Lipid Polymorphism of the Subchloroplast—Granum and Stroma Thylakoid Membrane–Particles. II. Structure and Functions
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Granum and Stroma Thylakoid Membranes
2.2. Lipase Treatments and Measurements at Different Temperatures
2.3. Circular Dichroism Spectroscopy
2.4. 77 K Chl a Fluorescence Spectroscopy
2.5. Fast Chl a Fluorescence Transients
2.6. Electrochromic Absorbance Transients, ΔA515
2.7. Data Analysis
2.8. SDS-PAGE and Western Blotting
2.9. VDE Protein Expression
2.10. Small-Angle X-ray Scattering (SAXS)
2.11. Freeze-Fracture Electron Microscopy (FF-EM)
2.12. Scanning Electron Microscopy (SEM)
2.13. Cryo-Electron Tomography (CET)
3. Results and Discussion
3.1. CD Spectroscopy
3.2. 77 K Chl a Fluorescence Spectroscopy
3.3. Fast Chl a Fluorescence Transients
3.4. Electrochromic Absorbance Transients, ΔA515
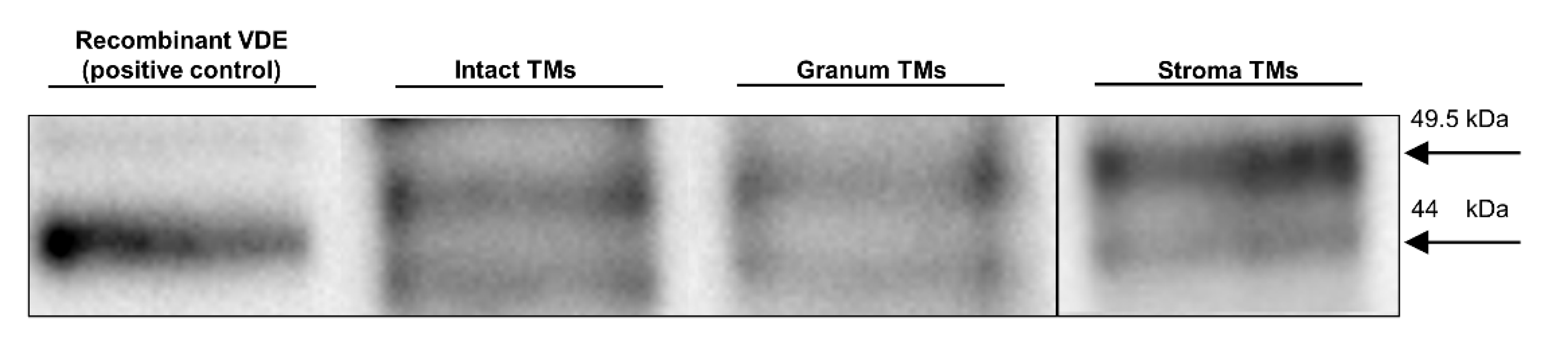
3.5. SDS-PAGE and Western Blotting
3.6. Small Angle X-ray Scattering (SAXS)
3.7. Freeze-Fracture Electron Microscopy (FF-EM)
3.8. Scanning Electron Microscopy (SEM)
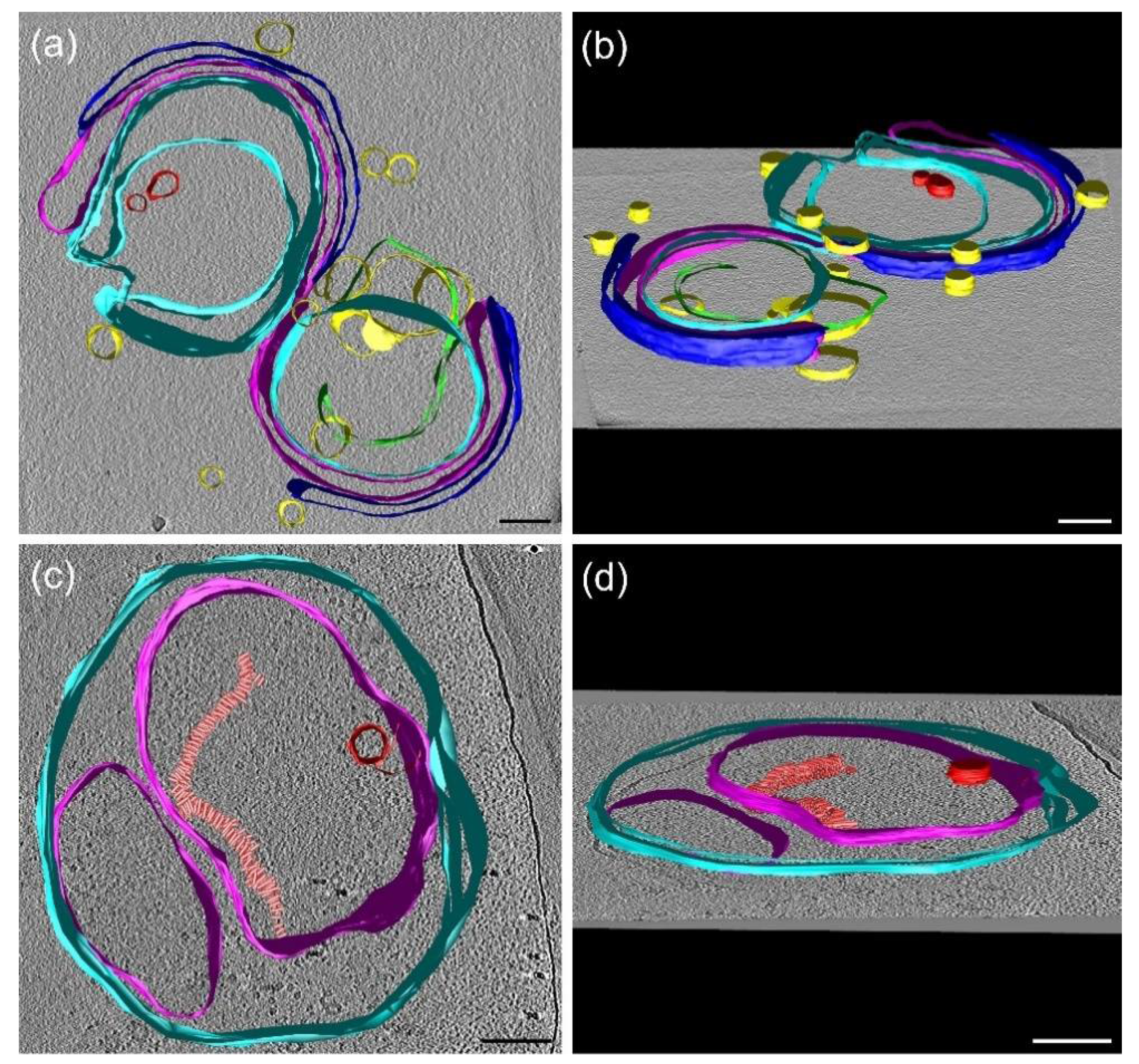
3.9. Cryo-Electron Tomography (CET)
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dlouhý, O.; Javornik, U.; Zsiros, O.; Šket, P.; Karlický, V.; Špunda, V.; Plavec, J.; Garab, G. Lipid polymorphism of the subchloroplast—granum and stroma thylakoid membrane—particles. I. 31P-NMR spectroscopy. Cells 2021, 10, 2354. [Google Scholar]
- Mitchell, P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol. Rev. Camb. Philos. Soc. 1966, 41, 445–502. [Google Scholar] [CrossRef]
- Douce, R.; Joyard, J. Biosynthesis of Thylakoid Membrane Lipids. In Oxygenic Photosynthesis: The Light Reactions; Ort, D.R., Yocum, C.F., Heichel, I.F., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 69–101. [Google Scholar]
- Krumova, S.B.; Dijkema, C.; de Waard, P.; Van As, H.; Garab, G.; van Amerongen, H. Phase behaviour of phosphatidylglycerol in spinach thylakoid membranes as revealed by P-31-NMR. Biochim. Biophys. Acta 2008, 1778, 997–1003. [Google Scholar] [CrossRef]
- Garab, G.; Ughy, B.; de Waard, P.; Akhtar, P.; Javornik, U.; Kotakis, C.; Šket, P.; Karlický, V.; Materová, Z.; Špunda, V.; et al. Lipid polymorphism in chloroplast thylakoid membranes—as revealed by P-31-NMR and timeresolved merocyanine fluorescence spectroscopy. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Ughy, B.; Karlický, V.; Dlouhý, O.; Javornik, U.; Materová, Z.; Zsiros, O.; Šket, P.; Plavec, J.; Špunda, V.; Garab, G. Lipid-polymorphism of plant thylakoid membranes. Enhanced non-bilayer lipid phases associated with increased membrane permeability. Physiol. Plant 2019, 166, 278–287. [Google Scholar] [CrossRef]
- Dlouhý, O.; Kurasová, I.; Karlický, V.; Javornik, U.; Šket, P.; Petrova, N.Z.; Krumova, S.B.; Plavec, J.; Ughy, B.; Špunda, V.; et al. Modulation of non-bilayer lipid phases and the structure and functions of thylakoid membranes: Effects on the water-soluble enzyme violaxanthin de-epoxidase. Sci. Rep. 2020, 10, 11959. [Google Scholar] [CrossRef]
- Garab, G.; van Amerongen, H. Linear dichroism and circular dichroism in photosynthesis research. Photosynth. Res. 2009, 101, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.J.; Rokke, G.; Hohmann-Marriott, M.F. Chlorophyll fluorescence emission spectroscopy of oxygenic organisms at 77 K. Photosynthetica 2018, 56, 105–124. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Lukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant 2016, 38, 102. [Google Scholar] [CrossRef]
- Lazár, D. Chlorophyll a fluorescence induction. Biochim. Biophys. Acta 1999, 1412, 1–28. [Google Scholar] [CrossRef]
- Sipka, G.; Magyar, M.; Mezzetti, A.; Akhtar, P.; Zhu, Q.; Xiao, Y.; Han, G.; Santabarbara, S.; Shen, J.-R.; Lambrev, P.H.; et al. Light-adapted charge-separated state of photosystem II: Structural and functional dynamics of the closed reaction center. Plant Cell 2021, 33, 1286–1302. [Google Scholar] [CrossRef]
- Bailleul, B.; Cardol, P.; Breyton, C.; Finazzi, G. Electrochromism: A useful probe to study algal photosynthesis. Photosynth. Res. 2010, 106, 179–189. [Google Scholar] [CrossRef]
- Pabst, G.; Koschuch, R.; Pozo-Navas, B.; Rappolt, M.; Lohner, K.; Laggner, P. Structural analysis of weakly ordered membrane stacks. J. Appl. Crystallogr. 2003, 36, 1378–1388. [Google Scholar] [CrossRef]
- Jakubauskas, D.; Mortensen, K.; Jensen, P.E.; Kirkensgaard, J.J.K. Small-Angle X-Ray and Neutron Scattering on Photosynthetic Membranes. Front. Chem. 2021, 9, 82. [Google Scholar] [CrossRef]
- Semenova, G.A. The relationship between the transformation of thylakoid acyl lipids and the formation of tubular lipid aggregates visible on fracture faces. J. Plant Physiol. 1999, 155, 669–677. [Google Scholar] [CrossRef]
- Williams, W.P. The Physical Properties of Thylakoid Membrane Lipids and Their Relation to Photosynthesis. In Lipids in Photosynthesis: Structure, Function and Genetics; Paul-André, S., Norio, M., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 103–118. [Google Scholar]
- Sprague, S.G.; Staehelin, L.A. Effects of reconstitution method on the structural organization of isolated chloroplast membrane-lipids. Biochim. Biophys. Acta 1984, 777, 306–322. [Google Scholar] [CrossRef]
- Daum, B.; Kuhlbrandt, W. Electron tomography of plant thylakoid membranes. J. Exp. Bot. 2011, 62, 2393–2402. [Google Scholar] [CrossRef]
- Bussi, Y.; Shimoni, E.; Weiner, A.; Kapon, R.; Charuvi, D.; Nevo, R.; Efrati, E.; Reich, Z. Fundamental helical geometry consolidates the plant photosynthetic membrane. Proc. Natl. Acad. Sci. USA 2019, 116, 22366–22375. [Google Scholar] [CrossRef] [PubMed]
- Mustárdy, L.A.; Janossy, A.G.S. Evidence of helical thylakoid arrangement by scanning electron-microscopy. Plant Sci. Lett. 1979, 16, 281–284. [Google Scholar] [CrossRef]
- Anderson, J.M.; Boardman, N.K. Fractionation of photochemical systems of photosynthesis. I. Chlorophyll contents and photochemical activities of particles isolated from spinach chloroplasts. Biochim. Biophys. Acta 1966, 112, 403–421. [Google Scholar] [CrossRef]
- Peters, F.; Vanwielink, J.E.; Sang, H.; Devries, S.; Kraayenhof, R. Studies on well coupled photosystem I-enriched subchloroplast vesicles—content and redox properties of electron-transfer components. Biochim. Biophys. Acta 1983, 722, 460–470. [Google Scholar] [CrossRef]
- Cuello, J.; Quiles, M.J. Fractionation of thylakoid membranes into grana and stroma thylakoids. Methods Mol. Biol. 2004, 274, 1–9. [Google Scholar] [CrossRef]
- Pavlovič, A.; Stolárik, T.; Nosek, L.; Kouřil, R.; Ilík, P. Light-induced gradual activation of photosystem II in dark-grown Norway spruce seedlings. Biochim. Biophys. Acta-Bioenerg. 2016, 1857, 799–809. [Google Scholar] [CrossRef]
- Saga, G.; Giorgetti, A.; Fufezan, C.; Giacometti, G.M.; Bassi, R.; Morosinotto, T. Mutation Analysis of Violaxanthin De-epoxidase Identifies Substrate-binding Sites and Residues Involved in Catalysis. J. Biol. Chem. 2010, 285, 23763–23778. [Google Scholar] [CrossRef] [PubMed]
- Wacha, A.; Varga, Z.; Bota, A. CREDO: A new general-purpose laboratory instrument for small-angle X-ray scattering. J. Appl. Crystallogr. 2014, 47, 1749–1754. [Google Scholar] [CrossRef]
- Wacha, A. Optimized pinhole geometry for small-angle scattering. J. Appl. Crystallogr. 2015, 48, 1843–1848. [Google Scholar] [CrossRef]
- Severs, N.J. Freeze-fracture electron microscopy. Nat. Protoc. 2007, 2, 547–576. [Google Scholar] [CrossRef]
- Arshad, R.; Calvaruso, C.; Boekema, E.J.; Büchel, C.; Kouřil, R. Revealing the architecture of the photosynthetic apparatus in the diatom Thalassiosira pseudonana. Plant Physiol. 2021. [Google Scholar] [CrossRef]
- Mastronarde, D.N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 2005, 152, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Peng, L.; Baldwin, P.R.; Mann, D.S.; Jiang, W.; Rees, I.; Ludtke, S.J. EMAN2: An extensible image processing suite for electron microscopy. J. Struct. Biol. 2007, 157, 38–46. [Google Scholar] [CrossRef]
- Frangakis, A.S.; Hegerl, R. Noise reduction in electron tomographic reconstructions using nonlinear anisotropic diffusion. J. Struct. Biol. 2001, 135, 239–250. [Google Scholar] [CrossRef]
- Kremer, J.R.; Mastronarde, D.N.; McIntosh, J.R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 1996, 116, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Dobrikova, A.G.; Várkonyi, Z.; Krumova, S.B.; Kovács, L.; Kostov, G.K.; Todinova, S.J.; Busheva, M.C.; Taneva, S.G.; Garab, G. Structural Rearrangements in chloroplast thylakoid membranes revealed by differential scanning calorimetry and circular dichroism spectroscopy. Thermo-optic effect. Biochemistry 2003, 42, 11272–11280. [Google Scholar] [CrossRef]
- Lambrev, P.H.; Varkonyi, Z.; Krumova, S.; Kovacs, L.; Miloslavina, Y.; Holzwarth, A.R.; Garab, G. Importance of trimer-trimer interactions for the native state of the plant light-harvesting complex II. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 847–853. [Google Scholar] [CrossRef]
- Berthold, D.A.; Babcock, G.T.; Yocum, C.F. A highly resolved, oxygen-evolving photosystem-II preparation from spinach thylakoid membranes—electron-paramagnetic-res and electron-transport properties. FEBS Lett. 1981, 134, 231–234. [Google Scholar] [CrossRef]
- Morosinotto, T.; Segalla, A.; Giacometti, G.M.; Bassi, R. Purification of structurally intact grana from plants thylakoids membranes. J. Bioenerg. Biomembr. 2010, 42, 37–45. [Google Scholar] [CrossRef]
- Miloslavina, Y.; Lambrev, P.H.; Jávorfi, T.; Várkonyi, Z.; Karlický, V.; Wall, J.S.; Hind, G.; Garab, G. Anisotropic circular dichroism signatures of oriented thylakoid membranes and lamellar aggregates of LHCII. Photosynth. Res. 2012, 111, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Nellaepalli, S.; Zsiros, O.; Toth, T.; Yadavalli, V.; Garab, G.; Subramanyam, R.; Kovacs, L. Heat- and light-induced detachment of the light harvesting complex from isolated photosystem I supercomplexes. J. Photochem. Photobiol. B-Biol. 2014, 137, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.J.; Xu, P.Q.; Chukhutsina, V.U.; Holzwarth, A.R.; Croce, R. Zeaxanthin-dependent nonphotochemical quenching does not occur in photosystem I in the higher plant Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, 4828–4832. [Google Scholar] [CrossRef]
- Krumova, S.B.; Várkonyi, Z.; Lambrev, P.H.; Kovács, L.; Todinova, S.J.; Busheva, M.C.; Taneva, S.G.; Garab, G. Heat- and light-induced detachment of the light-harvesting antenna complexes of photosystem I in isolated stroma thylakoid membranes. J. Photochem. Photobiol. B-Biol. 2014, 137, 4–12. [Google Scholar] [CrossRef]
- Akhtar, P.; Lingvay, M.; Kiss, T.; Deák, R.; Bóta, A.; Ughy, B.; Garab, G.; Lambrev, P.H. Excitation energy transfer between Light-harvesting complex II and Photosystem I in reconstituted membranes. Biochim. Biophys. Acta-Bioenerg. 2016, 1857, 462–472. [Google Scholar] [CrossRef]
- Xu, P.Q.; Tian, L.J.; Kloz, M.; Croce, R. Molecular insights into Zeaxanthin-dependent quenching in higher plants. Sci. Rep. 2015, 5, 13679. [Google Scholar] [CrossRef]
- Akhtar, P.; Dorogi, M.; Pawlak, K.; Kovács, L.; Bóta, A.; Kiss, T.; Garab, G.; Lambrev, P.H. Pigment Interactions in Light-harvesting Complex II in Different Molecular Environments. J. Biol. Chem. 2015, 290, 4877–4886. [Google Scholar] [CrossRef]
- Garab, G.I.; Horváth, G.; Faludi-Dániel, A. Resolution of fluorescence bands in greening chloroplasts of maize. Biochem. Biophys. Res. Commun. 1974, 56, 1004–1009. [Google Scholar] [CrossRef]
- Williams, W.P.; Brain, A.P.R.; Dominy, P.J. Induction of nonbilayer lipid phase separations in chloroplast thylakoid membranes by compatible co-solutes and its relation to the thermal-stability of photosystem-II. Biochim. Biophys. Acta 1992, 1099, 137–144. [Google Scholar] [CrossRef]
- Kotakis, C.; Akhtar, P.; Zsiros, O.; Garab, G.; Lambrev, P.H. Increased thermal stability of photosystem II and the macro-organization of thylakoid membranes, induced by co-solutes, associated with changes in the lipid-phase behaviour of thylakoid membranes. Photosynthetica 2018, 56, 254–264. [Google Scholar] [CrossRef]
- Mustardy, L.; Garab, G. Granum revisited. A three-dimensional model—where things fall into place. Trends Plant Sci. 2003, 8, 117–122. [Google Scholar] [CrossRef]
- Peters, F.; van Spanning, R.; Kraayenhof, R. Studies on well coupled photosystem I-enriched subchloroplast vesicles—optimization of ferredoxin-mediated cyclic phosphorylation and electric-potential generation. Biochim. Biophys. Acta 1983, 724, 159–165. [Google Scholar] [CrossRef]
- Latowski, D.; Akerlund, H.E.; Strzalka, K. Violaxanthin de-epoxidase, the xanthophyll cycle enzyme, requires lipid inverted hexagonal structures for its activity. Biochemistry 2004, 43, 4417–4420. [Google Scholar] [CrossRef]
- Goss, R.; Lohr, M.; Latowski, D.; Grzyb, J.; Vieler, A.; Wilhelm, C.; Strzalka, K. Role of hexagonal structure-forming lipids in diadinoxanthin and violaxanthin solubilization and de-epoxidation. Biochemistry 2005, 44, 4028–4036. [Google Scholar] [CrossRef]
- Rockholm, D.C.; Yamamoto, H.Y. Violaxanthin de-epoxidase—Purification of a 43-kilodalton lumenal protein from lettuce by lipid-affinity precipitation with monogalactosyldiacylglyceride. Plant Physiol. 1996, 110, 697–703. [Google Scholar] [CrossRef]
- Simionato, D.; Basso, S.; Zaffagnini, M.; Lana, T.; Marzotto, F.; Trost, P.; Morosinotto, T. Protein redox regulation in the thylakoid lumen: The importance of disulfide bonds for violaxanthin de-epoxidase. FEBS Lett. 2015, 589, 919–923. [Google Scholar] [CrossRef]
- Turner, D.C.; Gruner, S.M. X-ray-diffraction reconstruction of the inverted hexagonal (HII) phase in lipid water-systems. Biochemistry 1992, 31, 1340–1355. [Google Scholar] [CrossRef]
- Kirkensgaard, J.J.K.; Holm, J.K.; Larsen, J.K.; Posselt, D. Simulation of small-angle X-ray scattering from thylakoid membranes. J. Appl. Crystallogr. 2009, 42, 649–659. [Google Scholar] [CrossRef]
- Unnep, R.; Zsiros, O.; Hörcsik, Z.; Markó, M.; Jajoo, A.; Kohlbrecher, J.; Garab, G.; Nagy, G. Low-pH induced reversible reorganizations of chloroplast thylakoid membranes—As revealed by small-angle neutron scattering. Biochim. Biophys. Acta-Bioenerg. 2017, 1858, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Stock, D.; Leslie, A.G.W.; Walker, J.E. Molecular architecture of the rotary motor in ATP synthase. Science 1999, 286, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, H.; Haase, W.; Wegner, S.; Danielsson, R.; Ackermann, R.; Albertsson, P.A. Low-light-induced formation of semicrystalline photosystem II arrays in higher plant chloroplast. Biochemistry 2007, 46, 11169–11176. [Google Scholar] [CrossRef] [PubMed]
- Simidjiev, I.; Stoylova, S.; Amenitsch, H.; Javorfi, T.; Mustárdy, L.; Laggner, P.; Holzenburg, A.; Garab, G. Self-assembly of large, ordered lamellae from non-bilayer lipids and integral membrane proteins in vitro. Proc. Natl. Acad. Sci. USA 2000, 97, 1473–1476. [Google Scholar] [CrossRef]
- Holm, J.K. Structure and Structural Flexibility of Thylakoid Membranes; University of Roskilde: Roskilde, Denmark, 2004. [Google Scholar]
- Staehelin, L.A. Chloroplast Structure and Supramolecular Organization of Photosynthetic Membranes. In Photosynthesis III: Photosynthetic Membranes and Light Harvesting Systems; Staehelin, L.A., Arntzen, C.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 1–84. [Google Scholar]
- Gounaris, K.; Sen, A.; Brain, A.P.R.; Quinn, P.J.; Williams, W.P. The formation of non-bilayer structures in total polar lipid extracts of chloroplast membranes. Biochim. Biophys. Acta 1983, 728, 129–139. [Google Scholar] [CrossRef]
- Oszlánczi, A.; Bóta, A.; Klumpp, E. Influence of aminoglycoside antibiotics on the thermal behaviour and structural features of DPPE-DPPG model membranes. Colloids Surf. B Biointerfaces 2010, 75, 141–148. [Google Scholar] [CrossRef]
- Dekker, J.P.; Boekema, E.J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta Bioenerg. 2005, 1706, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Daum, B.; Nicastro, D.; Il, J.A.; McIntosh, J.R.; Kuhlbrandt, W. Arrangement of Photosystem II and ATP Synthase in Chloroplast Membranes of Spinach and Pea. Plant Cell 2010, 22, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Watts, A. NMR of Lipids. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1727–1738. [Google Scholar]
- Kouřil, R.; Oostergetel, G.T.; Boekema, E.J. Fine structure of granal thylakoid membrane organization using cryo electron tomography. Biochim. Biophys. Acta-Bioenerg. 2011, 1807, 368–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Eerden, F.J.; de Jong, D.H.; de Vries, A.H.; Wassenaar, T.A.; Marrink, S.J. Characterization of thylakoid lipid membranes from cyanobacteria and higher plants by molecular dynamics simulations. Biochim Biophys Acta 2015, 1848, 1319–1330. [Google Scholar] [CrossRef]
- Kirchhoff, H. Chloroplast ultrastructure in plants. New Phytol. 2019, 223, 565–574. [Google Scholar] [CrossRef]
- Seddon, J.M.; Templer, R.H. Chapter 3—Polymorphism of Lipid-Water Systems. In Handbook of Biological Physics; Lipowsky, R., Sackmann, E., Eds.; North-Holland: Amsterdam, The Netherlands, 1995; Volume 1, pp. 97–160. [Google Scholar]
- Chernomordik, L. Non-bilayer lipids and biological fusion intermediates. Chem. Phys. Lipids 1996, 81, 203–213. [Google Scholar] [CrossRef]
- Jouhet, J. Importance of the hexagonal lipid phase in biological membrane organization. Front Plant Sci. 2013, 4, 494. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, H.W.; Hong, M. Distinguishing Bicontinuous Lipid Cubic Phases from Isotropic Membrane Morphologies Using P-31 Solid-State NMR Spectroscopy. J. Phys. Chem. B 2015, 119, 4993–5001. [Google Scholar] [CrossRef]
- Mustárdy, L.; Buttle, K.; Steinbach, G.; Garab, G. The Three-Dimensional Network of the Thylakoid Membranes in Plants: Quasihelical Model of the Granum-Stroma Assembly. Plant Cell 2008, 20, 2552–2557. [Google Scholar] [CrossRef]
- Austin, J.R., II; Staehelin, L.A. Three-Dimensional Architecture of Grana and Stroma Thylakoids of Higher Plants as Determined by Electron Tomography. Plant Physiol. 2011, 155, 1601–1611. [Google Scholar] [CrossRef]
- de Kruijff, B. Biomembranes—Lipids beyond the bilayer. Nature 1997, 386, 129–130. [Google Scholar] [CrossRef]
- Brown, M.F. Curvature Forces in Membrane Lipid-Protein Interactions. Biochemistry 2012, 51, 9782–9795. [Google Scholar] [CrossRef]
- Ball, W.B.; Neff, J.K.; Gohil, V.M. The role of nonbilayer phospholipids in mitochondrial structure and function. FEBS Lett. 2018, 592, 1273–1290. [Google Scholar] [CrossRef]
- Zhang, M.; Mileykovskaya, E.; Dowhan, W. Gluing the respiratory chain together - Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J. Biol. Chem. 2002, 277, 43553–43556. [Google Scholar] [CrossRef]
- Pfeiffer, K.; Gohil, V.; Stuart, R.A.; Hunte, C.; Brandt, U.; Greenberg, M.L.; Schagger, H. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 2003, 278, 52873–52880. [Google Scholar] [CrossRef] [PubMed]
- Heinemeyer, J.; Braun, H.P.; Boekema, E.J.; Kouřil, R. A structural model of the cytochrome c reductase/oxidase supercomplex from yeast mitochondria. J. Biol. Chem. 2007, 282, 12240–12248. [Google Scholar] [CrossRef]
- Schuurmans, J.J.; Veerman, E.C.I.; Francke, J.A.; Torrespereira, J.M.G.; Kraayenhof, R. Temperature-dependence of energy-transducing functions and inhibitor sensitivity in chloroplasts. Plant Physiol. 1984, 74, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, A.N.; Vershubskii, A.V. Temperature-dependent regulation of electron transport and ATP synthesis in chloroplasts in vitro and in silico. Photosynth. Res. 2020, 146, 299–329. [Google Scholar] [CrossRef] [PubMed]
- Garab, G.; Lohner, K.; Laggner, P.; Farkas, T. Self-regulation of the lipid content of membranes by non-bilayer lipids: A hypothesis. Trends Plant Sci. 2000, 5, 489–494. [Google Scholar] [CrossRef]
- Gasanov, S.E.; Kim, A.A.; Yaguzhinsky, L.S.; Dagda, R.K. Non-bilayer structures in mitochondrial membranes regulate ATP synthase activity. Biochim. Biophys. Acta 2018, 1860, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.M.; Ravera, S.; Calzia, D.; Panfoli, I. An update of the chemiosmotic theory as suggested by possible proton currents inside the coupling membrane. Open Biol. 2019, 9, 180221. [Google Scholar] [CrossRef] [PubMed]

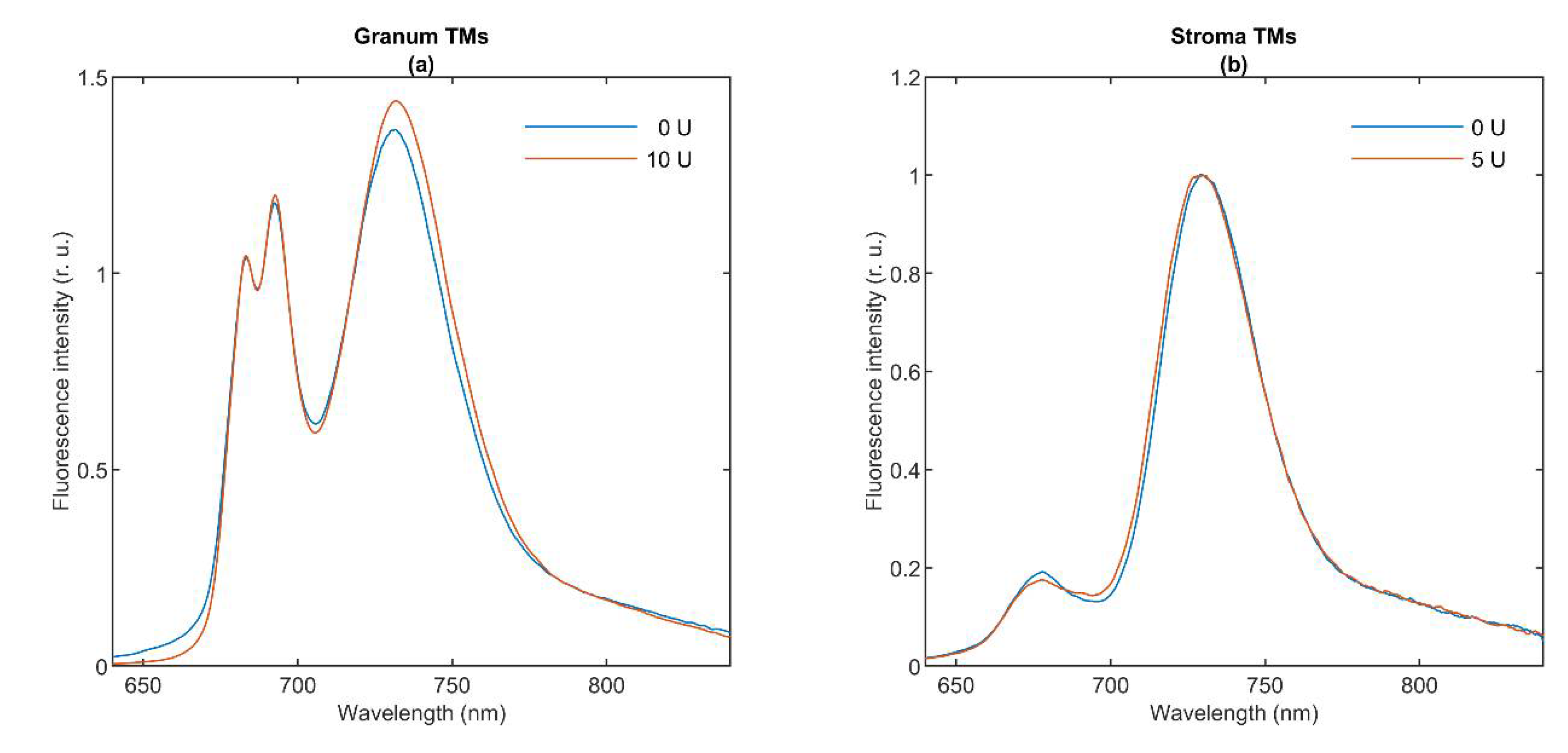



| Temperature | 5 °C | 15 °C | 25 °C |
| Fv/Fm | 0.61 ± 0.07 | 0.59 ± 0.06 | 0.46 ± 0.15 |
| Lipase activity | 0 U | 5 U | 10 U |
| Fv/Fm | 0.59 ± 0.05 | 0.58 ± 0.06 | 0.57 ± 0.04 |
| Granum TMs | 0 U | 10 U |
| (1.04 ± 0.20) × 10−3 | (0.92 ± 0.06) × 10−3 | |
| Stroma TMs | 0 U | 5 U |
| 0.95 × 10−3 | 0.78 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dlouhý, O.; Karlický, V.; Arshad, R.; Zsiros, O.; Domonkos, I.; Kurasová, I.; Wacha, A.F.; Morosinotto, T.; Bóta, A.; Kouřil, R.; et al. Lipid Polymorphism of the Subchloroplast—Granum and Stroma Thylakoid Membrane–Particles. II. Structure and Functions. Cells 2021, 10, 2363. https://doi.org/10.3390/cells10092363
Dlouhý O, Karlický V, Arshad R, Zsiros O, Domonkos I, Kurasová I, Wacha AF, Morosinotto T, Bóta A, Kouřil R, et al. Lipid Polymorphism of the Subchloroplast—Granum and Stroma Thylakoid Membrane–Particles. II. Structure and Functions. Cells. 2021; 10(9):2363. https://doi.org/10.3390/cells10092363
Chicago/Turabian StyleDlouhý, Ondřej, Václav Karlický, Rameez Arshad, Ottó Zsiros, Ildikó Domonkos, Irena Kurasová, András F. Wacha, Tomas Morosinotto, Attila Bóta, Roman Kouřil, and et al. 2021. "Lipid Polymorphism of the Subchloroplast—Granum and Stroma Thylakoid Membrane–Particles. II. Structure and Functions" Cells 10, no. 9: 2363. https://doi.org/10.3390/cells10092363
APA StyleDlouhý, O., Karlický, V., Arshad, R., Zsiros, O., Domonkos, I., Kurasová, I., Wacha, A. F., Morosinotto, T., Bóta, A., Kouřil, R., Špunda, V., & Garab, G. (2021). Lipid Polymorphism of the Subchloroplast—Granum and Stroma Thylakoid Membrane–Particles. II. Structure and Functions. Cells, 10(9), 2363. https://doi.org/10.3390/cells10092363







