Dermal Pericytes Exhibit Declined Ability to Promote Human Skin Regeneration with Ageing in 3D Organotypic Culture Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Tissue Collection
2.2. Isolation and Culture of Keratinocytes, Dermal Pericytes, and Fibroblasts
2.3. Organotypic Cultures
2.4. Immunostaining
2.5. Transmission Electron Microscopy
2.6. Quantification and Statistics
3. Results
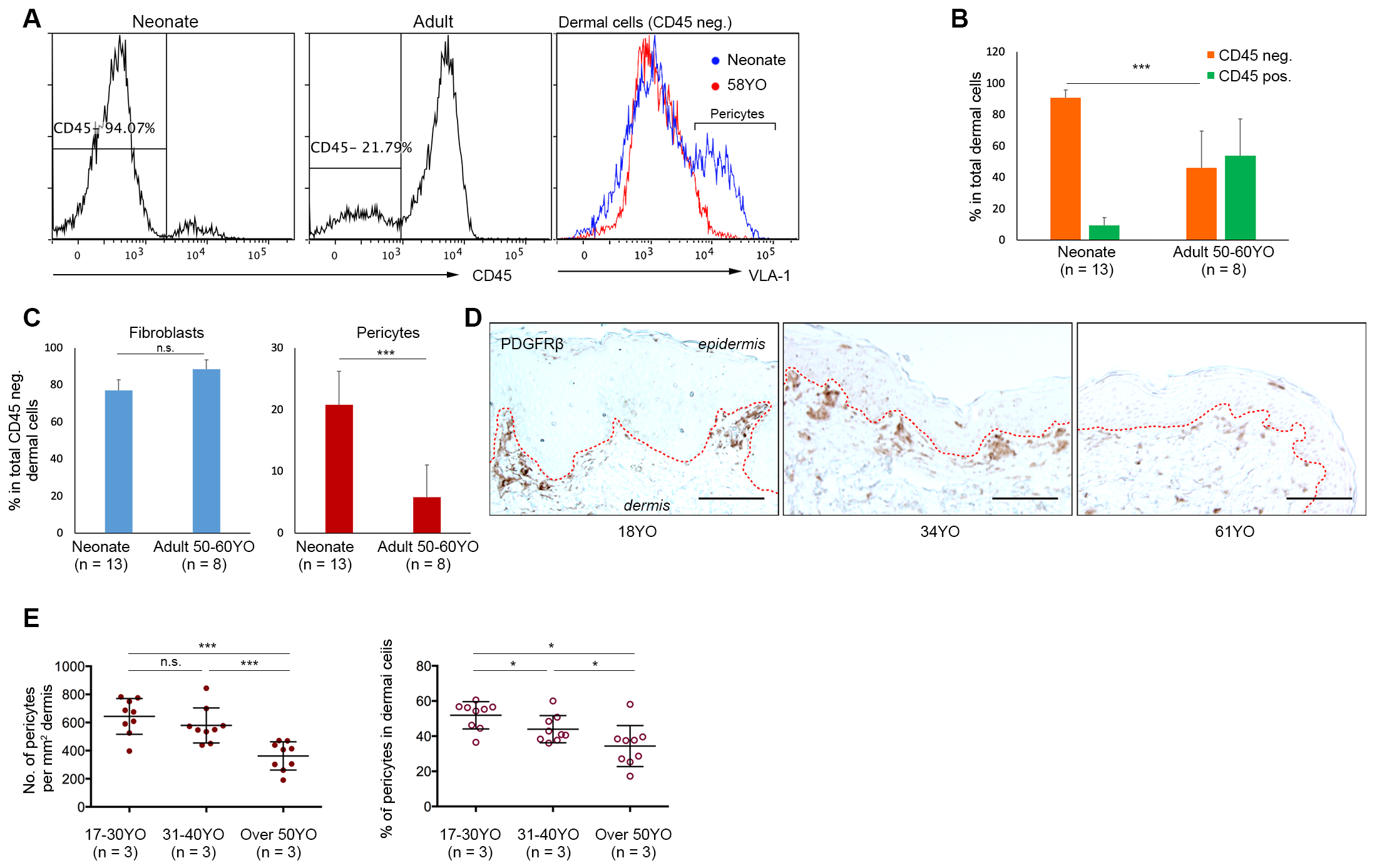
3.1. Aged Human Skin Exhibits a Decline in Pericyte Incidence
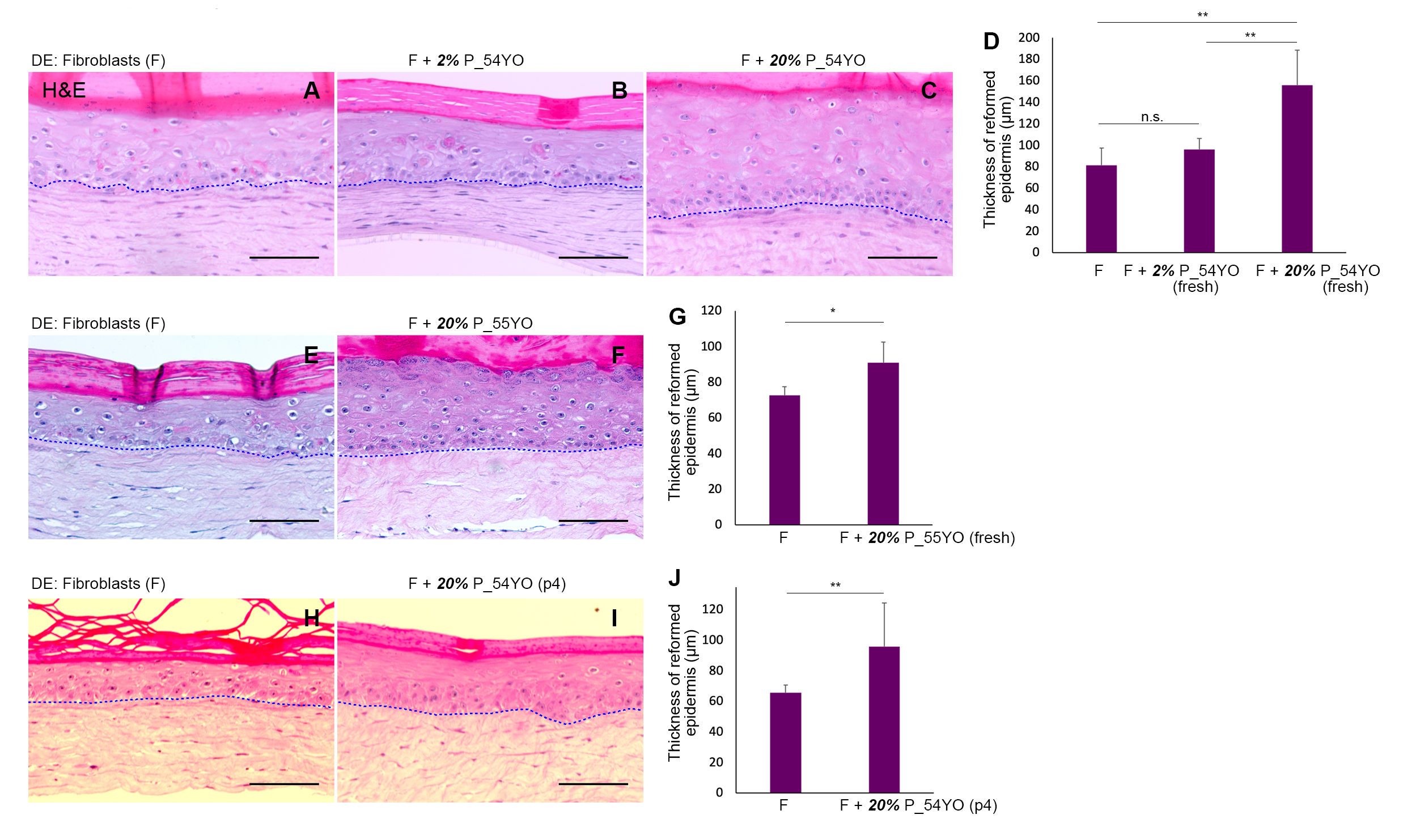
3.2. The Functional Capacity of Aged Pericytes to Promote Skin-Regeneration in Co-Operation with Neonatal Fibroblasts Is Affected by Their Lower Incidence
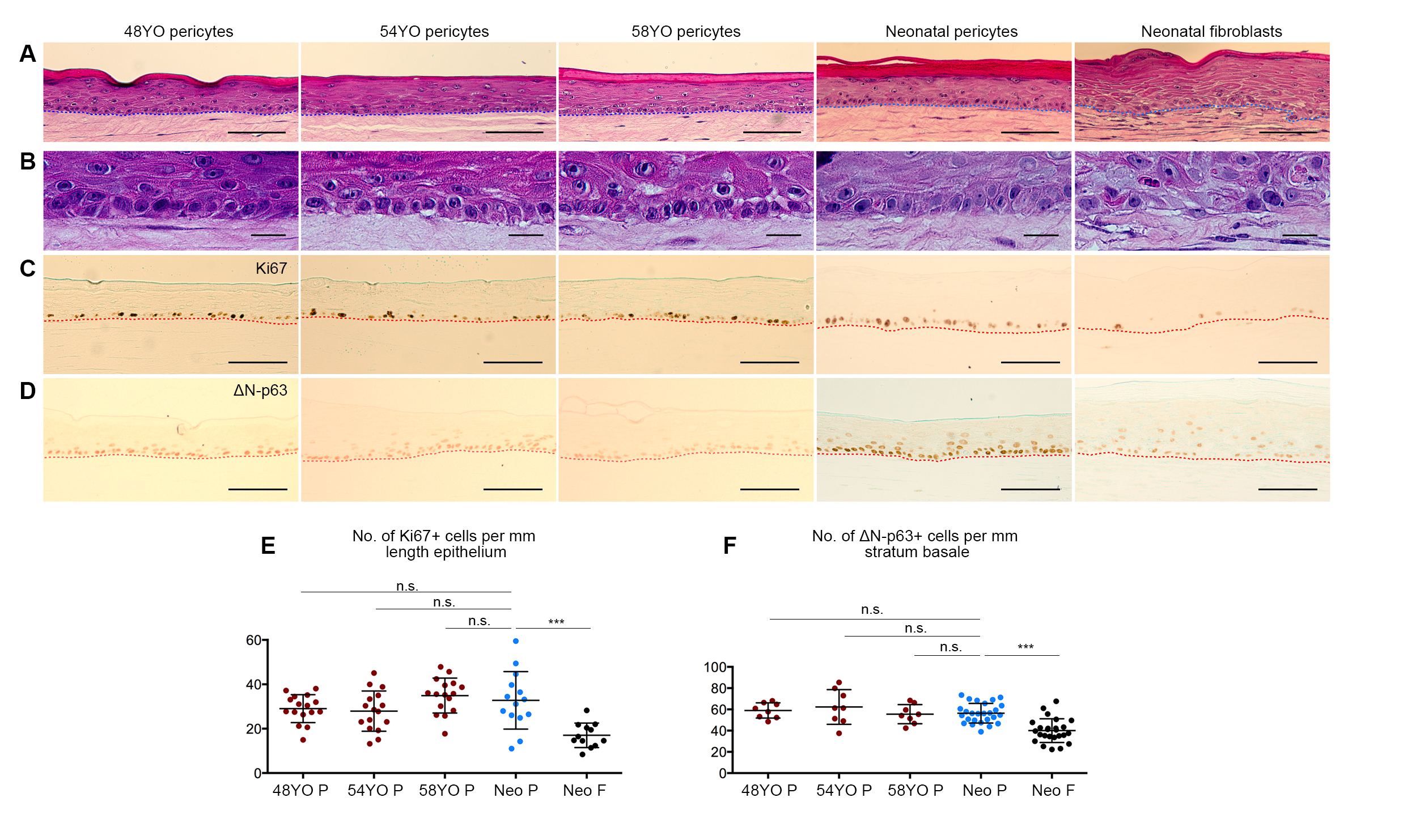
3.3. Pericytes from Ageing Human Skin Retain the Ability to Promote Epidermal Regeneration as the Sole Mesenchymal Element in OCs
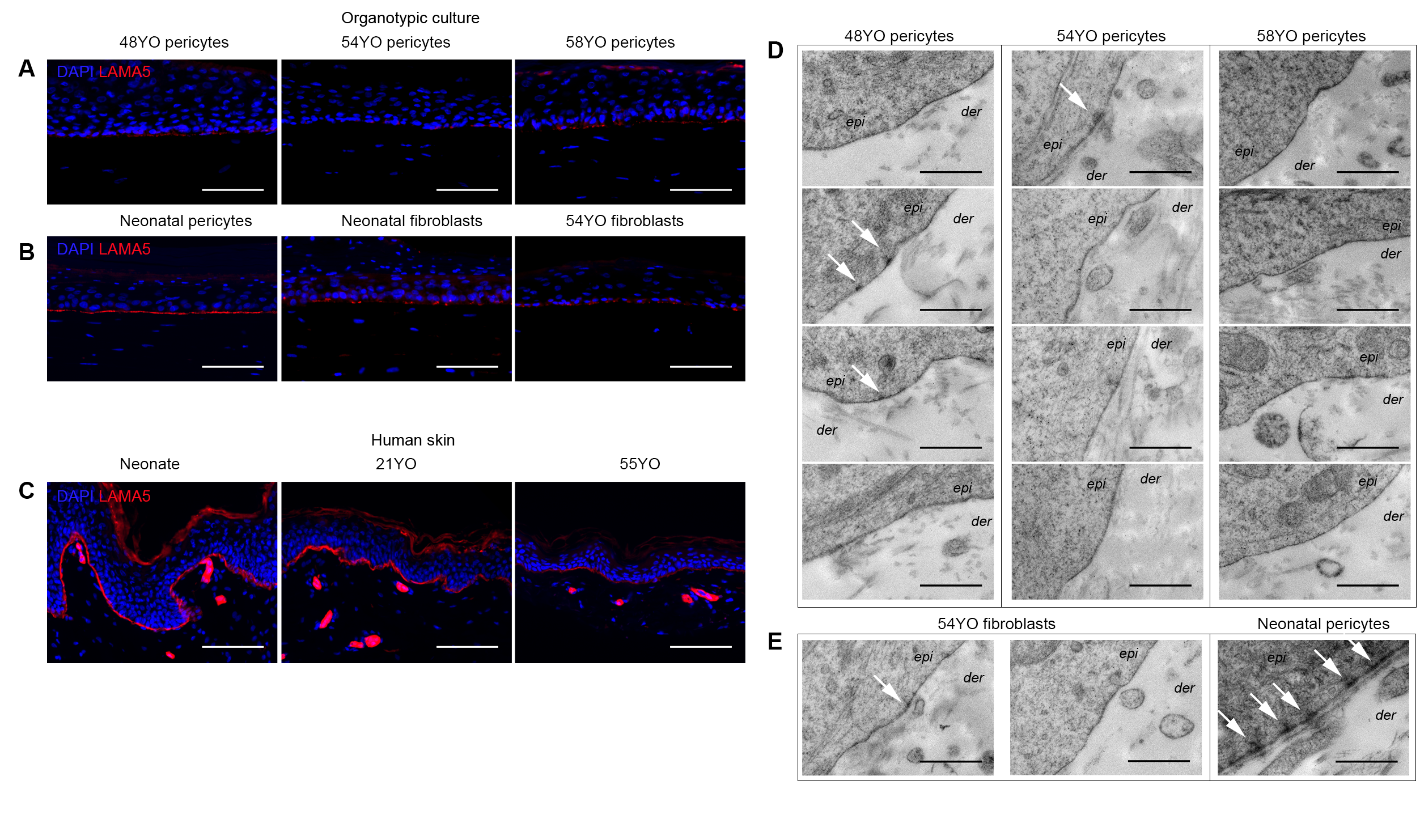
3.4. Aged Pericytes Do Not Restore Epidermal Cell Homeostasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gonzales, K.A.U.; Fuchs, E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev. Cell 2017, 43, 387–401. [Google Scholar] [PubMed] [Green Version]
- Miroshnikova, Y.A.; Le, H.Q.; Schneider, D.; Thalheim, T.; Rübsam, M.; Bremicker, N.; Polleux, J.; Kamprad, N.; Tarantola, M.; Wang, I.; et al. Adhesion forces and cortical tension couple cell proliferation and differentiation to drive epidermal stratification. Nat. Cell Biol. 2018, 20, 69–80. [Google Scholar] [PubMed]
- Mesa, K.R.; Kawaguchi, K.; Cockburn, K.; Gonzalez, D.; Boucher, J.; Xin, T.; Klein, A.M.; Greco, V. Homeostatic Epidermal Stem Cell Self-Renewal Is Driven by Local Differentiation. Cell Stem Cell 2018, 23, 677–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smola, H.; Thiekotter, G.; Fusenig, N.E. Mutual induction of growth factor gene expression by epidermal-dermal cell interaction. J. Cell Biol. 1993, 122, 417–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maas-Szabowski, N.; Stark, H.J.; Fusenig, N.E. Keratinocyte growth regulation in defined organotypic cultures through IL-1-induced keratinocyte growth factor expression in resting fibroblasts. J. Investig. Dermatol. 2000, 114, 1075–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabowski, A.; Maas-Szabowski, N.; Andrecht, S.; Kolbus, A.; Schorpp-Kistner, M.; Fusenig, N.E.; Angel, P. c-Jun and JunB antagonistically control cytokine-regulated mesenchymal-epidermal interaction in skin. Cell 2000, 103, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Miki, T.; Fleming, T.P.; Bottaro, D.P.; Rubin, J.S.; Ron, D.; Aaronson, S.A. Expression cDNA cloning of the KGF receptor by creation of a transforming autocrine loop. Science 1991, 251, 72–75. [Google Scholar]
- Aaronson, S.A.; Rubin, J.S.; Finch, P.W.; Wong, J.; Marchese, C.; Falco, J.; Taylor, W.G.; Kraus, M.H. Growth factor-regulated pathways in epithelial cell proliferation. Am. Rev. Respir. Dis. 1990, 142, S7–S10. [Google Scholar] [CrossRef]
- Marchese, C.; Felici, A.; Visco, V.; Lucania, G.; Igarashi, M.; Picardo, M.; Frati, L.; Torrisi, M.R. Fibroblast growth factor 10 induces proliferation and differentiation of human primary cultured keratinocytes. J. Investig. Dermatol. 2001, 116, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.H.; Leimeister, C.; Gessler, M.; Kopan, R. Activation of the Notch pathway in the hair cortex leads to aberrant differentiation of the adjacent hair-shaft layers. Development 2000, 127, 2421–2432. [Google Scholar] [CrossRef]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. Beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar]
- Sheng, H.; Goich, S.; Wang, A.; Grachtchouk, M.; Lowe, L.; Mo, R.; Lin, K.; de Sauvage, F.J.; Sasaki, H.; Hui, C.; et al. Dissecting the oncogenic potential of Gli2: Deletion of an NH(2)-terminal fragment alters skin tumor phenotype. Cancer Res. 2002, 62, 5308–5316. [Google Scholar] [PubMed]
- Mill, P.; Mo, R.; Fu, H.; Grachtchouk, M.; Kim, P.C.; Dlugosz, A.A.; Hui, C.-C. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 2003, 17, 282–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, L.K.; Kaur, P. The Ageing Epidermal Skin Niche Chapter 4. In Advances in Stem Cells and their Niches; Nilssoned, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 4. [Google Scholar]
- Rheinwald, J.G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef]
- Ghalbzouri, A.E.; Lamme, E.; Ponec, M. Crucial role of fibroblasts in regulating epidermal morphogenesis. Cell Tissue Res. 2002, 310, 189–199. [Google Scholar]
- Salzer, M.C.; Lafzi, A.; Berenguer-Llergo, A.; Youssif, C.; Castellanos, A.; Solanas, G.; Peixoto, F.O.; Attolini, C.S.-O.; Prats, N.; Aguilera, M.; et al. Identity Noise and Adipogenic Traits Characterize Dermal Fibroblast Aging. Cell 2018, 175, 1575–1590. [Google Scholar] [CrossRef] [Green Version]
- Sorrell, J.M.; Caplan, A.I. Fibroblast heterogeneity: More than skin deep. J. Cell Sci. 2004, 117, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, R.A.; Grove, G. Human skin fibroblasts derived from papillary and reticular dermis: Differences in growth potential in vitro. Science 1979, 204, 526–527. [Google Scholar] [CrossRef] [PubMed]
- Mine, S.; Fortunel, N.O.; Pageon, H.; Asselineau, D. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: A new view of skin morphogenesis and aging. PLoS ONE 2008, 3, e4066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schonherr, E.; Beavan, L.A.; Hausser, H.; Kresse, H.; Culp, L.A. Differences in decorin expression by papillary and reticular fibroblasts in vivo and In Vitro. Biochem. J. 1993, 290, 893–899. [Google Scholar]
- Jiang, D.; Rinkevich, Y. Defining Skin Fibroblastic Cell Types Beyond CD90. Front. Cell Dev. Biol. 2018, 6, 133. [Google Scholar] [CrossRef] [Green Version]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [PubMed] [Green Version]
- Lynch, M.D.; Watt, F.M. Fibroblast heterogeneity: Implications for human disease. J. Clin. Investig. 2018, 128, 26–35. [Google Scholar] [PubMed] [Green Version]
- Dulauroy, S.; Dulauroy, S.; Di Carlo, S.E.; Langa, F.; Eberl, G.; Peduto, L. Lineage tracing and genetic ablation of ADAM12 (+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 2012, 18, 1262–1270. [Google Scholar] [CrossRef]
- Paquet-Fifield, S.; Schlüter, H.; Li, A.; Aitken, T.; Gangatirkar, P.; Blashki, D.; Koelmeyer, R.; Pouliot, N.; Palatsides, M.; Ellis, S.; et al. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J. Clin. Investig. 2009, 119, 2795–2806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, L.; Lawlor, K.T.; Schlueter, H.; Pieterse, Z.; Yu, Y.; Kaur, P. Pericytes promote skin regeneration by inducing epidermal cell polarity and planar cell divisions. Life Sci. Alliance 2018, 1, e201700009. [Google Scholar]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncol. 2005, 7, 452–464. [Google Scholar]
- Esteves, C.L.; Donadeu, F.X. Pericytes and their potential in regenerative medicine across species. Cytom. A 2018, 93, 50–59. [Google Scholar]
- Gangatirkar, P.; Paquet-Fifield, S.; Li, A.; Rossi, R.; Kaur, P. Establishment of 3D organotypic cultures using human neonatal epidermal cells. Nat. Protoc. 2007, 2, 178–186. [Google Scholar]
- Li, A.; Pouliot, N.; Redvers, R.; Kaur, P. Extensive tissue-regenerative capacity of neonatal human keratinocyte stem cells and their progeny. J. Clin. Investig. 2004, 113, 390–400. [Google Scholar] [PubMed] [Green Version]
- Helmbold, P.; Lautenschläger, C.; WCh, M.; Nayak, R.C. Detection of a physiological juvenile phase and the central role of pericytes in human dermal microvascular aging. J. Investig. Dermatol. 2006, 126, 1419–1421. [Google Scholar] [CrossRef] [Green Version]
- Grove, G.L.; Kligman, A.M. Age-associated changes in human epidermal cell renewal. J. Gerontol. 1983, 38, 137–142. [Google Scholar]
- Gilchrest, B.A. In Vitro assessment of keratinocyte aging. J. Investig. Dermatol. 1983, 81, S184–S189. [Google Scholar] [CrossRef] [Green Version]
- Gunin, A.G.; Kornilova, N.K.; Vasilieva, O.V.; Petrov, V.V. Age-Related Changes in Proliferation, the Numbers of Mast Cells, Eosinophils, and cd45-Positive Cells in Human Dermis. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 385–392. [Google Scholar]
- Petrov, V.V.; Vasilyeva, O.V.; Kornilova, N.K.; Gunin, A.G. Age-related changes in mast cells and eosinophils of human dermis. Russ. J. Dev. Biol. 2013, 44, 139–143. [Google Scholar] [CrossRef]
- Kurban, R.S.; Bhawan, J. Histologic changes in skin associated with aging. J. Dermatol. Surg. Oncol. 1990, 16, 908–914. [Google Scholar] [PubMed]
- Hayflick, L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Mills, A.A.; Zheng, B.; Wang, X.-J.; Vogel, O.H.; Roop, D.R.; Bradley, A. P63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999, 398, 708–713. [Google Scholar] [CrossRef]
- Yang, A.; Schweitzer, R.; Sun, D.; Kaghad, M.; Walker, N.; Bronson, R.T.; Tabin, C.; Sharpe, A.; Caput, D.; Crum, C.; et al. P63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999, 398, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Lechler, T.; Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 2005, 437, 275–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, L.; Visalakshan, R.M.; Kaur, P. Dermal Pericytes Exhibit Declined Ability to Promote Human Skin Regeneration with Ageing in 3D Organotypic Culture Models. Cells 2021, 10, 3051. https://doi.org/10.3390/cells10113051
Zhuang L, Visalakshan RM, Kaur P. Dermal Pericytes Exhibit Declined Ability to Promote Human Skin Regeneration with Ageing in 3D Organotypic Culture Models. Cells. 2021; 10(11):3051. https://doi.org/10.3390/cells10113051
Chicago/Turabian StyleZhuang, Lizhe, Rahul M. Visalakshan, and Pritinder Kaur. 2021. "Dermal Pericytes Exhibit Declined Ability to Promote Human Skin Regeneration with Ageing in 3D Organotypic Culture Models" Cells 10, no. 11: 3051. https://doi.org/10.3390/cells10113051
APA StyleZhuang, L., Visalakshan, R. M., & Kaur, P. (2021). Dermal Pericytes Exhibit Declined Ability to Promote Human Skin Regeneration with Ageing in 3D Organotypic Culture Models. Cells, 10(11), 3051. https://doi.org/10.3390/cells10113051





