; Carriero, M.V.; Cavalcanti, E.; Palmieri, G.; et al. AurkA inhibitors enhance the effects of B-RAF and MEK inhibitors in melanoma treatment. J. Transl. Med. 2014, 12, 216. [Google Scholar] [CrossRef]
- Kim, D.E.; Chang, B.Y.; Ham, S.O.; Kim, Y.C.; Kim, S.Y. Neobavaisoflavone Inhibits Melanogenesis through the Regulation of Akt/GSK-3β and MEK/ERK Pathways in B16F10 Cells and a Reconstructed Human 3D Skin Model. Molecules 2020, 25, 2683. [Google Scholar] [CrossRef]
- Choi, S.G.; Kim, J.H.; Hong, S.H.; Lee, O.Y.; Kang, N.G. Exogenous pyruvate alleviates UV-induced hyperpigmentation via restraining dendrite outgrowth and Rac1 GTPase activity. J. Dermatol. Sci. 2021, 101, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Vultur, A.; Schanstra, T.; Herlyn, M. The promise of 3D skin and melanoma cell bioprinting. Melanoma Res. 2016, 26, 205–206. [Google Scholar] [CrossRef]
- Peng, W.; Datta, P.; Ayan, B.; Ozbolat, V.; Sosnoski, D.; Ozbolat, I.T. 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomater. 2017, 57, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.K.; Schmid, R.; Arkudas, A.; Kengelbach-Weigand, A.; Bosserhoff, A.K. Tumor cells develop defined cellular phenotypes after 3D-bioprinting in different bioinks. Cells 2019, 8, 1295. [Google Scholar] [CrossRef] [PubMed]
- Tarassoli, S.P.; Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Whitaker, S.; Doak, S.; Whitaker, I.S. Skin tissue engineering using 3D bioprinting: An evolving research field. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 615–623. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Sadangi, S.; Lares, M.; Rehman, S.; Humayun, M.; Denecke, K.M.; Skala, M.C.; Beebe, D.J.; Setaluri, V. Microfluidic model with air-walls reveals fibroblasts and keratinocytes modulate melanoma cell phenotype, migration, and metabolism. Lab Chip 2021, 21, 1139–1149. [Google Scholar] [CrossRef]
- Patel, D.; Gao, Y.; Son, K.; Siltanen, C.; Neve, R.M.; Ferrara, K.; Revzin, A. Microfluidic co-cultures with hydrogel-based ligand trap to study paracrine signals giving rise to cancer drug resistance. Lab Chip 2015, 15, 4614–4624. [Google Scholar] [CrossRef] [PubMed]
- Quaresmini, D.; Guida, M. Neoangiogenesis in melanoma: An issue in biology and systemic treatment. Front. Immunol. 2020, 11, 584903. [Google Scholar] [CrossRef]
- Esteves, M.; Monteiro, M.P.; Duarte, J.A. The effects of vascularization on tumor development: A systematic review and meta-analysis of pre-clinical studies. Crit. Rev. Oncol. Hematol. 2021, 159, 103245. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Chen, H.; Bauer, R.J.; Bauer, S.M.; Velazquez, O.C. Normal human fibroblasts enable melanoma cells to induce angiogenesis in type I collagen. Surgery 2005, 138, 439–449. [Google Scholar] [CrossRef]
- Kumar, S.R.; Gajagowni, S.; Bryan, J.N.; Bodenhausen, H.M. Molecular targets for tivantinib (ARQ 197) and vasculogenic mimicry in human melanoma cells. Eur. J. Pharmacol. 2019, 853, 316–324. [Google Scholar] [CrossRef]
- Treps, L.; Faure, S.; Clere, N. Vasculogenic mimicry, a complex and devious process favoring tumorigenesis—Interest in making it a therapeutic target. Pharmacol. Ther. 2021, 223, 107805. [Google Scholar] [CrossRef]
- Mabeta, P. Paradigms of vascularization in melanoma: Clinical significance and potential for therapeutic targeting. Biomed. Pharmacother. 2020, 127, 110135. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, M.J.C.; Seftor, E.A.; Seftor, R.E.B.; Chao, J.T.; Chien, D.S.; Chu, Y.W. Tumor cell vascular mimicry: Novel targeting opportunity in melanoma. Pharmacol. Ther. 2016, 159, 83–92. [Google Scholar] [CrossRef]
- Liu, Z.J.L.; Zhou, Y.J.; Ding, R.L.; Xie, F.; Fu, S.Z.; Wu, J.B.; Yang, L.L.; Wen, Q.L. In vitro and in vivo apatinib inhibits vasculogenic mimicry in melanoma MUM-2B cells. PLoS ONE 2018, 13, e0200845. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, O.; Greenberg, E.; Shalmon, B.; Kubi, A.; Treves, A.J.; Shapira-Frommer, R.; Avivi, C.; Ortenberg, R.; Ben-Ami, E.; Schachter, J.; et al. Nicotinamide inhibits vasculogenic mimicry, an alternative vascularization pathway observed in highly aggressive melanoma. PLoS ONE 2013, 8, e57160. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.E.; Junttila, M.R.; De Sauvage, F.J. Translational value of mouse models in oncology drug development. Nat. Med. 2015, 21, 431–439. [Google Scholar] [CrossRef]
- Ireson, C.R.; Alavijeh, M.S.; Palmer, A.M.; Fowler, E.R.; Jones, H.J. The role of mouse tumour models in the discovery and development of anticancer drugs. Br. J. Cancer 2019, 121, 101–108. [Google Scholar] [CrossRef]
- Fonseca, N.A.; Gregório, A.C.; Mendes, V.M.; Lopes, R.; Abreu, T.; Gonçalves, N.; Manadas, B.; Lacerda, M.; Figueiredo, P.; Pereira, M.; et al. GMP-grade nanoparticle targeted to nucleolin downregulates tumor molecular signature, blocking growth and invasion, at low systemic exposure. Nano Today 2021, 37, 101095. [Google Scholar] [CrossRef]
- Rangarajan, A.; Weinberg, R.A. Comparative biology of mouse versus human cells: Modelling human cancer in mice. Nat. Rev. Cancer 2003, 3, 952–959. [Google Scholar] [CrossRef]
- Kuzu, O.F.; Nguyen, F.D.; Noory, M.A.; Sharma, A. Current State of Animal (Mouse) Modeling in Melanoma Research. Cancer Growth Metastasis 2015, 8, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Saleh, J. Murine models of melanoma. Pathol. Res. Pract. 2018, 214, 1235–1238. [Google Scholar] [CrossRef]
- Kato, R.; Haratani, K.; Hayashi, H.; Sakai, K.; Sakai, H.; Kawakami, H.; Tanaka, K.; Takeda, M.; Yonesaka, K.; Nishio, K.; et al. Nintedanib promotes antitumour immunity and shows antitumour activity in combination with PD-1 blockade in mice: Potential role of cancer-associated fibroblasts. Br. J. Cancer 2021, 124, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.H.; Ribeiro, A.B.; Rinaldi-Neto, F.; Fernandes, F.S.; do Nascimento, S.; Braz, W.R.; Nassar, E.J.; Tavares, D.C. Anti-melanoma activity of indomethacin incorporated into mesoporous silica nanoparticles. Pharm. Res. 2020, 37, 172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bush, X.; Yan, B.; Chen, J.A. Gemcitabine nanoparticles promote antitumor immunity against melanoma. Biomaterials 2019, 189, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, A.; Attias, M.; Mahmood, N.; Arakelian, A.; Mihalcioiu, C.; Piccirillo, C.A.; Szyf, M.; Rabbani, S.A. Enhanced anticancer effect of a combination of S-adenosylmethionine (SAM) and immune checkpoint inhibitor (ICPi) in a syngeneic mouse model of advanced melanoma. Front. Oncol. 2020, 10, 1361. [Google Scholar] [CrossRef]
- Lee, Y.T.; Lim, S.H.; Lee, B.; Kang, I.; Yeo, E.J. Compound C inhibits B16-F1 tumor growth in a syngeneic mouse model via the blockage of cell cycle progression and angiogenesis. Cancers 2019, 11, 823. [Google Scholar] [CrossRef]
- Timmons, J.J.; Cohessy, S.; Wong, E.T. Injection of syngeneic murine melanoma cells to determine their metastatic potential in the lungs. J. Vis. Exp. 2016, 111, e54039. [Google Scholar] [CrossRef]
- Liu, W.; Stachura, P.; Xu, H.C.; Umesh Ganesh, N.; Cox, F.; Wang, R.; Lang, K.S.; Gopalakrishnan, J.; Häussinger, D.; Homey, B.; et al. Repurposing the serotonin agonist Tegaserod as an anticancer agent in melanoma: Molecular mechanisms and clinical implications. J. Exp. Clin. Cancer Res. 2020, 39, 38. [Google Scholar] [CrossRef]
- Chao, W.W.; Cheng, Y.W.; Chen, Y.R.; Lee, S.H.; Chiou, C.Y.; Shyur, L.F. Phyto-sesquiterpene lactone deoxyelephantopin and cisplatin synergistically suppress lung metastasis of B16 melanoma in mice with reduced nephrotoxicity. Phytomedicine 2019, 56, 194–206. [Google Scholar] [CrossRef]
- Krajnović, T.; Drača, D.; Kaluđerović, G.N.; Dunđerović, D.; Mirkov, I.; Wessjohann, L.A.; Maksimović-Ivanić, D.; Mijatović, S. The hop-derived prenylflavonoid isoxanthohumol inhibits the formation of lung metastasis in B16-F10 murine melanoma model. Food Chem. Toxicol. 2019, 129, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Seitz, T.; Hackl, C.; Freese, K.; Dietrich, P.; Mahli, A.; Thasler, R.M.; Thasler, W.E.; Lang, S.A.; Bosserhoff, A.K.; Hellerbrand, C. Xanthohumol, a prenylated chalcone derived from hops, inhibits growth and metastasis of melanoma cells. Cancers 2021, 13, 511. [Google Scholar] [CrossRef]
- Guenzle, J.; Akasaka, H.; Joechle, K.; Reichardt, W.; Venkatasamy, A.; Hoeppner, J.; Hellerbrand, C.; Fichtner-Feigl, S.; Lang, S.A. Pharmacological inhibition of mTORC2 reduces migration and metastasis in melanoma. Int. J. Mol. Sci. 2021, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Carlson, P.M.; Mohan, M.; Rodriguez, M.; Subbotin, V.; Sun, C.X.; Patel, R.B.; Birstler, J.; Hank, J.A.; Rakhmilevich, A.L.; Morris, Z.S.; et al. Depth of tumor implantation affects response to in situ vaccination in a syngeneic murine melanoma model. J. Immunother. Cancer 2021, 9, e002107. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Wang, Y.; Mier, J.W. CXCR4 inhibition modulates the tumor microenvironment and retards the growth of B16-OVA melanoma and Renca tumors Ruchi. Melanoma Res. 2020, 30, 14–25. [Google Scholar] [CrossRef]
- Yoncheva, K.; Merino, M.; Shenol, A.; Daskalov, N.T.; Petkov, P.S.; Vayssilov, G.N.; Garrido, M.J. Optimization and in-vitro/in-vivo evaluation of doxorubicin-loaded chitosan-alginate nanoparticles using a melanoma mouse model. Int. J. Pharm. 2019, 556, 1–8. [Google Scholar] [CrossRef]
- Shen, S.; Hadley, M.; Ustinova, K.; Pavlicek, J.; Knox, T.; Noonepalle, S.; Tavares, M.T.; Zimprich, C.A.; Zhang, G.; Robers, M.B.; et al. Discovery of a new isoxazole-3-hydroxamate-based histone deacetylase 6 inhibitor SS-208 with antitumor activity in syngeneic melanoma mouse models. J. Med. Chem. 2019, 62, 8557–8577. [Google Scholar] [CrossRef]
- Erkes, D.A.; Rosenbaum, S.R.; Field, C.O.; Chervoneva, I.; Villanueva, J.; Aplin, A.E. PLX3397 inhibits the accumulation of intra-tumoral macrophages and improves BET inhibitor efficacy in melanoma. Pigment Cell Melanoma Res. 2020, 33, 372–377. [Google Scholar] [CrossRef]
- McGonigle, P.; Ruggeri, B. Animal models of human disease: Challenges in enabling translation. Biochem. Pharmacol. 2014, 87, 162–171. [Google Scholar] [CrossRef]
- Suggitt, M.; Bibby, M.C. 50 Years of preclinical anticancer drug screening: Empirical to target-driven approaches. Clin. Cancer Res. 2005, 11, 971–981. [Google Scholar]
- Elmets, C.A.; Yusuf, N. Murine skin carcinogenesis and the role of immune system dysregulation in the tumorigenicity of 2-ethylhexyl acrylate. Biomed. Hub 2020, 5, 508295. [Google Scholar] [CrossRef]
- Hirenallur-Shanthappa, D.K.; Ramírez, J.A.; Iritani, B.M. Immunodeficient mice: The backbone of patient-derived tumor xenograft models. In Patient Derived Tumor Xenograft Models: Promise, Potential and Practice; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 57–73. [Google Scholar]
- Okada, S.; Vaeteewoottacharn, K.; Kariya, R. Application of highly immunocompromised mice for the establishment of patient-derived xenograft (PDX) models. Cells 2019, 8, 889. [Google Scholar] [CrossRef]
- Alves da Costa, T.; Lang, J.; Torres, R.M.; Pelanda, R. The development of human immune system mice and their use to study tolerance and autoimmunity. J. Transl. Autoimmun. 2019, 2, 100021. [Google Scholar] [CrossRef] [PubMed]
- Bosma, M.J.; Carrol, A.M. The SCID mouse mutant: Definition, characterization, and potential uses. Annu. Rev. Immunol. 1991, 9, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Kikutani, H.; Makino, S. The murine autoimmune diabetes model: NOD and related strains. Adv. Immunol. 1992, 51, 258–322. [Google Scholar]
- Mosier, D.E.; Stell, K.L.; Gulizia, R.J.; Torbett, B.E.; Gilmore, G.L. Homozygous scid/scid;beige/beige mice have low levels of spontaneous or neonatal T cell-induced B cell generation. J. Exp. Med. 1993, 177, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Lyons, B.L.; Burzenski, L.M.; Gott, B.; Chen, X.; Chaleff, S.; Kotb, M.; Gillies, S.D.; King, M.; Mangada, J.; et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R γ null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 2005, 174, 6477–6489. [Google Scholar] [CrossRef]
- Ritsma, L.; Dey-Guha, I.; Talele, N.; Sole, X.; Salony; Chowdhury, J.; Ross, K.N.; Ramaswamy, S. Integrin β1 activation induces an antimelanoma host response. PLoS ONE 2017, 12, e0175300. [Google Scholar] [CrossRef]
- Du, L.; Anderson, A.; Nguyen, K.; Ojeda, S.S.; Ortiz-Rivera, I.; Nguyen, T.N.; Zhang, T.; Kaoud, T.S.; Gray, N.S.; Dalby, K.N.; et al. JNK2 is required for the tumorigenic properties of melanoma cells. ACS Chem. Biol. 2019, 14, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Hamamura, K.; Takei, Y.; Bhuiyan, R.H.; Ohkawa, Y.; Ohmi, Y.; Nakashima, H.; Furukawa, K.; Furukawa, K. A therapeutic trial of human melanomas with combined small interfering RNAs targeting adaptor molecules p130Cas and paxillin activated under expression of ganglioside GD3. Biochim. Biophys. Acta 2016, 1860, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, D.; Lee, D.; Kapoor, S.; Gibson-Corley, K.N.; Quinn, T.P.; Sagastume, E.A.; Mott, S.L.; Walsh, S.A.; Acevedo, M.R.; et al. Enhancing the efficacy of melanocortin 1 receptor-targeted radiotherapy by pharmacologically upregulating the receptor in metastatic melanoma. Mol. Pharm. 2019, 16, 3904–3915. [Google Scholar] [CrossRef]
- Tiago, M.; Capparelli, C.; Erkes, D.A.; Purwin, T.J.; Heilman, S.A.; Berger, A.C.; Davies, M.A.; Aplin, A.E. Targeting BRD/BET proteins inhibits adaptive kinome upregulation and enhances the effects of BRAF/MEK inhibitors in melanoma. Br. J. Cancer 2020, 122, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Zalba, S.; Seynhaeve, A.L.B.; Debets, R.; ten Hagen, T.L.M. Liposomes targeted to MHC-restricted antigen improve drug delivery and antimelanoma response. Int. J. Nanomed. 2019, 14, 2069–2089. [Google Scholar] [CrossRef]
- White, J.B.; Hu, L.Y.; Boucher, D.L.; Sutcliffe, J.L. ImmunoPET imaging of αvβ6 expression using an engineered anti-αvβ6 Cys-diabody site-specifically radiolabeled with Cu-64: Considerations for optimal imaging with antibody fragments. Mol. Imaging Biol. 2018, 20, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Pampalakis, G.; Zingkou, E.; Zoumpourlis, V.; Sotiropoulou, G. Ectopic expression of KLK6 in MDA-MB-435 melanoma cells reduces tumorigenicity in vivo. Pathol. Res. Pract. 2021, 217, 153276. [Google Scholar] [CrossRef] [PubMed]
- Georgouli, M.; Herraiz, C.; Crosas-Molist, E.; Fanshawe, B.; Maiques, O.; Perdrix, A.; Pandya, P.; Rodriguez-Hernandez, I.; Ilieva, K.M.; Cantelli, G.; et al. Regional activation of myosin II in cancer cells drives tumor progression via a secretory cross-talk with the immune microenvironment. Cell 2019, 176, 757–774. [Google Scholar] [CrossRef]
- Kanygin, V.; Zaboronok, A.; Taskaeva, I.; Zavjalov, E.; Mukhamadiyarov, R.; Kichigin, A.; Kasatova, A.; Razumov, I.; Sibirtsev, R.; Mathis, B.J. In vitro and in vivo evaluation of fluorescently labeled borocaptate-containing liposomes. J. Fluoresc. 2021, 31, 73–83. [Google Scholar] [CrossRef]
- Tichacek, C.J.; Tafreshi, N.K.; Kil, H.; Engelman, R.W.; Doligalski, M.L.; Budzevich, M.M.; Gage, K.L.; Mclaughlin, M.L.; Wadas, T.J.; Silva, A.; et al. Biodistribution and multicompartment pharmacokinetic analysis of a targeted α particle therapy. Mol. Pharm. 2020, 17, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Orouji, E.; Federico, A.; Larribère, L.; Novak, D.; Lipka, D.B.; Assenov, Y.; Sachindra, S.; Hüser, L.; Granados, K.; Gebhardt, C.; et al. Histone methyltransferase SETDB1 contributes to melanoma tumorigenesis and serves as a new potential therapeutic target. Int. J. Cancer 2019, 145, 3462–3477. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, X.; Wang, L.; Gao, X.; Xiong, Y.; Liu, L.; Wei, F.; Yang, L.; Ren, X. T-cell receptor gene therapy targeting melanoma-associated antigen-A4 by silencing of endogenous TCR inhibits tumor growth in mice and human. Cell Death Dis. 2019, 10, 475. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, K.; Ji, J.; Parajuli, H.; Aasen, S.N.; Espedal, H.; Huang, B.; Chen, A.; Wang, J.; Li, X.; et al. Trifluoperazine prolongs the survival of experimental brain metastases by STAT3-dependent lysosomal membrane permeabilization. Am. J. Cancer Res. 2020, 10, 545–563. [Google Scholar]
- Yoshida, H.; Koodie, L.; Jacobsen, K.; Hanzawa, K.; Miyamoto, Y.; Yamamoto, M. B4GALNT1 induces angiogenesis, anchorage independence growth and motility, and promotes tumorigenesis in melanoma by induction of ganglioside GM2/GD2. Sci. Rep. 2020, 10, 1199. [Google Scholar] [CrossRef] [PubMed]
- Phung, B.; Cieśla, M.; Sanna, A.; Guzzi, N.; Beneventi, G.; Cao Thi Ngoc, P.; Lauss, M.; Cabrita, R.; Cordero, E.; Bosch, A.; et al. The X-linked DDX3X RNA helicase dictates translation reprogramming and metastasis in melanoma. Cell Rep. 2019, 27, 3573–3586. [Google Scholar] [CrossRef]
- Benito-Jardon, L.; Díaz-Martínez, M.; Arellano-Sanchez, N.; Vaquero-Morales, P.; Esparís-Ogando, A.; Teixido, J. Resistance to MAPK inhibitors in melanoma involves activation of the IGF1R-MEK5-Erk5 pathway. Cancer Res. 2019, 79, 2244–2256. [Google Scholar] [CrossRef] [PubMed]
- de Araujo Farias, V.; O’Valle, F.; Serrano-Saenz, S.; Anderson, P.; Andrés, E.; López-Peñalver, J.; Tovar, I.; Nieto, A.; Santos, A.; Martín, F.; et al. Exosomes derived from mesenchymal stem cells enhance radiotherapy-induced cell death in tumor and metastatic tumor foci. Mol. Cancer 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Lelliott, E.J.; Cullinane, C.; Martin, C.A.; Walker, R.; Ramsbottom, K.M.; Souza-Fonseca-Guimaraes, F.; Abuhammad, S.; Michie, J.; Kirby, L.; Young, R.J.; et al. A novel immunogenic mouse model of melanoma for the preclinical assessment of combination targeted and immune-based therapy. Sci. Rep. 2019, 9, 1225. [Google Scholar] [CrossRef]
- Kumar, D.; Rahman, H.; Tyagi, E.; Liu, T.; Li, C.; Lu, R.; Lum, D.; Holmen, S.L.; Maschek, J.A.; Cox, J.E.; et al. Aspirin suppresses PGE2 and activates AMP kinase to inhibit melanoma cell motility, pigmentation, and selective tumor growth in vivo. Cancer Prev. Res. 2018, 11, 629–641. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Merkens, H.; Zeisler, J.; Colpo, N.; Hundal-Jabal, N.; Perrin, D.M.; Lin, K.S.; Bénard, F. 18F-Labeled cyclized α-melanocyte-stimulating hormone derivatives for imaging human melanoma xenograft with positron emission tomography. Sci. Rep. 2019, 9, 13575. [Google Scholar] [CrossRef]
- Wang, J.T.W.; Hodgins, N.O.; Al-Jamal, W.T.; Maher, J.; Sosabowski, J.K.; Al-Jamal, K.T. Organ biodistribution of radiolabelled δγ t cells following liposomal alendronate administration in different mouse tumour models. Nanotheranostics 2020, 4, 71–82. [Google Scholar] [CrossRef]
- Snyder, D.; Wang, Y.; Kaetzel, D.M. A rare subpopulation of melanoma cells with low expression of metastasis suppressor NME1 is highly metastatic in vivo. Sci. Rep. 2020, 10, 1971. [Google Scholar] [CrossRef]
- Jespersen, H.; Lindberg, M.F.; Donia, M.; Söderberg, E.M.V.; Andersen, R.; Keller, U.; Ny, L.; Svane, I.M.; Nilsson, L.M.; Nilsson, J.A. Clinical responses to adoptive T-cell transfer can be modeled in an autologous immune-humanized mouse model. Nat. Commun. 2017, 8, 707. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Patel, C.B.; Habte, F.; Gambhir, S.S. Dosimetry prediction for clinical translation of 64Cu-pembrolizumab immunoPET targeting human PD-1 expression. Sci. Rep. 2018, 8, 633. [Google Scholar] [CrossRef]
- Morton, J.J.; Alzofon, N.; Keysar, S.B.; Chimed, T.S.; Reisinger, J.; Perrenoud, L.; Le, P.N.; Nieto, C.; Gomez, K.; Miller, B.; et al. Studying immunotherapy resistance in a melanoma autologous humanized mouse xenograft. Mol. Cancer Res. 2021, 19, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, R.; Connelly, T.; Choi, R.; Choi, H.; Samarkina, A.; Li, L.; Gregorio, E.; Chen, Y.; Thakur, R.; Abdel-Mohsen, M.; et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. Nat. Commun. 2021, 12, 346. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, P.; Guo, S.; Yi, X.; Wang, H.; Yang, Y.; Liu, L.; Shi, Q.; Gao, T.; Li, C. Metastatic melanoma cells rely on Sestrin2 to acquire anoikis resistance via detoxifying intracellular ROS. J. Investig. Dermatol. 2020, 140, 666–675. [Google Scholar] [CrossRef]
- Shi, S.; Li, C.; Zhang, Y.; Deng, C.; Tan, M.; Pan, G.; Du, J.; Ji, Y.; Li, Q.; Liang, H.; et al. Lycorine hydrochloride inhibits melanoma cell proliferation, migration and invasion via down-regulating p21Cip1/WAF1. Am. J. Cancer Res. 2021, 11, 1391–1409. [Google Scholar] [PubMed]
- Cvetanova, B.; Li, M.Y.; Yang, C.C.; Hsiao, P.W.; Yang, Y.C.; Feng, J.H.; Shen, Y.C.; Nakagawa-Goto, K.; Lee, K.H.; Shyur, L.F. Sesquiterpene lactone deoxyelephantopin isolated from Elephantopus scaber and its derivative detd-35 suppress BRAFV600E mutant melanoma lung metastasis in mice. Int. J. Mol. Sci. 2021, 22, 3226. [Google Scholar] [CrossRef]
- Boyle, S.E.; Fedele, C.G.; Shackleton, M. Patient-derived xenografting of human melanoma. In Patient Derived Tumor Xenograft Models: Promise, Potential and Practice; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 341–363. [Google Scholar]
- Krepler, C.; Sproesser, K.; Brafford, P.; Beqiri, M.; Garman, B.; Xiao, M.; Shannan, B.; Watters, A.; Perego, M.; Zhang, G.; et al. A comprehensive patient-derived xenograft collection representing the heterogeneity of melanoma. Cell Rep. 2017, 21, 1953–1967. [Google Scholar] [CrossRef]
- Quintana, E.; Shackleton, M.; Sabel, M.S.; Fullen, D.R.; Johnson, T.M.; Morrison, S.J. Efficient tumour formation by single human melanoma cells. Nature 2008, 456, 593–598. [Google Scholar] [CrossRef]
- Tentler, J.J.; Tan, A.C.; Weekes, C.D.; Jimeno, A.; Leong, S.; Pitts, T.M.; Arcaroli, J.J.; Messersmith, W.A.; Eckhardt, S.G. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 2012, 9, 338–350. [Google Scholar] [CrossRef]
- Aladowicz, E.; Granieri, L.; Marocchi, F.; Punzi, S.; Giardina, G.; Ferrucci, P.F.; Mazzarol, G.; Capra, M.; Viale, G.; Confalonieri, S.; et al. ShcD binds DOCK4, promotes ameboid motility and metastasis dissemination, predicting poor prognosis in melanoma. Cancers 2020, 12, 3366. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, B.A.; Camp, F.; Miknyoczki, S. Animal models of disease: Pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem. Pharmacol. 2014, 87, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Guijarro, E.; Day, C.P.; Merlino, G.; Zaidi, R. Genetically engineered mouse models of melanoma. Cancer 2017, 123, 2089–2103. [Google Scholar] [CrossRef] [PubMed]
- Lelliott, E.J.; Mangiola, S.; Ramsbottom, K.M.; Zethoven, M.; Lim, L.; Lau, P.K.; Oliver, A.J.; Martelotto, L.G.; Kirby, L.; Martin, C.; et al. Combined BRAF, MEK, and CDK4/6 inhibition depletes intratumoral immune-potentiating myeloid populations in melanoma. Cancer Immunol. Res. 2021, 9, 136–146. [Google Scholar] [CrossRef]
- Di Leo, L.; Bodemeyer, V.; Bosisio, F.M.; Claps, G.; Carretta, M.; Rizza, S.; Faienza, F.; Frias, A.; Khan, S.; Bordi, M.; et al. Loss of Ambra1 promotes melanoma growth and invasion. Nat. Commun. 2021, 12, 2550. [Google Scholar] [CrossRef] [PubMed]
- Giblin, W.; Bringman-Rodenbarger, L.; Guo, A.H.; Kumar, S.; Monovich, A.C.; Mostafa, A.M.; Skinner, M.E.; Azar, M.; Mady, A.S.; Chung, C.H.; et al. The deacylase SIRT5 supports melanoma viability by influencing chromatin dynamics. J. Clin. Investig. 2021, 138926. [Google Scholar] [CrossRef]
- Hao, X.; Falo, L.D.; Chen, G.; Zhang, J.; Carey, C.D.; Storkus, W.J.; Falo, L.D.; You, Z. Skin immunization for effective treatment of multifocal melanoma refractory to PD1 blockade and Braf inhibitors. J. Immunother. Cancer 2021, 9, e001179. [Google Scholar] [CrossRef]
- Vitiello, M.; Evangelista, M.; Di Lascio, N.; Kusmic, C.; Massa, A.; Orso, F.; Sarti, S.; Marranci, A.; Rodzik, K.; Germelli, L.; et al. Antitumoral effects of attenuated Listeria monocytogenes in a genetically engineered mouse model of melanoma. Oncogene 2019, 38, 3756–3762. [Google Scholar] [CrossRef]
- Tandukar, B.; Kalapurakal, E.; Hornyak, T.J. B6-Dct-H2BGFP bitransgenic mice: A standardized mouse model for in vivo characterization of melanocyte development and stem cell differentiation. Pigment Cell Melanoma Res. 2021, 34, 905–917. [Google Scholar] [CrossRef]
- Leonard, M.K.; Pamidimukkala, N.; Puts, G.S.; Snyder, D.E.; Slominski, A.T.; Kaetzel, D.M. The HGF/SF mouse model of UV-induced melanoma as an in vivo sensor for metastasis-regulating gene. Int. J. Mol. Sci. 2017, 18, 1647. [Google Scholar] [CrossRef]
- Pamidimukkala, N.; Puts, G.S.; Kathryn Leonard, M.; Snyder, D.; Dabernat, S.; De Fabo, E.C.; Noonan, F.P.; Slominski, A.; Merlino, G.; Kaetzel, D.M. Nme1 and Nme2 genes exert metastasis-suppressor activities in a genetically engineered mouse model of UV-induced melanoma. Br. J. Cancer 2021, 124, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Urtatiz, O.; Cook, C.; Huang, J.L.; Yeh, I.; Van Raamsdonk, C.D. GNAQQ209L expression initiated in multipotent neural crest cells drives aggressive melanoma of the central nervous system. Pigment Cell Melanoma Res. 2020, 33, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Loo, K.; Soliman, I.; Renzetti, M.; Li, T.; Wu, H.; Reddy, S.; Olszanski, A.J.; Farma, J.M. Impact of sun exposure and tanning patterns on next-generation sequencing mutations in melanoma. J. Surg. Res. 2020, 254, 147–153. [Google Scholar] [CrossRef]
- Day, C.P.; Maschalik, R.; Merlino, G.; Michael, H.T. Mouse models of UV-induced melanoma: Genetics, pathology, and clinical relevance. Lab. Investig. 2017, 97, 698–705. [Google Scholar] [CrossRef]
- De Luca, D.A.; Sterniczky, B.; Kimeswenger, S.; Födinger, D.; Schwarz, A.; Schwarz, T.; Jantschitsch, C. Ultraviolet radiation induces Melan-A-expressing cells in interfollicular epidermis in wild-type mice. Arch. Dermatol. Res. 2018, 310, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Yamashita, Y.; Umezawa, K.; Hirobe, T.; Ito, S.; Wakamatsu, K. The pro-oxidant activity of pheomelanin is significantly enhanced by UVA irradiation: Benzothiazole moieties are more reactive than benzothiazine moieties. Int. J. Mol. Sci. 2018, 19, 2889. [Google Scholar] [CrossRef]
- Moon, H.; Donahue, L.R.; Choi, E.; Scumpia, P.O.; Lowry, W.E.; Grenier, J.K.; Zhu, J.; White, A.C. Melanocyte stem cell activation and translocation initiate cutaneous melanoma in response to UV exposure. Cell Stem Cell 2017, 21, 665–678. [Google Scholar] [CrossRef]
- Jegal, J.; Chung, K.W.; Chung, H.Y.; Jeong, E.J.; Yang, M.H. The standardized extract of Juniperus communis alleviates hyperpigmentation in vivo HRM-2 hairless mice and in vitro murine B16 melanoma cells. Biol. Pharm. Bull. 2017, 40, 1381–1388. [Google Scholar] [CrossRef]
- Saba, E.; Kim, S.H.; Lee, Y.Y.; Kim, H.K.; Roh, S.S.; Kwak, Y.S.; Park, C.K.; Kim, S.D.; Rhee, M.H. Anti-melanogenic effects of Korean Red Ginseng Oil in an ultraviolet B-induced hairless mouse model. Molecules 2020, 25, 4755. [Google Scholar] [CrossRef]
- Hwang, G.Y.; Choung, S.Y. Anti-melanogenic effects of Aster spathulifolius extract in UVB-exposed C57BL/6J mice and B16F10 melanoma cells through the regulation of MAPK/ERK and AKT/GSK3β signalling. J. Pharm. Pharmacol. 2016, 68, 503–513. [Google Scholar] [CrossRef]
- Katiyar, S.K. Proanthocyanidins from grape seeds inhibit UV-radiation-induced immune suppression in mice: Detection and analysis of molecular and cellular targets. Photochem. Photobiol. 2015, 91, 156–162. [Google Scholar] [CrossRef]
- Ferguson, B.; Handoko, H.Y.; Mukhopadhyay, P.; Chitsazan, A.; Balmer, L.; Morahan, G.; Walker, G.J. Different genetic mechanisms mediate spontaneous versus UVR-induced malignant melanoma. eLife 2019, 8, e42424. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, R.C.; Holderbaum, A.M.; Bonilla, A.; Delaney, C.; Gillahan, J.E.; Tober, K.L.; Oberyszyn, T.M.; Zippin, J.H.; Burd, C.E. Ultraviolet radiation accelerates NRas-mutant melanomagenesis: A cooperative effect blocked by sunscreen. Pigment Cell Melanoma Res. 2017, 30, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Ferguson, B.; Muller, H.K.; Handoko, H.Y.; Walker, G.J. Murine melanomas accelerated by a single UVR exposure carry photoproduct footprints but lack UV signature C>T mutations in critical genes. Oncogene 2016, 35, 3342–3350. [Google Scholar] [CrossRef] [PubMed]
- Chagani, S.; Wang, R.; Carpenter, E.L.; Löhr, C.V.; Ganguli-Indra, G.; Indra, A.K. Ablation of epidermal RXRα in cooperation with activated CDK4 and oncogenic NRAS generates spontaneous and acute neonatal UVB induced malignant metastatic melanomas. BMC Cancer 2017, 17, 736. [Google Scholar] [CrossRef]
- Kligman, L.H.; Elenitsas, R. Melanoma induction in a hairless mouse with short-term application of dimethylbenz[a]anthracene. Melanoma Res. 2001, 11, 319–324. [Google Scholar] [CrossRef]
- Manna, D.; Akhtar, S.; Maiti, P.; Mondal, S.; Kumar Mandal, T.; Ghosh, R. Anticancer activity of a 1,4-dihydropyridine in DMBA-induced mouse skin tumor model. Anticancer Drugs 2020, 31, 394–402. [Google Scholar] [CrossRef]
- Nasti, T.H.; Cochran, J.B.; Tsuruta, Y.; Yusuf, N.; Mckay, K.M.; Athar, M.; Timares, L.; Elmets, C.A. A murine model for the development of melanocytic nevi and their progression to melanoma. Mol. Carcinog. 2016, 55, 646–658. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Du, H.; Huang, V.; Sun, B.; Harris, J.P.; Richardson, Q.; Shen, X.; Jin, R.; Li, G.; et al. Withaferin A suppresses the up-regulation of acetyl-coA carboxylase 1 and skin tumor formation in a skin carcinogenesis mouse model. Mol. Carcinog. 2016, 55, 1739–1746. [Google Scholar] [CrossRef]
- Ghiciuc, C.M.; Strat, A.L.; Ochiuz, L.; Lupusoru, C.E.; Ignat, M.; Vasile, A.; Grigorovici, A.; Stoleriu, I.; Solcan, C. Inhibition of bcl-2 and cox-2 protein expression after local application of a new carmustine-loaded clinoptilolite-based delivery system in a chemically induced skin cancer model in mice. Molecules 2017, 22, 2014. [Google Scholar] [CrossRef]
- Kircher, D.A.; Silvis, M.R.; Cho, J.H.; Holmen, S.L. Melanoma brain metastasis: Mechanisms, models, and medicine. Int. J. Mol. Sci. 2016, 17, 1468. [Google Scholar] [CrossRef]
- Cheng, X.; Cheng, K. Visualizing cancer extravasation: From mechanistic studies to drug development. Cancer Metastasis Rev. 2021, 40, 71–88. [Google Scholar] [CrossRef]
- Travnickova, J.; Patton, E.E. Deciphering melanoma cell states and plasticity with zebrafish models. J. Investig. Dermatol. 2021, 141, 1389–1394. [Google Scholar] [CrossRef]
- Frantz, W.T.; Ceol, C.J. From tank to treatment: Modeling melanoma in zebrafish. Cells 2020, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Dilshat, R.; Fock, V.; Kenny, C.; Gerritsen, I.; Lasseur, R.M.J.; Travnickova, J.; Eichhoff, O.; Cerny, P.; Möller, K.; Sigurbjörnsdóttir, S.; et al. MITF reprograms the extracellular matrix and focal adhesion in melanoma. eLife 2021, 10, e63093. [Google Scholar] [CrossRef] [PubMed]
- Ablain, J.; Liu, S.; Moriceau, G.; Lo, R.S.; Zon, L.I. SPRED1 deletion confers resistance to MAPK inhibition in melanoma. J. Exp. Med. 2021, 218, e20201097. [Google Scholar] [CrossRef] [PubMed]
- Fazio, M.; van Rooijen, E.; Mito, J.K.; Modhurima, R.; Weiskopf, E.; Yang, S.; Zon, L.I. Recurrent co-alteration of HDGF and SETDB1 on chromosome 1q drives cutaneous melanoma progression and poor prognosis. Pigment Cell Melanoma Res. 2021, 34, 641–647. [Google Scholar] [CrossRef]
- Mahmood, I.; Azfaralariff, A.; Mohamad, A.; Airianah, O.B.; Law, D.; Dyari, H.R.E.; Lim, Y.C.; Fazry, S. Mutated Shiitake extracts inhibit melanin-producing neural crest-derived cells in zebrafish embryo. Comp. Biochem. Physiol. Part C 2021, 245, 109033. [Google Scholar]
- Jeon, H.J.; Kim, K.; Kim, C.; Kim, M.J.; Kim, T.O.; Lee, S.E. Molecular mechanisms of anti-melanogenic gedunin derived from neem tree (Azadirachta indica) using B16F10 mouse melanoma cells and early-stage zebrafish. Plants 2021, 10, 330. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Y.; Im, S.T.; Myung, S.; Kim, H.-S.; Lee, S.-H. Diphlorethohydroxycarmalol inhibits melanogenesis via protein kinase A/cAMP response element-binding protein and extracellular signal-regulated kinase-mediated microphthalmia-associated transcription factor downregulation in α-melanocyte stimulating hormo. Cell Biochem. Funct. 2021, 39, 546–554. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, B.; Meng, S.; Zhou, L.; Xu, Y.; Du, W.; Shan, L. Theaflavin induces apoptosis of A375 human melanoma cells and inhibits tumor growth in xenograft zebrafishes through P53- and JNK-related mechanism. Front. Pharmacol. 2020, 11, 1317. [Google Scholar] [CrossRef]
- Yang, H.L.; Lin, C.P.; Vudhya Gowrisankar, Y.; Huang, P.J.; Chang, W.L.; Shrestha, S.; Hseu, Y.C. The anti-melanogenic effects of ellagic acid through induction of autophagy in melanocytes and suppression of UVA-activated α-MSH pathways via Nrf2 activation in keratinocytes. Biochem. Pharmacol. 2021, 185, 11445. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.K.; Jung, S.H.; Jeong, J.H.; Yoo, H.M.; Lee, I.S.; Shin, H.S. The antimelanogenic effect of inularin isolated from flowers of Inula britannica on B16F10 melanoma cells and zebrafish embryos. J. Microbiol. Biotechnol. 2020, 30, 749–752. [Google Scholar] [CrossRef]
- Valenti, M.T.; Marchetto, G.; Perduca, M.; Tiso, N.; Mottes, M.; Carbonare, L.D. Bel β-trefoil reduces the migration ability of RUNX2 expressing melanoma cells in xenotransplanted zebrafish. Molecules 2020, 25, 1270. [Google Scholar] [CrossRef]
- Di Palma, S.; McConnell, A.; Verganti, S.; Starkey, M. Review on canine oral melanoma: An undervalued authentic genetic model of human oral melanoma. Vet. Pathol. 2021, 58, 300985821996658. [Google Scholar] [CrossRef]
- Yasumaru, C.C.; Xavier, J.G.; Strefezzi, R.D.F.; Salles-Gomes, C.O.M. Intratumoral T-lymphocyte subsets in canine oral Melanoma and their association with clinical and histopathological parameters. Vet. Pathol. 2021, 58, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Brocca, G.; Poncina, B.; Sammarco, A.; Cavicchioli, L.; Castagnaro, M. Kit somatic mutations and immunohistochemical expression in canine oral melanoma. Animals 2020, 10, 2370. [Google Scholar] [CrossRef]
- Hernandez, B.; Adissu, H.A.; Wei, B.R.; Michael, H.T.; Merlino, G.; Mark Simpson, R. Naturally occurring canine melanoma as a predictive comparative oncology model for human mucosal and other triple wild-type melanomas. Int. J. Mol. Sci. 2018, 19, 394. [Google Scholar] [CrossRef] [PubMed]
- Prouteau, A.; André, C. Canine melanomas as models for human melanomas: Clinical, histological, and genetic comparison. Genes 2019, 10, 501. [Google Scholar] [CrossRef]
- van der Weyden, L.; Brenn, T.; Patton, E.E.; Wood, G.A.; Adams, D.J. Spontaneously occurring melanoma in animals and their relevance to human melanoma. J. Pathol. 2020, 252, 4–21. [Google Scholar] [CrossRef]
- Monteiro, A.C.; Muenzner, J.K.; Andrade, F.; Rius, F.E.; Ostalecki, C.; Geppert, C.I.; Agaimy, A.; Hartmann, A.; Fujita, A.; Schneider-Stock, R.; et al. Gene expression and promoter methylation of angiogenic and lymphangiogenic factors as prognostic markers in melanoma. Mol. Oncol. 2019, 13, 1433–1449. [Google Scholar] [CrossRef] [PubMed]
- Strnadová, K.; Španko, M.; Dvořánková, B.; Lacina, L.; Kodet, O.; Shbat, A.; Klepáček, I.; Smetana, K. Melanoma xenotransplant on the chicken chorioallantoic membrane: A complex biological model for the study of cancer cell behaviour. Histochem. Cell Biol. 2020, 154, 177–188. [Google Scholar] [CrossRef]
- Avram, S.; Coricovac, D.E.; Pavel, I.Z.; Pinzaru, I.; Ghiulai, R.; Baderca, F.; Soica, C.; Muntean, D.; Branisteanu, D.E.; Spandidos, D.A.; et al. Standardization of A375 human melanoma models on chicken embryo chorioallantoic membrane and Balb/c nude mice. Oncol. Rep. 2017, 38, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yu, D.; Fu, R.; An, P.; Sun, R.; Wang, Z.; Guo, R.; Li, H.; Zhang, Y.; Li, Z.; et al. IL2RG-deficient minipigs generated via CRISPR/Cas9 technology support the growth of human melanoma-derived tumours. Cell Prolif. 2020, 53, e12863. [Google Scholar] [CrossRef]
- Marthey, S.; Estellé, J.; Blin, A.; Wahlberg, P.; Créchet, F.; Lecardonnel, J.; Tessiot, F.; Rogel-Gaillard, C.; Bourneuf, E. Transcription from a gene desert in a melanoma porcine model. Mol. Genet. Genom. 2020, 295, 1239–1252. [Google Scholar] [CrossRef] [PubMed]
- Horak, V.; Palanova, A.; Cizkova, J.; Miltrova, V.; Vodicka, P.; Skalnikova, H.K. Melanoma-bearing libechov minipig (MeLiM): The unique swine model of hereditary metastatic melanoma. Genes 2019, 10, 915. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmann, V.K.; Gellrich, D.; Ullrich, A.; Zahler, S.; Vollmar, A.M.; Kazmaier, U.; Fürst, R. Novel tubulin antagonist pretubulysin displays antivascular properties in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 294–303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodríguez-García, A.; Giménez-Alejandre, M.; Rojas, J.J.; Moreno, R.; Bazan-Peregrino, M.; Cascalló, M.; Ramon, A. Safety and efficacy of VCN-01, an oncolytic adenovirus combining fiber HSG-binding domain replacement with RGD and hyaluronidase expression. Clin. Cancer Res. 2015, 21, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Śniegocka, M.; Podgórska, E.; Plonka, P.M.; Elas, M.; Romanowska-Dixon, B.; Szczygiel, M.; Żmijewski, M.A.; Cichorek, M.; Markiewicz, A.; Brożyna, A.A.; et al. Transplantable melanomas in hamsters and gerbils as models for human melanoma. Sensitization in melanoma radiotherapy—From animal models to clinical trials. Int. J. Mol. Sci. 2018, 19, 1048. [Google Scholar] [CrossRef]
- Liebscher, G.; Vanchangiri, K.; Mueller, T.; Feige, K.; Cavalleri, J.M.V.; Paschke, R. In vitro anticancer activity of Betulinic acid and derivatives thereof on equine melanoma cell lines from grey horses and in vivo safety assessment of the compound NVX-207 in two horses. Chem. Biol. Interact. 2016, 246, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; VandeBerg, J.L. Immunotolerance in the laboratory opossum (Monodelphis domestica) to xenografted mouse melanoma. Contemp. Top. Lab. Anim. Sci. 2005, 44, 39–42. [Google Scholar]
- Lu, Y.; Sandoval, A.; Voss, S.; Lai, Z.; Kneitz, S.; Boswell, W.; Boswell, M.; Savage, M.; Walter, C.; Warren, W.; et al. Oncogenic allelic interaction in Xiphophorus highlights hybrid incompatibility. Proc. Natl. Acad. Sci. USA 2020, 117, 29786–29794. [Google Scholar] [CrossRef] [PubMed]
- Klotz, B.; Kneitz, S.; Lu, Y.; Boswell, W.; Postlethwait, J.; Warren, W.; Walter, R.B.; Schartl, M. Expression signatures of cisplatin- and trametinib-treated early-stage medaka melanomas. G3 2019, 9, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials. Available online: https://clinicaltrials.gov/ (accessed on 13 June 2021).
- Aguilera, J.V.; Paludo, J.; McWilliams, J.J.; Zhang, H.; Li, Y.; Kumar, A.B.; Failing, J.; Kottschade, L.A.; Block, M.S.; Markovic, S.N. Chemo-immunotherapy combination after PD-1 inhibitor failure improves clinical outcomes in metastatic melanoma patients. Melanoma Res. 2020, 30, 364–375. [Google Scholar] [CrossRef]
- Wróbel, S.; Przybyło, M.; Stępień, E. The clinical trial landscape for melanoma therapies. J. Clin. Med. 2019, 8, 368. [Google Scholar] [CrossRef]
- Velho, T.R. Metastatic melanoma—A review of current and future drugs. Drugs Context. 2012, 2012, 212242. [Google Scholar] [CrossRef]
- Soltantoyeh, T.; Akbari, B.; Karimi, A.; Chalbatani, G.M.; Ghahri-Saremi, N.; Hadjati, J.; Hamblin, M.R.; Mirzaei, H.R. Chimeric Antigen Receptor (CAR) T Cell Therapy for Metastatic Melanoma: Challenges and Road Ahead. Cells 2021, 10, 1450. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Pala, L.; Conforti, F.; Cocorocchio, E. Talimogene laherparepvec (T-vec): An intralesional cancer immunotherapy for advanced melanoma. Cancers 2021, 13, 1383. [Google Scholar] [CrossRef]
- Revythis, A.; Shah, S.; Kutka, M.; Moschetta, M.; Ozturk, M.A.; Pappas-Gogos, G.; Ioannidou, E.; Sheriff, M.; Rassy, E.; Boussios, S. Unraveling the wide spectrum of melanoma biomarkers. Diagnostics 2021, 11, 1341. [Google Scholar] [CrossRef]
- Indini, A.; Roila, F.; Grossi, F.; Massi, D.; Mandalà, M. Impact of Circulating and Tissue Biomarkers in Adjuvant and Neoadjuvant Therapy for High-Risk Melanoma: Ready for Prime Time? Am. J. Clin. Dermatol. 2021, 22, 511–522. [Google Scholar] [CrossRef] [PubMed]
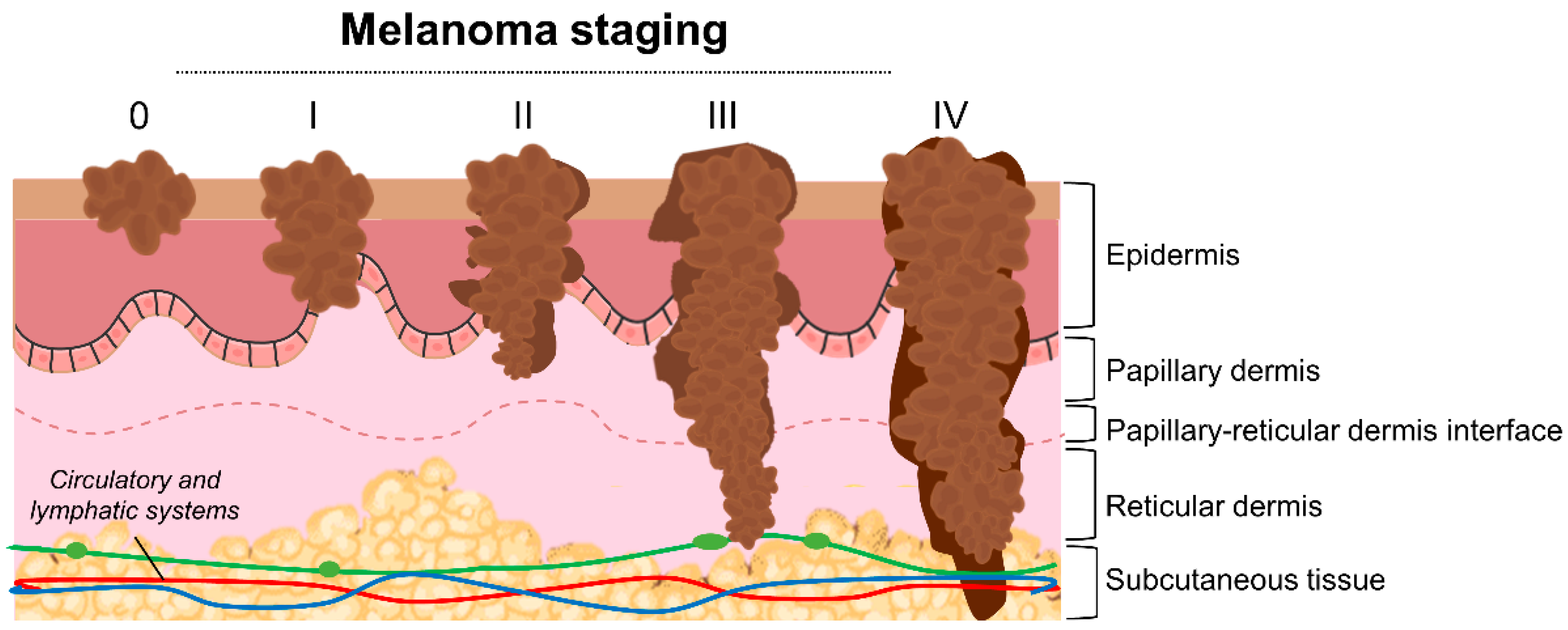
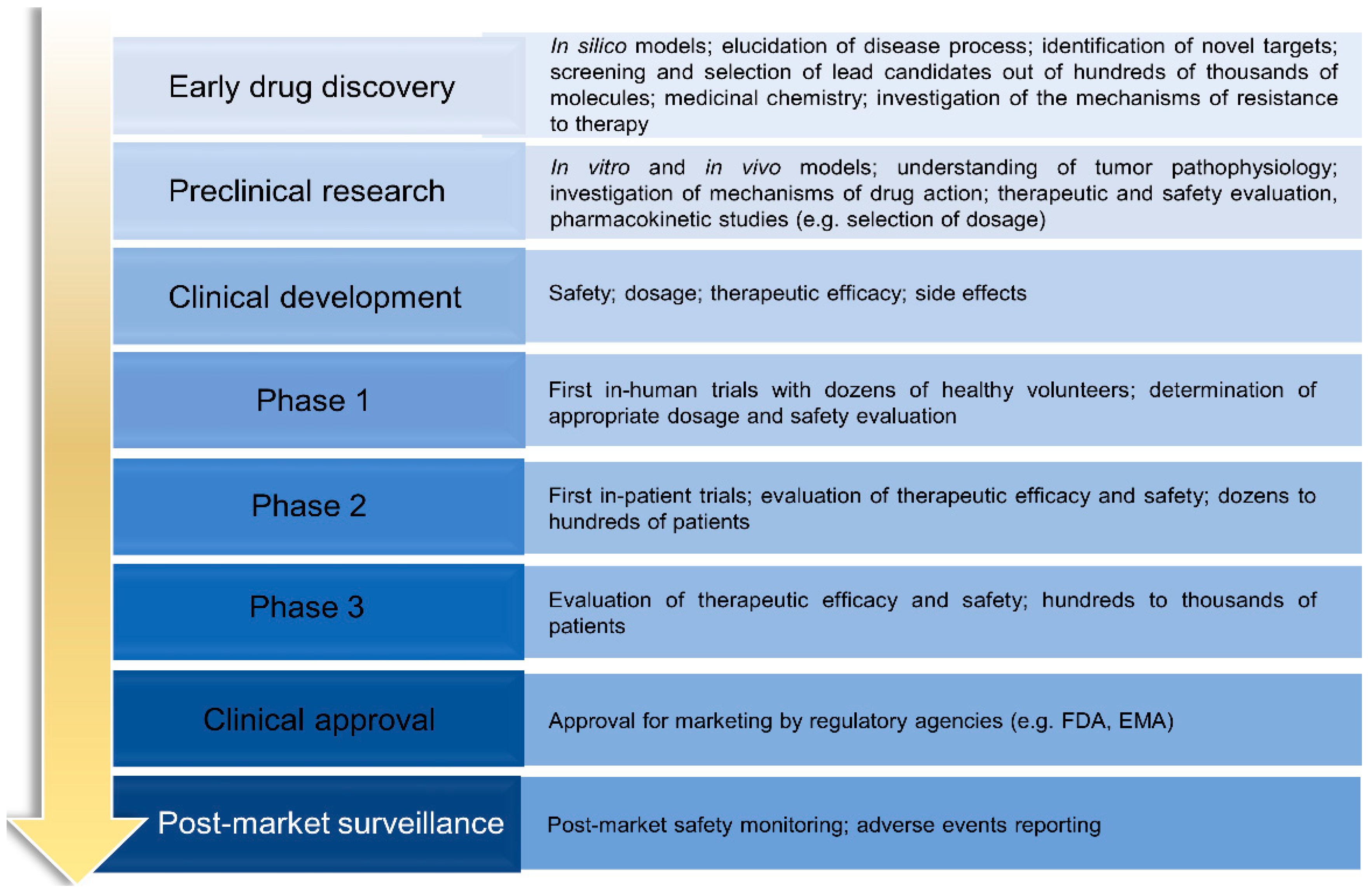

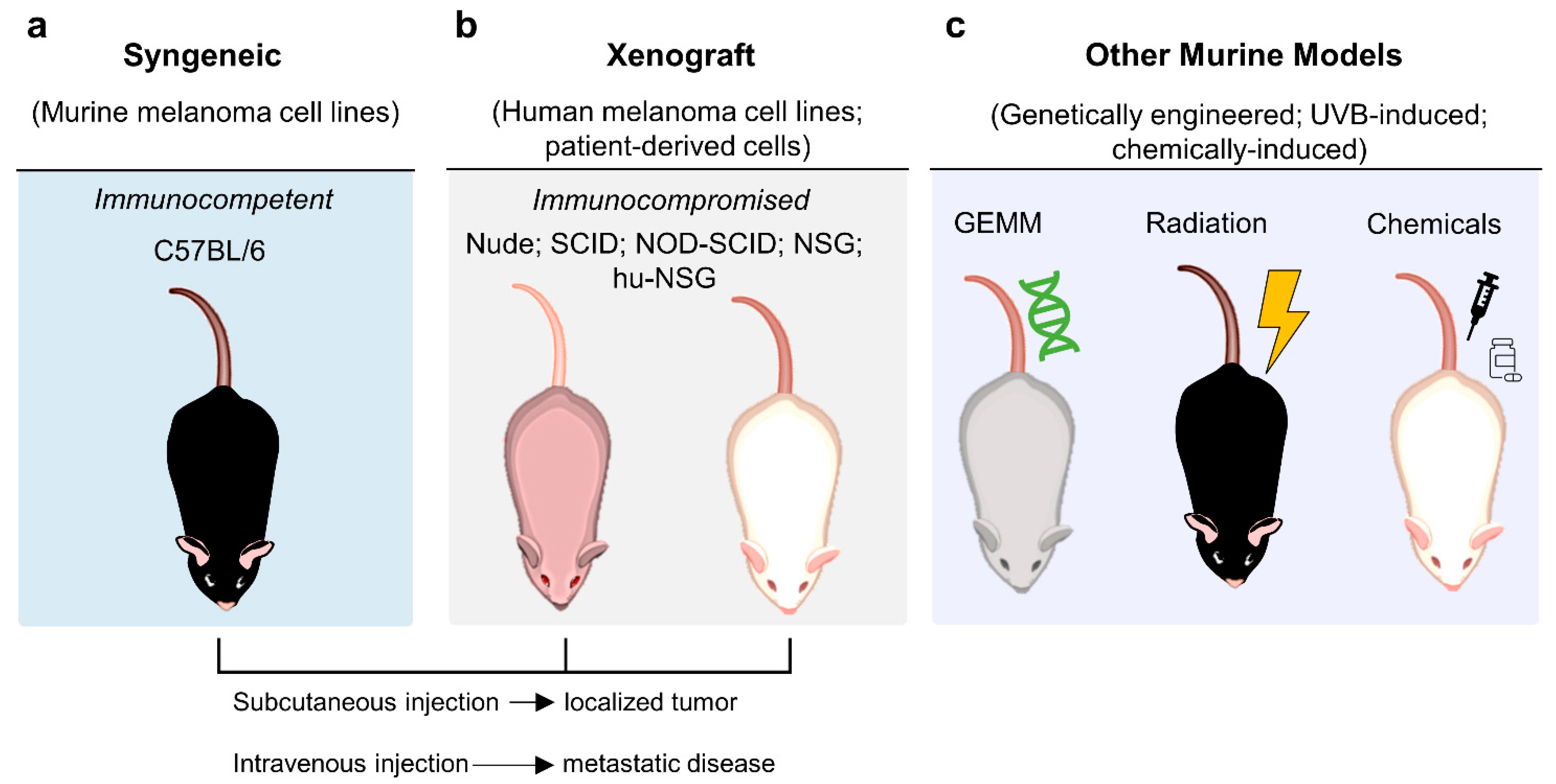
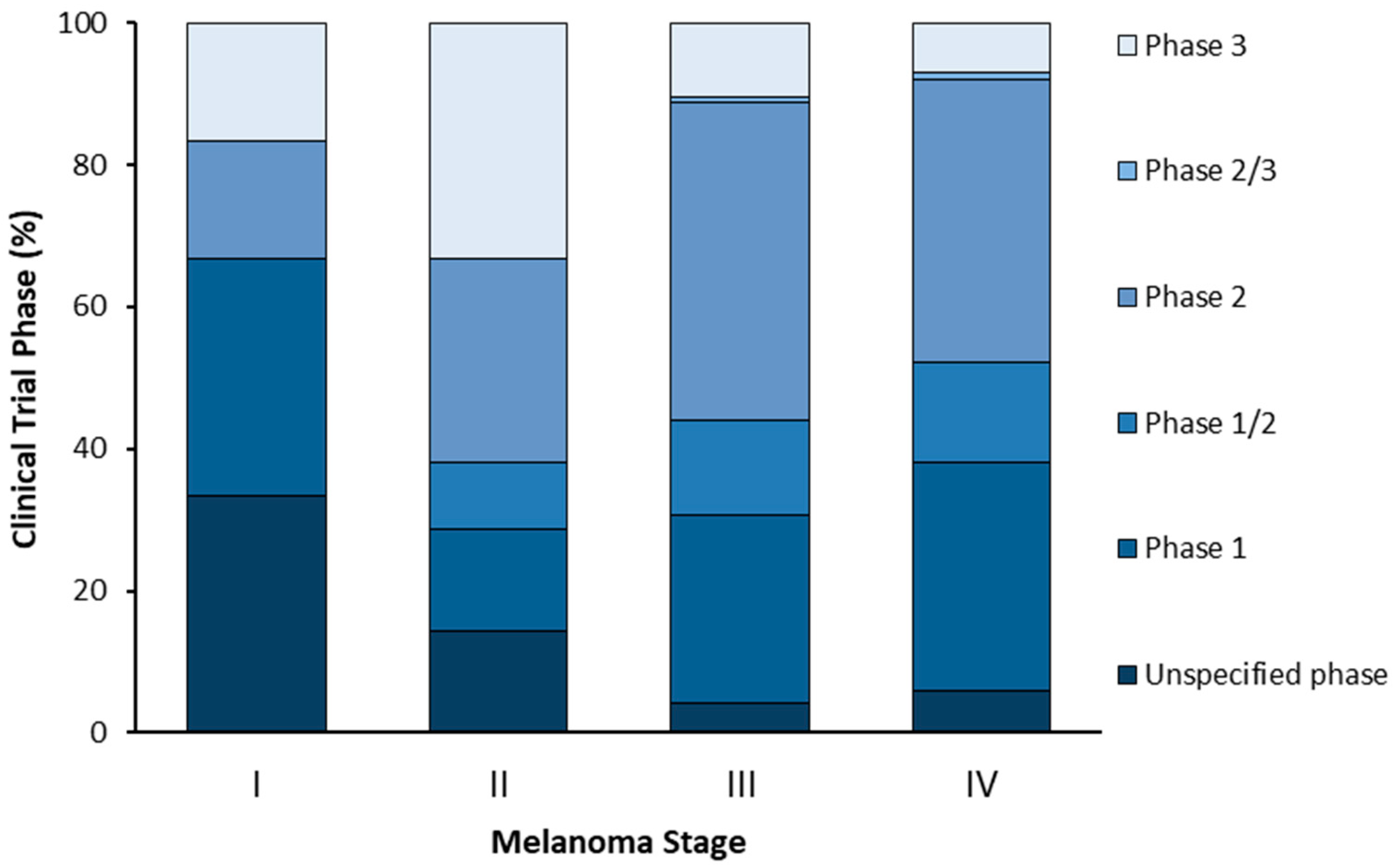
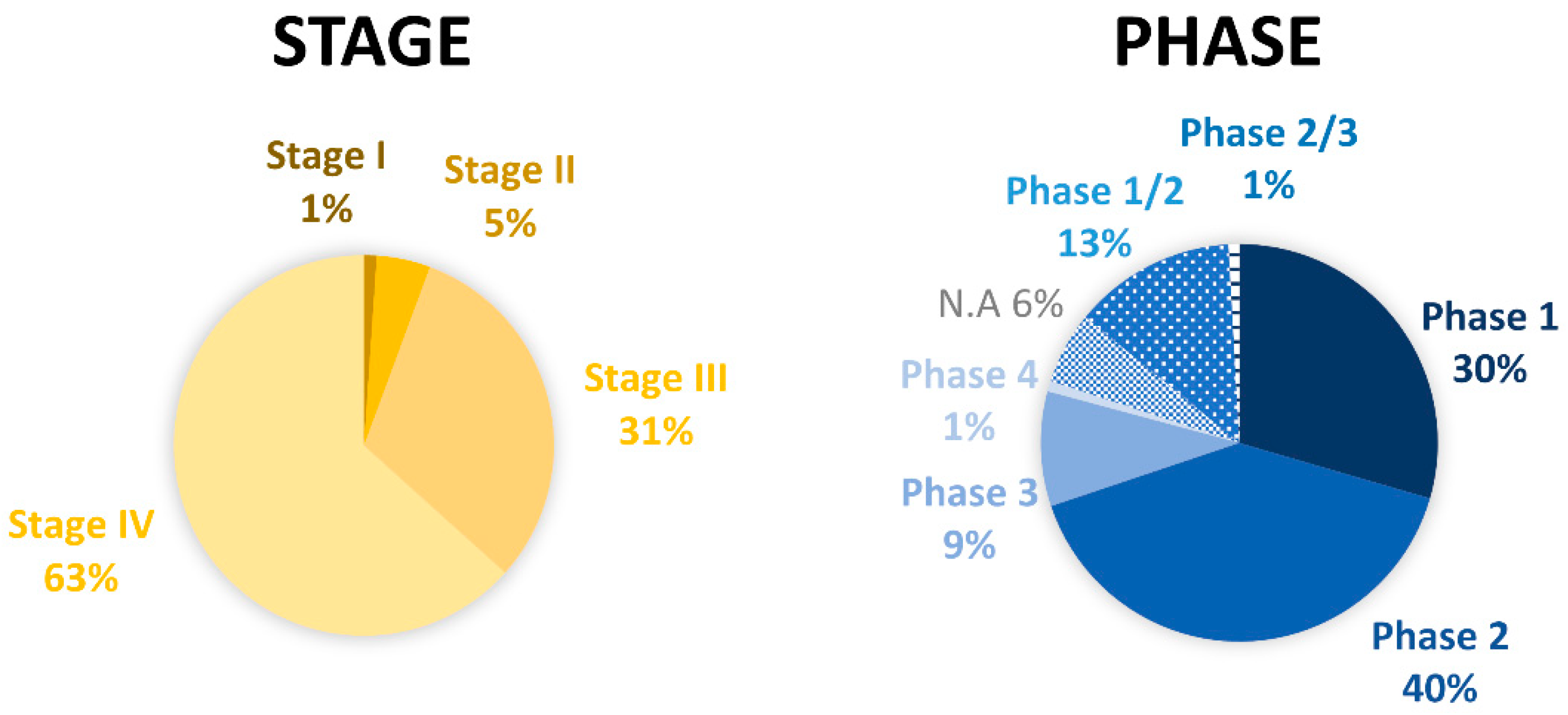

| Therapeutic Option | Advantages | Limitations |
|---|---|---|
| Surgery | Prevention of melanoma systemic spreading Reduction of local recurrence risk | Associated severe comorbidities Unsuitable for systemic disease |
| Radiotherapy | Good local tumor control Useful for palliative care | Associated intrinsic resistance Severe side effects |
| Chemotherapy | Effective in highly proliferating disease conditions Indicated for palliative care | Reduced specificity Severe side effects |
| Targeted therapy | Reduction of side effects Improvement of response and survival rates Personalized therapy | Emergence of resistances High cost |
| Immunotherapy | Improvement of clinical outcomes | Severe side effects High cost |
| Experimental Model | Advantages | Disadvantages | References |
|---|---|---|---|
| 2D models | Initial screening and selection of new molecules Basic research of tumor cell biology Easy to perform Compliant with HTS High reproducibility Cost-effective Pure and free from contaminating cells | Unable to mimic in vivo tumor microenvironment Loss of stromal, vascular, and immune cellular populations Lack of heterogeneity Alterations after long-term culture Cannot reproduce melanoma cell interactions with extracellular matrix | [90,108,109] |
| 3D models | |||
| Spheroid | Easy to perform Compliant with HTS Co-culture ability Relative low cost High reproducibility Good representation of oxygen, nutrient, and other soluble factors Simulation of tumor heterogeneity and drug resistance | Simplified architecture Unsuitable for longitudinal studies Limited number of cell types for co-culture | [75,110,111,112] |
| Tumorosphere | Preservation of cancer stem cell features Initiating ability Self-renewal potential Study of drug resistance | Excessive sensitivity to the culture method Cell fusion and aggregation Low reproducibility Unable to reproduce the variety of cell types | [74,111,113,114] |
| Organoid | Compliant with HTS Patient specific Simulation of in vivo tumor complexity and architecture | Low reproducibility Lack of vascular system and/or key cell types Reduced number of cells available Reduced heterogeneity compared to original tumor | [75,110] |
| Skin Reconstruct | Simulation of in vivo tumor architecture Controlled tissue organization Co-culture ability | Time-consuming procedure Constant monitoring required | [111,115] |
| Melanoma-on-chip | Simulation of in vivo tumor architecture, microenvironment, chemical and physical gradients Accurate and rapid procedure Cost-effective (small scale) | Lack of vascular system Unsuitable for HTS | [74,110] |
| Neoangiogenesis | Simulation of in vivo tumor microenvironment Co-culture ability Time-effective | Insufficient vessel stabilization due to the short duration of assays Formation of vessel like-structures instead of capillaries | [75,116,117] |
| Clinical Trial (NCT Number) | Melanoma Stage | Clinical Phase | Start Date | Sponsor |
|---|---|---|---|---|
| NCT04697576 | I/II/IV | 1 | 2021 | Carlo Contreras |
| NCT03819296 | I/II/III/IV | 1/2 | 2021 | M. D. Anderson Cancer Center |
| NCT03757689 | II | 2 | 2019 | Abramson Cancer Center |
| NCT03860883 | II | 3 | 2019 | Melanoma and Skin Cancer Trials Ltd. |
| NCT04309409 | II | 3 | 2020 | University Hospital, Essen |
| NCT03554083 | III | 2 | 2018 | Mayo Clinic |
| NCT03021460 | III/IV | 1 | 2017 | Mayo Clinic |
| NCT03132675 | III/IV | 2 | 2017 | OncoSec Medical Inc. |
| NCT02816021 | III/IV | 2 | 2017 | M. D. Anderson Cancer Center |
| NCT03991130 | III/IV | 2 | 2019 | Gregory Daniels |
| NCT04356729 | III/IV | 2 | 2020 | Elizabeth Buchbinder |
| NCT02506153 | III/IV | 3 | 2015 | National Cancer Institute |
| NCT04410445 | III/IV | 3 | 2020 | Nektar Therapeutics |
| NCT01993719 | IV | 2 | 2013 | National Cancer Institute |
| NCT03928275 | IV | 2/3 | 2021 | Carman Giacomantonio |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matias, M.; Pinho, J.O.; Penetra, M.J.; Campos, G.; Reis, C.P.; Gaspar, M.M. The Challenging Melanoma Landscape: From Early Drug Discovery to Clinical Approval. Cells 2021, 10, 3088. https://doi.org/10.3390/cells10113088
Matias M, Pinho JO, Penetra MJ, Campos G, Reis CP, Gaspar MM. The Challenging Melanoma Landscape: From Early Drug Discovery to Clinical Approval. Cells. 2021; 10(11):3088. https://doi.org/10.3390/cells10113088
Chicago/Turabian StyleMatias, Mariana, Jacinta O. Pinho, Maria João Penetra, Gonçalo Campos, Catarina Pinto Reis, and Maria Manuela Gaspar. 2021. "The Challenging Melanoma Landscape: From Early Drug Discovery to Clinical Approval" Cells 10, no. 11: 3088. https://doi.org/10.3390/cells10113088
APA StyleMatias, M., Pinho, J. O., Penetra, M. J., Campos, G., Reis, C. P., & Gaspar, M. M. (2021). The Challenging Melanoma Landscape: From Early Drug Discovery to Clinical Approval. Cells, 10(11), 3088. https://doi.org/10.3390/cells10113088










