Plasma Membrane Fluidity: An Environment Thermal Detector in Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Membrane Isolation
2.3. Measurement of Membrane Fluidity
2.4. Electron Microscopy
2.5. Protein Determination
2.6. Immunoblotting
2.7. ATP Hydrolysis Assays
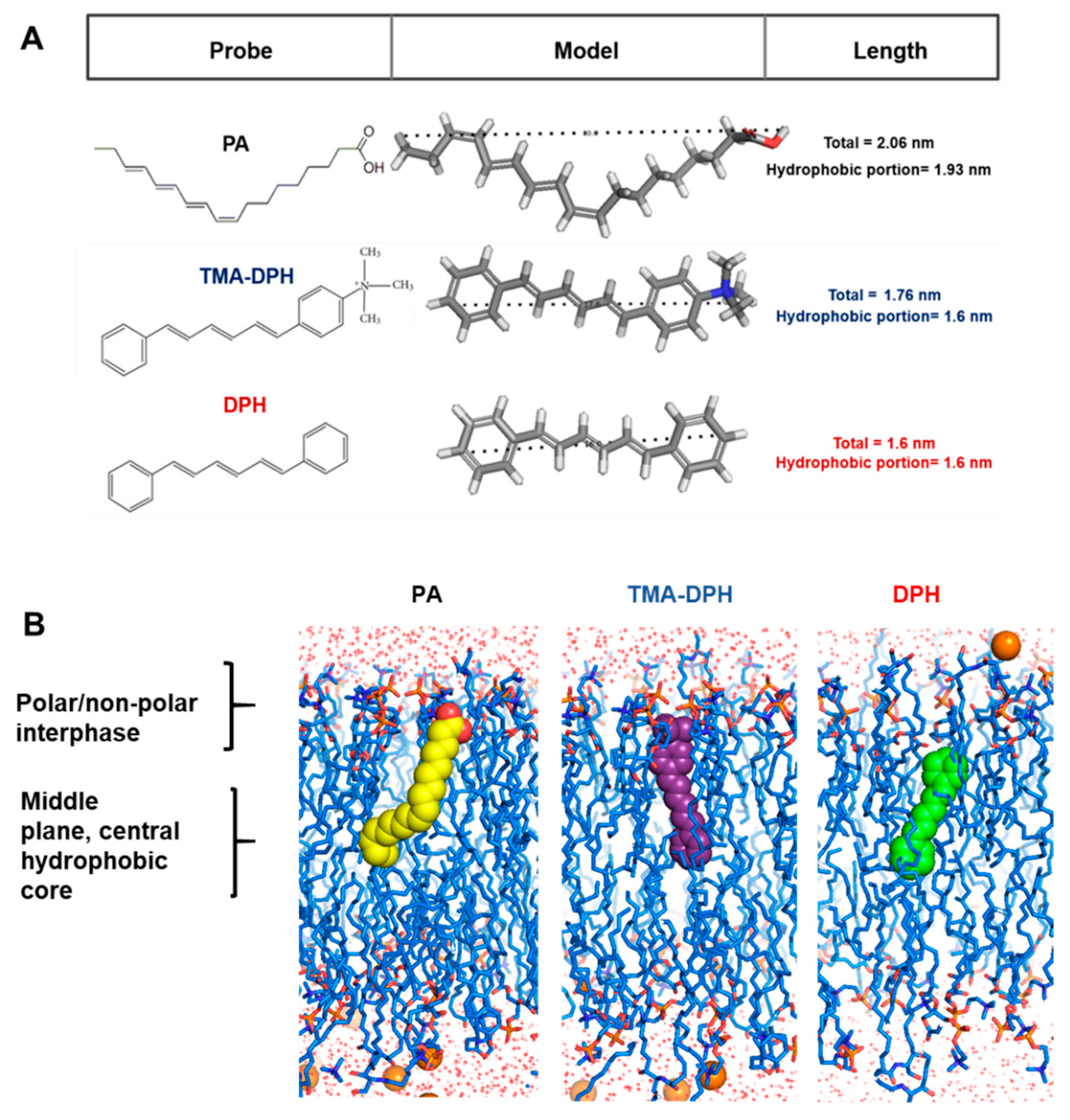
2.8. Membrane and Probe Models
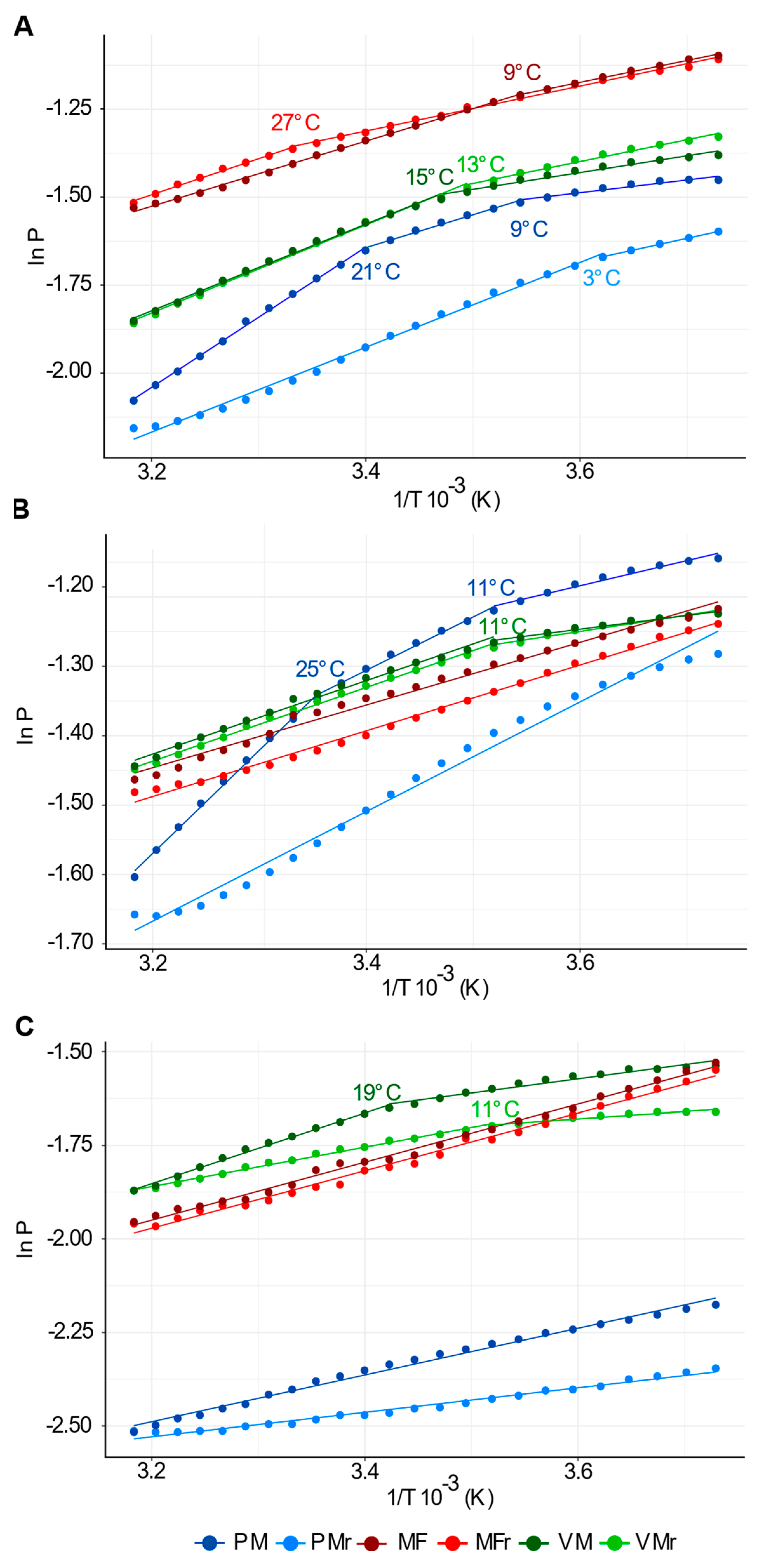
2.9. Segment Analysis of Arrhenius Plots
2.10. Statistics
3. Results
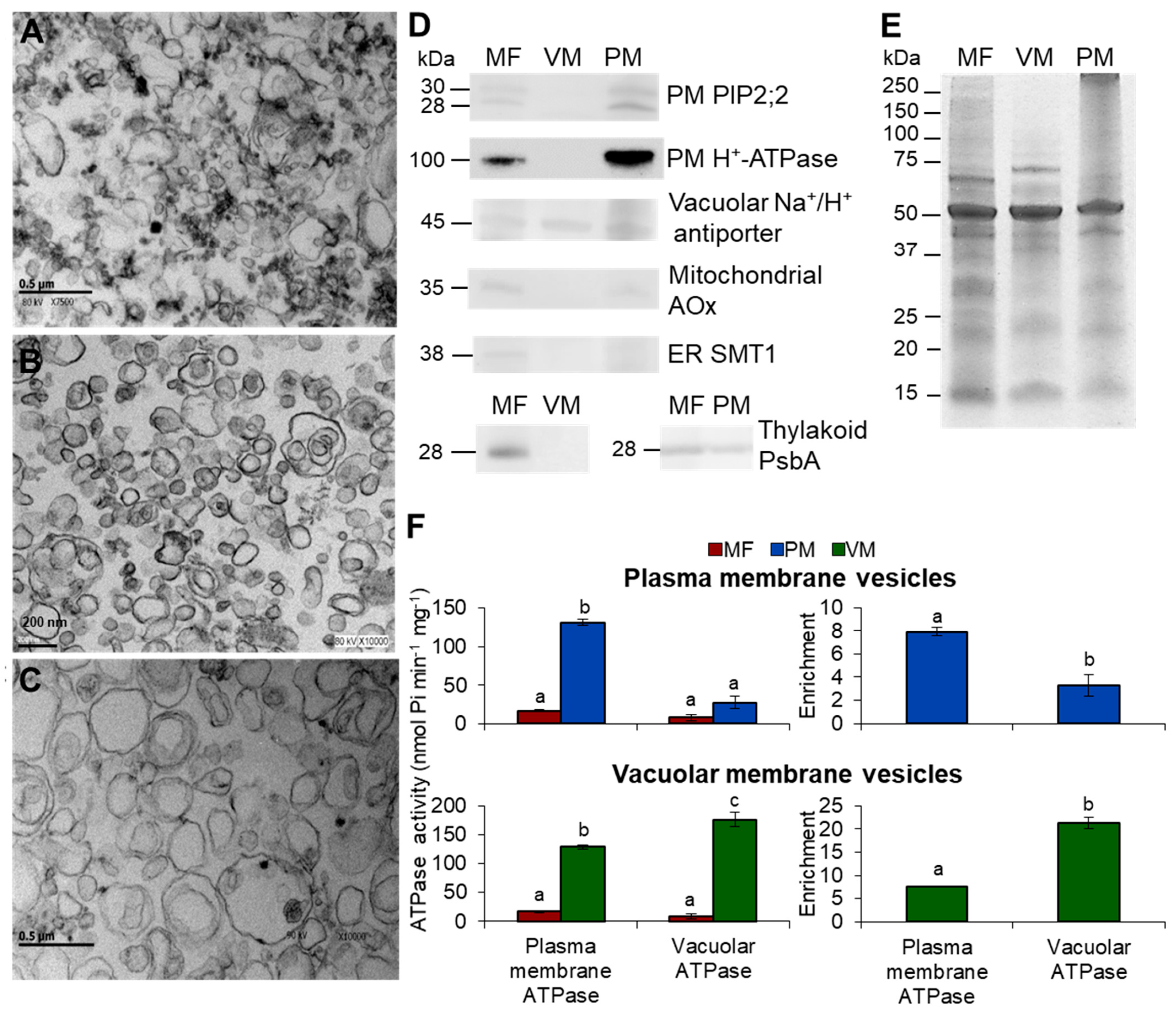
3.1. Purity Assessment of Total, Vacuolar, and Plasma Membrane Vesicles Isolated from Arabidopsis Thaliana
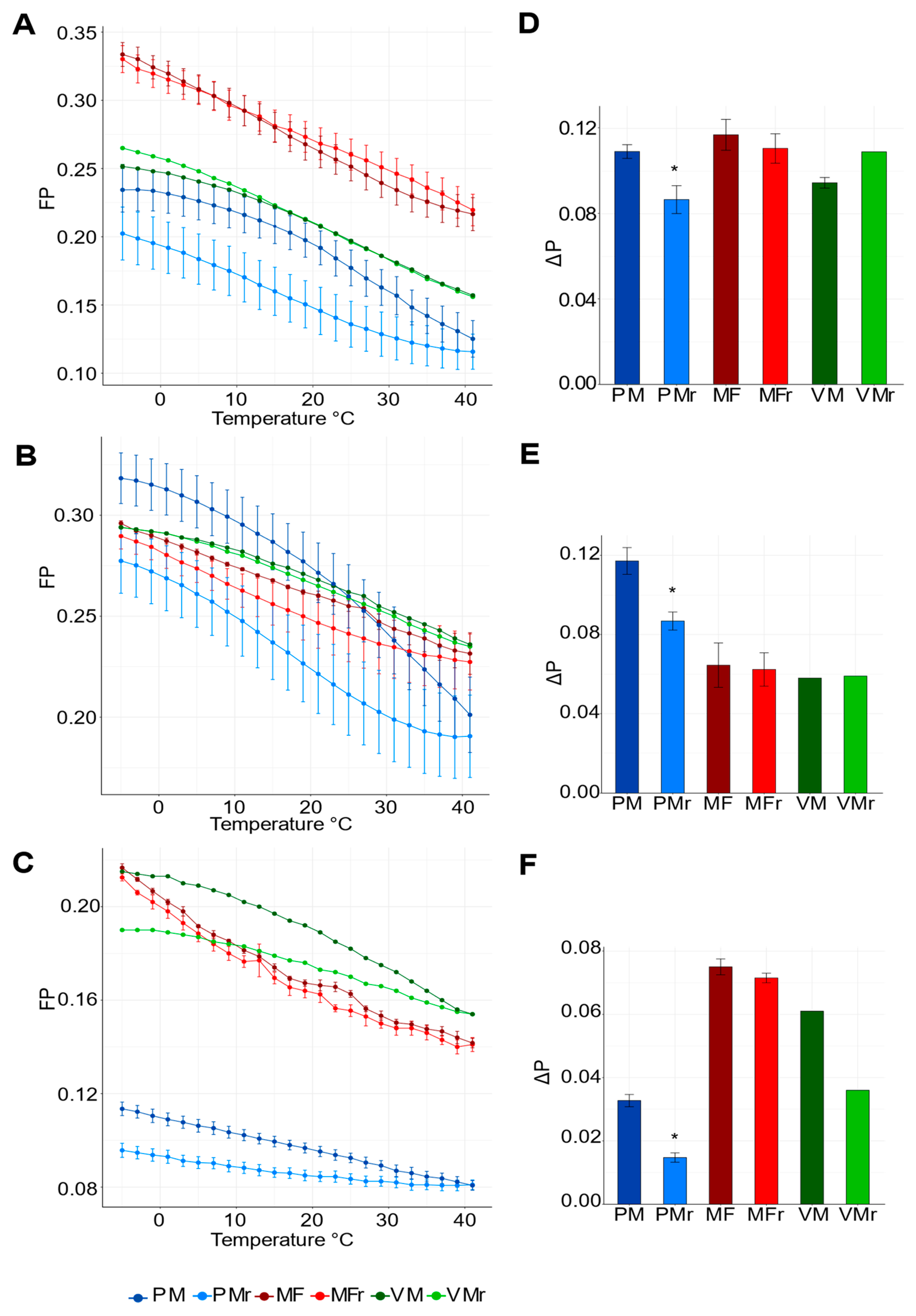
3.2. PM, but Not VM or MF, Show the Highest Fluidity Values and Responds to External Temperature Variations
4. Discussion
4.1. PM Exhibits High Fluidity, Phase Transitions, and Irreversible Behavior during a Heating/Cooling Cycle
4.2. Identification of a Temperature Sensor in Plants
4.3. The Support for the Hypothesis of the PM Fluidity as a Temperature Detector
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cullis, P.; Kruijff, B. Lipid Polymorphism and the Functional Roles of Lipids in Biological Membranes. Biochim. Biophys. Acta 1979, 559, 399–420. [Google Scholar] [CrossRef]
- Frolov, V.A.; Shnyrova, A.V.; Zimmerberg, J. Lipid Polymorphisms and Membrane Shape. Cold Spring Harb. Perspect. Biol. 2011, 3, a004747. [Google Scholar] [CrossRef] [Green Version]
- Garidel, P.; Kaconis, Y.; Heinbockel, L.; Wulf, M.; Gerber, S.; Munk, A.; Vill, V.; Brandenburg, K. Self-Organisation, Thermotropic and Lyotropic Properties of Glycolipids Related to Their Biological Implications. Open Biochem. J. 2015, 9, 49–72. [Google Scholar] [CrossRef]
- Uemura, M.; Steponkus, P.L. A Contrast of the Plasma Membrane Lipid Composition of Oat and Rye Leaves in Relation to Freezing Tolerance. Plant Physiol. 1994, 104, 479–496. [Google Scholar] [CrossRef] [Green Version]
- Los, D.A.; Murata, N. Membrane Fluidity and Its Roles in the Perception of Environmental Signals. Biochim. Biophys. Acta BBA—Biomembr. 2004, 1666, 142–157. [Google Scholar] [CrossRef] [Green Version]
- Contreras, F.-X.; Ernst, A.M.; Wieland, F.; Brügger, B. Specificity of Intramembrane Protein–Lipid Interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004705. [Google Scholar] [CrossRef] [Green Version]
- Iba, K. Acclimative Response to Temperature Stress in Higher Plants: Approaches of Gene Engineering for Temperature Tolerance. Annu. Rev. Plant Biol. 2002, 53, 225–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Los, D.A.; Mironov, K.S.; Allakhverdiev, S.I. Regulatory Role of Membrane Fluidity in Gene Expression and Physiological Functions. Photosynth. Res. 2013, 116, 489–509. [Google Scholar] [CrossRef]
- Ernst, R.; Ejsing, C.S.; Antonny, B. Homeoviscous Adaptation and the Regulation of Membrane Lipids. J. Mol. Biol. 2016, 428, 4776–4791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidi, Y.; Finka, A.; Muriset, M.; Bromberg, Z.; Weiss, Y.G.; Maathuis, F.J.M.; Goloubinoff, P. The Heat Shock Response in Moss Plants Is Regulated by Specific Calcium-Permeable Channels in the Plasma Membrane. Plant Cell 2009, 21, 2829–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, S.; Schachtschabel, J.; Mishkind, M.; Munnik, T.; Arisz, S.A. Hot Topic: Thermosensing in Plants. Plant Cell Environ. 2020, 44, 2018–2033. [Google Scholar] [CrossRef]
- Sung, D.Y.; Kaplan, F.; Lee, K.J.; Guy, C.L. Acquired Tolerance to Temperature Extremes. Trends Plant Sci. 2003, 8, 179–187. [Google Scholar] [CrossRef]
- Martinière, A.; Shvedunova, M.; Thomson, A.J.W.; Evans, N.H.; Penfield, S.; Runions, J.; McWatters, H.G. Homeostasis of Plasma Membrane Viscosity in Fluctuating Temperatures. New Phytol. 2011, 192, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.V.; Steponkus, P.L. Plasma Membrane Lipid Alterations Associated with Cold Acclimation of Winter Rye Seedlings (Secale cereale L. Cv Puma). Plant Physiol. 1987, 83, 761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miquel, M.; James, D.; Dooner, H.; Browse, J. Arabidopsis Requires Polyunsaturated Lipids for Low-Temperature Survival. Proc. Natl. Acad. Sci. USA 1993, 90, 6208–6212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.-E.; Rajashekar, C.B.; Williams, T.D.; Wang, X. Profiling Membrane Lipids in Plant Stress Responses. Role of Phospholipase D Alpha in Freezing-Induced Lipid Changes in Arabidopsis. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcone, D.L.; Ogas, J.P.; Somerville, C.R. Regulation of Membrane Fatty Acid Composition by Temperature in Mutants of Arabidopsis with Alterations in Membrane Lipid Composition. BMC Plant Biol. 2004, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Minami, A.; Fujiwara, M.; Furuto, A.; Fukao, Y.; Yamashita, T.; Kamo, M.; Kawamura, Y.; Uemura, M. Alterations in Detergent-Resistant Plasma Membrane Microdomains in Arabidopsis thaliana during Cold Acclimation. Plant Cell Physiol. 2009, 50, 341–359. [Google Scholar] [CrossRef] [Green Version]
- Uemura, M.; Joseph, R.A.; Steponkus, P.L. Cold Acclimation of Arabidopsis thaliana (Effect on Plasma Membrane Lipid Composition and Freeze-Induced Lesions). Plant Physiol. 1995, 109, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Murakami, Y.; Tsuyama, M.; Kobayashi, Y.; Kodama, H.; Iba, K. Trienoic Fatty Acids and Plant Tolerance of High Temperature. Science 2000, 287, 476–479. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, Q.; Shen, W.; Cram, D.; Fowler, D.B.; Wei, Y.; Zou, J. Understanding the Biochemical Basis of Temperature-Induced Lipid Pathway Adjustments in Plants. Plant Cell 2015, 27, 86–103. [Google Scholar] [CrossRef] [Green Version]
- Carmona-Salazar, L.; Hafidi, M.E.; Enríquez-Arredondo, C.; Vázquez-Vázquez, C.; Vara, L.E.G.D.L.; Gavilanes-Ruíz, M. Isolation of Detergent-Resistant Membranes from Plant Photosynthetic and Non-Photosynthetic Tissues. Anal. Biochem. 2011, 417, 220–227. [Google Scholar] [CrossRef]
- Barkla, B.J.; Vera-Estrella, R.; Pantoja, O. Enhanced Separation of Membranes during Free Flow Zonal Electrophoresis in Plants. Anal. Chem. 2007, 79, 5181–5187. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.L. A Simplification of the Protein Assay Method of Lowry et al. Which Is More Generally Applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Tricine–sodium Dodecyl Sulfate–polyacrylamide Gel Electrophoresis for the Separation of Proteins in the Range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L.; Mackerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The Biomolecular Simulation Program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Zoete, V.; Cuendet, M.A.; Grosdidier, A.; Michielin, O. SwissParam: A Fast Force Field Generation Tool for Small Organic Molecules. J. Comput. Chem. 2011, 32, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Shinitzky, M.; Barenholz, Y. Fluidity Parameters of Lipid Regions Determined by Fluorescence Polarization. Biochim. Biophys. Acta BBA—Rev. Biomembr. 1978, 515, 367–394. [Google Scholar] [CrossRef]
- Sklar, L.A.; Miljanich, G.P.; Dratz, E.A. Phospholipid Lateral Phase Separation and the Partition of Cis-Parinaric Acid and Trans-Parinaric Acid among Aqueous, Solid Lipid, and Fluid Lipid Phases. Biochemistry 2002, 18, 1707–1716. [Google Scholar] [CrossRef]
- Lentz, B.R. Use of Fluorescent Probes to Monitor Molecular Order and Motions within Liposome Bilayers. Chem. Phys. Lipids 1993, 64, 99–116. [Google Scholar] [CrossRef]
- do Canto, A.M.T.M.; Robalo, J.R.; Santos, P.D.; Carvalho, A.J.P.; Ramalho, J.P.P.; Loura, L.M.S. Diphenylhexatriene Membrane Probes DPH and TMA-DPH: A Comparative Molecular Dynamics Simulation Study. Biochim. Biophys. Acta BBA—Biomembr. 2016, 1858, 2647–2661. [Google Scholar] [CrossRef]
- Schroeder, F.; Soler-Argilaga, C. Calcium Modulates Fatty Acid Dynamics in Rat Liver Plasma Membranes. Eur. J. Biochem. 1983, 132, 517–524. [Google Scholar] [CrossRef]
- Graham, D.; Patterson, B.D. Responses of Plants to Low, Nonfreezing Temperatures: Proteins, Metabolism, and Acclimation. Ann. Rev. Plant Physiol. 1982, 33, 347–372. [Google Scholar] [CrossRef]
- Yoshida, S. Studies on Freezing Injury of Plant Cells: I. Relation Between Thermotropic Properties of Isolated Plasma Membrane Vesicles and Freezing Injury. Plant Physiol. 1984, 75, 38–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behzadipour, M.; Ratajczak, R.; Faist, K.; Pawlitschek, P.; Trémolières, A.; Kluge, M. Phenotypic Adaptation of Tonoplast Fluidity to Growth Temperature in the CAM Plant Kalanchoë Daigremontiana Ham. et Per. Is Accompanied by Changes in the Membrane Phospholipid and Protein Composition. J. Membr. Biol. 1998, 166, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.; Ford, R.C.; Mitchell, R.A.C.; Millner, P.A. Chloroplast Thylakoid Membrane Fluidity and Its Sensitivity to Temperature. Planta 1984, 161, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Bohn, M.; Heinz, E.; Lüthje, S. Lipid Composition and Fluidity of Plasma Membranes Isolated from Corn (Zea mays L.) Roots. Arch. Biochem. Biophys. 2001, 387, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Yoshida, S. Involvement of Plasma Membrane Alterations in Cold Acclimation of Winter Rye Seedlings (Secale cereale L. Cv Puma). Plant Physiol. 1984, 75, 818–826. [Google Scholar] [CrossRef] [Green Version]
- Hellgren, L.I.; Selldén, G.; Sandelius, A.S. Effects of Moderately Enhanced Levels of Ozone on the Acyl Lipid Composition and Dynamical Properties of Plasma Membranes Isolated From Garden Pea (Pisum sativum). Physiol. Plant. 2011, 11, 165–171. [Google Scholar] [CrossRef]
- Hellgren, L.I.; Sandelius, A.S. The Impact of Different Phytosterols on the Molecular Dynamics in the Hydrophobic/Hydrophilic Interface Phosphatidylcholine- Liposomes. Physiol. Plant. 2001, 113, 23–32. [Google Scholar] [CrossRef]
- Roche, Y.; Gerbeau-Pissot, P.; Buhot, B.; Thomas, D.; Bonneau, L.; Gresti, J.; Mongrand, S.; Perrier-Cornet, J.-M.; Simon-Plas, F. Depletion of Phytosterols from the Plant Plasma Membrane Provides Evidence for Disruption of Lipid Rafts. FASEB J. 2008, 22, 3980–3991. [Google Scholar] [CrossRef] [Green Version]
- Sha, W.; Moore, J.; Chen, K.; Lassaletta, A.D.; Yi, C.-S.; Tyson, J.J.; Sible, J.C. Hysteresis Drives Cell-Cycle Transitions in Xenopus Laevis Egg Extracts. Proc. Natl. Acad. Sci. USA 2003, 100, 975–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holopainen, J.M.; Lemmich, J.; Richter, F.; Mouritsen, O.G.; Rapp, G.; Kinnunen, P.K.J. Dimyristoylphosphatidylcholine/C16:0-Ceramide Binary Liposomes Studied by Differential Scanning Calorimetry and Wide-and Small-Angle x-Ray Scattering. Biophys. J. 2000, 78, 2459–2469. [Google Scholar] [CrossRef] [Green Version]
- Urrutia, M.E.; Duman, J.G.; Knight, C.A. Plant Thermal Hysteresis Proteins. Biochim. Biophys. Acta BBA—Protein Struct. Mol. Enzymol. 1992, 1121, 199–206. [Google Scholar] [CrossRef]
- Sengupta, P.; Garrity, P. Sensing Temperature. Curr. Biol. 2013, 23, R304–R307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saita, E.; Abriata, L.A.; Tsai, Y.T.; Trajtenberg, F.; Lemmin, T.; Buschiazzo, A.; Peraro, M.D.; de Mendoza, D.; Albanesi, D. A Coiled Coil Switch Mediates Cold Sensing by the Thermosensory Protein DesK. Mol. Microbiol. 2015, 98, 258–271. [Google Scholar] [CrossRef]
- Horváth, I.; Glatz, A.; Varvasovszki, V.; Török, Z.; Páli, T.; Balogh, G.; Kovács, E.; Nádasdi, L.; Benkö, S.; Joó, F.; et al. Membrane Physical State Controls the Signaling Mechanism of the Heat Shock Response in Synechocystis PCC 6803: Identification of Hsp17 as a “Fluidity Gene”. Proc. Natl. Acad. Sci. USA 1998, 95, 3513–3518. [Google Scholar] [CrossRef] [Green Version]
- Mironov, K.S.; Sidorov, R.A.; Trofimova, M.S.; Bedbenov, V.S.; Tsydendambaev, V.D.; Allakhverdiev, S.I.; Los, D.A. Light-Dependent Cold-Induced Fatty Acid Unsaturation, Changes in Membrane Fluidity, and Alterations in Gene Expression in Synechocystis. Biochim. Biophys. Acta 2012, 1817, 1352–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruelland, E.; Zachowski, A. How Plants Sense Temperature. Environ. Exp. Bot. 2010, 69, 225–232. [Google Scholar] [CrossRef]
- Knight, M.R.; Knight, H. Low-Temperature Perception Leading to Gene Expression and Cold Tolerance in Higher Plants. New Phytol. 2012, 195, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Knight, H.; Trewavas, A.J.; Knight, M.R. Cold Calcium Signaling in Arabidopsis Involves Two Cellular Pools and a Change in Calcium Signature after Acclimation. Plant Cell 1996, 8, 489–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Örvar, B.L.; Sangwan, V.; Omann, F.; Dhindsa, R.S. Early Steps in Cold Sensing by Plant Cells: The Role of Actin Cytoskeleton and Membrane Fluidity. Plant J. 2000, 23, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Pokorná, J.; Schwarzerová, K.; Zelenková, S.; Petrášek, J.; Janotová, I.; Čapková, V.; Opatrný, Z. Sites of Actin Filament Initiation and Reorganization in Cold-Treated Tobacco Cells. Plant Cell Environ. 2004, 27, 641–653. [Google Scholar] [CrossRef]
- Vaultier, M.; Cantrel, C.; Vergnolle, C.; Justin, A.; Demandre, C.; Benhassaine-Kesri, G.; Ciçek, D.; Zachowski, A.; Ruelland, E. Desaturase Mutants Reveal That Membrane Rigidification Acts as a Cold Perception Mechanism Upstream of the Diacylglycerol Kinase Pathway in Arabidopsis Cells. FEBS Lett. 2006, 580, 4218–4223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saucedo-García, M.; Gavilanes-Ruíz, M.; Arce-Cervantes, O. Long-Chain Bases, Phosphatidic Acid, MAPKs, and Reactive Oxygen Species as Nodal Signal Transducers in Stress Responses in Arabidopsis. Front. Plant Sci. 2015, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Saidi, Y.; Peter, M.; Finka, A.; Cicekli, C.; Vigh, L.; Goloubinoff, P. Membrane Lipid Composition Affects Plant Heat Sensing and Modulates Ca2+-Dependent Heat Shock Response. Plant Signal. Behav. 2010, 5, 1530–1533. [Google Scholar] [CrossRef] [Green Version]
- Saidi, Y.; Finka, A.; Goloubinoff, P. Heat Perception and Signalling in Plants: A Tortuous Path to Thermotolerance. New Phytol. 2011, 190, 556–565. [Google Scholar] [CrossRef]
- Niu, Y.; Xiang, Y. An Overview of Biomembrane Functions in Plant Responses to High-Temperature Stress. Front. Plant Sci. 2018, 9, 915. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Xu, Y.; Zhu, Z. Emerging Plant Thermosensors: From RNA to Protein. Trends Plant Sci. 2020, 25, 1187–1189. [Google Scholar] [CrossRef]
- Legris, M.; Klose, C.; Burgie, E.S.; Rojas, C.C.; Neme, M.; Hiltbrunner, A.; Wigge, P.A.; Schäfer, E.; Vierstra, R.D.; Casal, J.J. Phytochrome B Integrates Light and Temperature Signals in Arabidopsis. Science 2016, 354, 897–900. [Google Scholar] [CrossRef] [Green Version]
- Biswal, B.; Joshi, P.N.; Raval, M.K.; Biswal, U.C. Photosynthesis, a Global Sensor of Environmental Stress in Green Plants: Stress Signalling and Adaptation. Curr. Sci. 2011, 101, 47–56. Available online: http://www.jstor.org/stable/24077862 (accessed on 9 October 2021).
- Mittler, R.; Finka, A.; Goloubinoff, P. How Do Plants Feel the Heat? Trends Biochem Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, P.J.; Kumar, M.; Martinho, C.; Yoo, S.J.; Lan, H.; Artavanis, G.; Charoensawan, V.; Schöttler, M.A.; Bock, R.; Jaeger, K.E.; et al. Chloroplast Signaling Gates Thermotolerance in Arabidopsis. Cell Rep. 2018, 22, 1657–1665. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and Responses of Chloroplasts to Heat Stress in Plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu, L.D.; Gevaert, K.; De Smet, I. Feeling the Heat: Searching for Plant Thermosensors. Trends Plant Sci. 2019, 24, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Lamers, J.; van der Meer, T.; Testerink, C. How Plants Sense and Respond to Stressful Environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Liu, B.; Qin, F. Modular Thermal Sensors in Temperature-Gated Transient Receptor Potential (TRP) Channels. Proc. Natl. Acad. Sci. USA 2011, 108, 11109–11114. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Ramirez, D.L.; Carmona-Salazar, L.; Morales-Cedillo, F.; Ramírez-Salcedo, J.; Cahoon, E.B.; Gavilanes-Ruíz, M. Plasma Membrane Fluidity: An Environment Thermal Detector in Plants. Cells 2021, 10, 2778. https://doi.org/10.3390/cells10102778
Cano-Ramirez DL, Carmona-Salazar L, Morales-Cedillo F, Ramírez-Salcedo J, Cahoon EB, Gavilanes-Ruíz M. Plasma Membrane Fluidity: An Environment Thermal Detector in Plants. Cells. 2021; 10(10):2778. https://doi.org/10.3390/cells10102778
Chicago/Turabian StyleCano-Ramirez, Dora L., Laura Carmona-Salazar, Francisco Morales-Cedillo, Jorge Ramírez-Salcedo, Edgar B. Cahoon, and Marina Gavilanes-Ruíz. 2021. "Plasma Membrane Fluidity: An Environment Thermal Detector in Plants" Cells 10, no. 10: 2778. https://doi.org/10.3390/cells10102778
APA StyleCano-Ramirez, D. L., Carmona-Salazar, L., Morales-Cedillo, F., Ramírez-Salcedo, J., Cahoon, E. B., & Gavilanes-Ruíz, M. (2021). Plasma Membrane Fluidity: An Environment Thermal Detector in Plants. Cells, 10(10), 2778. https://doi.org/10.3390/cells10102778





