Basophils and Mast Cells in COVID-19 Pathogenesis
Abstract
:1. Introduction
Basophils and Mast Cells in Viral Infections
2. Search Strategy
3. Results
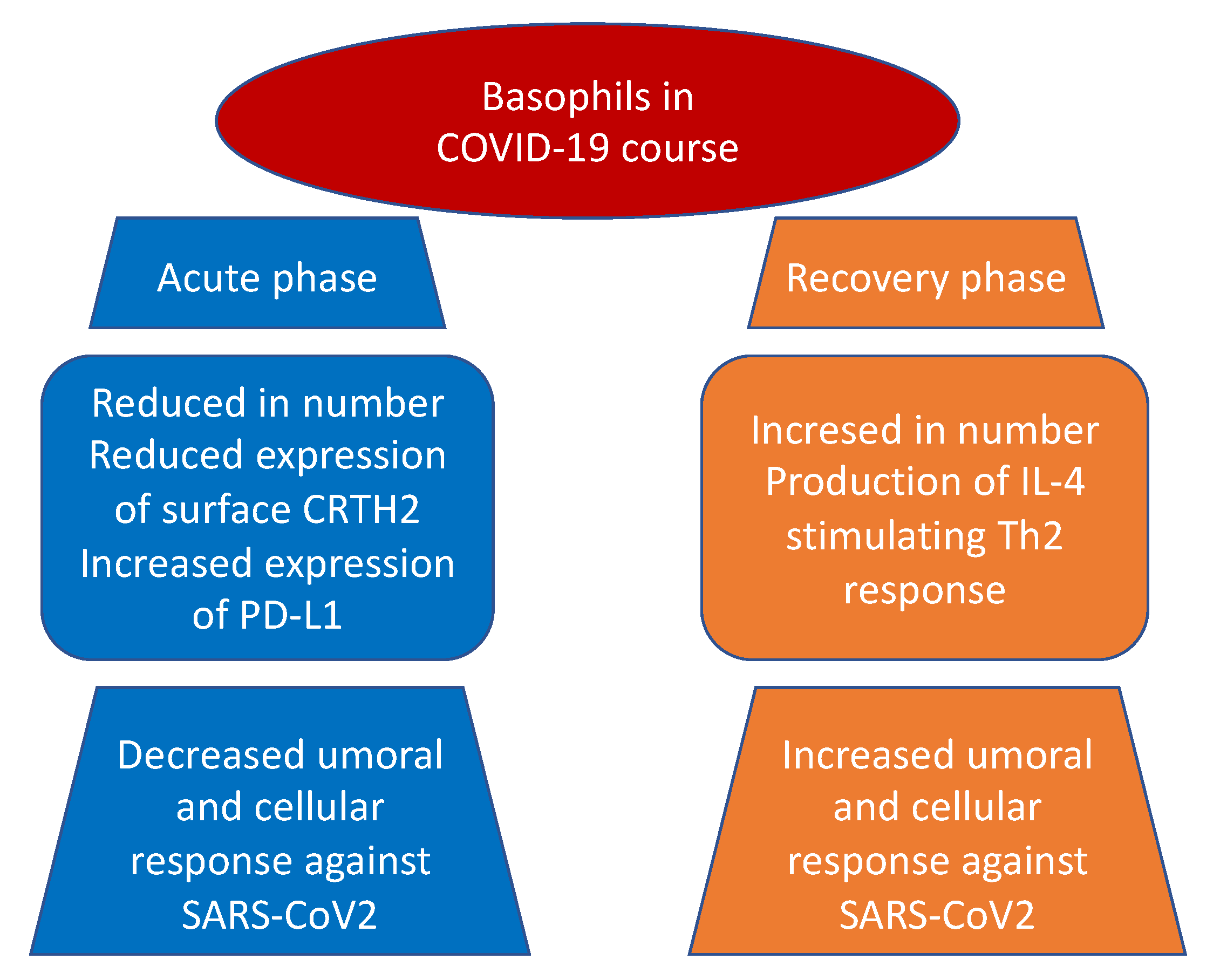
3.1. Basophils and COVID-19 Disease
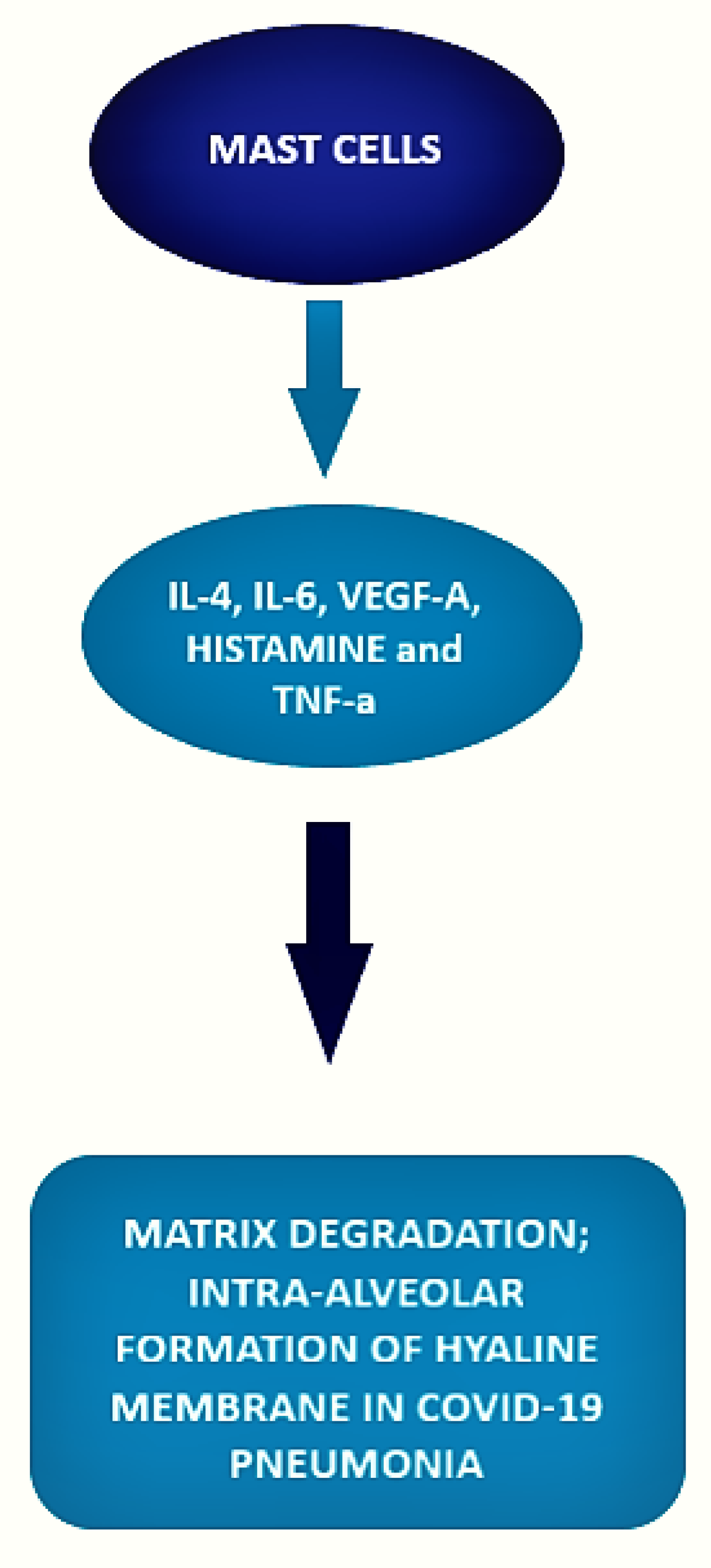
3.2. Mast Cell and COVID-19 Disease
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.-M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.Y.; Thone, M.N.; Kwon, Y.J. COVID-19 vaccines: The status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2020, 170, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Marovich, M.; Mascola, J.R.; Cohen, M.S. Monoclonal Antibodies for Prevention and Treatment of COVID-19. JAMA 2020, 324, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Brown, E.S.; Cheeseman, H.M.; Flight, K.E.; Higham, S.L.; Lemm, N.M.; Pierce, B.F.; Stirling, D.C.; Wang, Z.; Pollock, K.M. Vaccines for COVID-19. Clin. Exp. Immunol. 2020, 202, 162–192. [Google Scholar] [CrossRef]
- Brant, A.C.; Tian, W.; Majerciak, V.; Yang, W.; Zheng, Z.M. SARS-CoV-2: From its discovery to genome structure, transcrip-tion, and replication. Cell Biosci. 2021, 11, 136. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Lam, C.S.; Lau, C.C.; Tsang, A.K.; Lau, J.H.; Bai, R.; Teng, J.L.; Tsang, C.C.; Wanget, M.; et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and be-tacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar]
- Mohamadian, M.; Chiti, H.; Shoghli, A.; Biglari, S.; Parsamanesh, N.; Esmaeilzadeh, A. COVID-19: Virology, biology and novel laboratory diagnosis. J. Gene Med. 2020, 23, e3303. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.-Y.; Zhao, R.; Gao, L.-J.; Gao, X.-F.; Wang, D.-P.; Cao, J.-M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell. Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treat-ment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Taghiloo, S.; Aliyali, M.; Abedi, S.; Mehravaran, H.; Sharifpour, A.; Zaboli, E.; Eslami-Jouybari, M.; Ghasemian, R.; Vahedi-Larijani, L.; Hossein-Nattaj, H.; et al. Apoptosis and immunophenotyping of peripheral blood lymphocytes in Iranian COVID-19 patients: Clinical and laboratory characteristics. J. Med. Virol. 2020, 93, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, T. CD4+T, CD8+T counts and severe COVID-19: A meta-analysis. J. Infect. 2020, 81, e82–e84. [Google Scholar] [CrossRef]
- Mazzoni, A.; Salvati, L.; Maggi, L.; Capone, M.; Vanni, A.; Spinicci, M.; Mencarini, J.; Caporale, R.; Peruzzi, B.; Antonelli, A.; et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Investig. 2020, 130, 4694–4703. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Allegra, A.; Di Gioacchino, M.; Tonacci, A.; Musolino, C.; Gangemi, S. Immunopathology of SARS-CoV-2 Infection: Immune Cells and Mediators, Prognostic Factors, and Immune-Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 13. [Google Scholar] [CrossRef]
- Marone, G.; Varricchi, G.; Loffredo, S.; Galdiero, M.R.; Rivellese, F.; De Paulis, A. Are Basophils and Mast Cells Masters in HIV Infection? Int. Arch. Allergy Immunol. 2016, 171, 158–165. [Google Scholar] [CrossRef]
- Jiang, A.-P.; Jiang, J.-F.; Guo, M.-G.; Jin, Y.-M.; Li, Y.-Y.; Wang, J.-H. Human Blood-Circulating Basophils Capture HIV-1 and Mediate Viral trans -Infection of CD4 + T Cells. J. Virol. 2015, 89, 8050–8062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, F.W.; Prevete, N.; Rivellese, F.; Lobasso, A.; Napolitano, F.; Granata, F.; Selleri, C.; De Paulis, A. HIV-1 Nef promotes migration and chemokine synthesis of human basophils and mast cells through the interaction with CXCR4. Clin. Mol. Allergy 2016, 14, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, A.-P.; Jiang, J.-F.; Wei, J.-F.; Guo, M.-G.; Qin, Y.; Guo, Q.-Q.; Ma, L.; Liu, B.-C.; Wang, X.; Veazey, R.S.; et al. Human Mucosal Mast Cells Capture HIV-1 and Mediate Viral trans -Infection of CD4 + T Cells. J. Virol. 2016, 90, 2928–2937. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, R.; Wisniewski, J.; Yu, M.D.; Kennedy, J.L.; Platts-Mills, T.; Heymann, P.W.; Woodfolk, J.A. Infection with human rhinovirus 16 promotes enhanced IgE responsiveness in basophils of atopic asthmatics. Clin. Exp. Allergy 2014, 44, 1266–1273. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.L.; Newcomb, D.C.; Parekh, V.V.; Van Kaer, L.; Collins, R.D.; Zhou, W.; Goleniewska, K.; Chi, M.H.; Mitchell, D.; Boyce, J.A.; et al. STAT1 Negatively Regulates Lung Basophil IL-4 Expression Induced by Respiratory Syncytial Virus Infection. J. Immunol. 2009, 183, 2016–2026. [Google Scholar] [CrossRef] [Green Version]
- Huftel, M.A.; Swensen, C.A.; Borcherding, W.R.; Dick, E.C.; Hong, R.; Kita, H.; Gleich, G.J.; Busse, W.W. The Effect of T-Cell Depletion on Enhanced Basophil Histamine Release afterIn VitroIncubation with Live Influenza A Virus. Am. J. Respir. Cell Mol. Biol. 1992, 7, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.; John, A.L.S. Protective and pathogenic roles for mast cells during viral infections. Curr. Opin. Immunol. 2020, 66, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Witczak, P.; Brzezińska-Błaszczyk, E. Mast cells in viral infections. Adv. Hyg. Exp. Med. 2012, 66, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.; Cheng, J.; Xiao, J.; Chen, M.; Zou, S.; Tian, H.; Wang, M.; Sun, L.; Hao, Z.; Hu, Y. Defective Viral Particles Produced in Mast Cells Can Effectively Fight Against Lethal Influenza A Virus. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Desheva, Y.; Mamontov, A.; Petkova, N.; Karev, V.; Nazarov, P. Mast cell degranulation and histamine release during A/H5N1 influenza infection in influenza-sensitized mice. Life Sci. 2020, 258, 118230. [Google Scholar] [CrossRef] [PubMed]
- King, C.A.; Anderson, R.; Marshall, J.S. Dengue Virus Selectively Induces Human Mast Cell Chemokine Production. J. Virol. 2002, 76, 8408–8419. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.G.; McAlpine, S.M.; Huang, Y.Y.; Haidl, I.D.; Al-Afif, A.; Marshall, J.S.; Anderson, R. RNA Sensors Enable Human Mast Cell Anti-Viral Chemokine Production and IFN-Mediated Protection in Response to Antibody-Enhanced Dengue Virus Infection. PLoS ONE 2012, 7, e34055. [Google Scholar] [CrossRef] [PubMed]
- Londono-Renteria, B.; Marinez-Angarita, J.C.; Troupin, A.; Colpitts, T.M. Role of Mast Cells in Dengue Virus Pathogenesis. DNA Cell Biol. 2017, 36, 423–427. [Google Scholar] [CrossRef] [PubMed]
- King, C.A.; Marshall, J.S.; Alshurafa, H.; Anderson, R. Release of Vasoactive Cytokines by Antibody-Enhanced Dengue Virus Infection of a Human Mast Cell/Basophil Line. J. Virol. 2000, 74, 7146–7150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, R.; Kawamura, T.; Goshima, F.; Ogawa, Y.; Nakae, S.; Nakao, A.; Moriishi, K.; Nishiyama, Y.; Shimada, S. Mast Cells Play a Key Role in Host Defense against Herpes Simplex Virus Infection through TNF-α and IL-6 Production. J. Investig. Dermatol. 2013, 133, 2170–2179. [Google Scholar] [CrossRef] [Green Version]
- Karasuyama, H.; Yamanishi, Y. Basophils have emerged as a key player in immunity. Curr. Opin. Immunol. 2014, 31, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Koketsu, R.; Suzukawa, M.; Kawakami, A.; Iikura, M. Human Basophils and Cytokines/Chemokines. Allergol. Int. 2009, 58, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Karasuyama, H.; Obata, K.; Wada, T.; Tsujimura, Y.; Mukai, K. Newly appreciated roles for basophils in allergy and protective immunity. Allergy 2011, 66, 1133–1141. [Google Scholar] [CrossRef]
- Van Panhuys, N.; Prout, M.; Forbes, E.; Min, B.; Paul, W.E.; Le Gros, G. Basophils are the major producers of IL-4 during pri-mary helminth infection. J. Immunol. 2011, 186, 2719–2728. [Google Scholar] [CrossRef] [PubMed]
- Stephen-Victor, E.; Das, M.; Sharma, M.; Galeotti, C.; Fohrer-Ting, H.; Sendid, B.; Darnige, L.; Terris, B.; Badoual, C.; Bruneval, P.; et al. Demystification of enigma on antigen-presenting cell features of human basophils: Data from secondary lymphoid organs. Haematologica 2017, 102, e233–e237. [Google Scholar] [CrossRef] [Green Version]
- Denzel, A.; Maus, U.A.; Gomez, M.R.; Moll, C.; Niedermeier, M.; Winter, C.; Maus, R.; Hollingshead, S.; Briles, D.E.; Kunz-Schughart, L.A.; et al. Basophils enhance immunological memory responses. Nat. Immunol. 2008, 9, 733–742. [Google Scholar] [CrossRef]
- Kawakami, T. Basophils now enhance memory. Nat. Immunol. 2008, 9, 720–721. [Google Scholar] [CrossRef]
- Wakahara, K.; Baba, N.; Van, V.Q.; Bégin, P.; Rubio, M.; Ferraro, P.; Panzini, B.; Wassef, R.; Lahaie, R.; Caussignac, Y.; et al. Human basophils interact with memory T cells to augment Th17 responses. Blood 2012, 120, 4761–4771. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, L.; Pekkarinen, P.T.; Lakshmikanth, T.; Tan, Z.; Consiglio, C.R.; Pou, C.; Chen, Y.; Mugabo, C.H.; Nguyen, N.A.; Nowlan, K.; et al. Systems-Level Immunomonitoring from Acute to Recovery Phase of Severe COVID-19. Cell Rep. Med. 2020, 1, 100078. [Google Scholar] [CrossRef]
- Mao, J.; Dai, R.; Du, R.-C.; Zhu, Y.; Shui, L.-P.; Luo, X.-H. Hematologic changes predict clinical outcome in recovered patients with COVID-19. Ann. Hematol. 2021, 100, 675–689. [Google Scholar] [CrossRef]
- Conceição-Silva, F.; Reis, C.; De Luca, P.; Leite-Silva, J.; Santiago, M.; Morrot, A.; Morgado, F. The Immune System Throws Its Traps: Cells and Their Extracellular Traps in Disease and Protection. Cells 2021, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Ten-Caten, F.; Gonzalez-Dias, P.; Castro, L.; Ogava, R.L.; Giddaluru, J.; Silva, J.C.S.; Martins, F.; Gonçalves, A.N.; Costa-Martins, A.G.; Araujo, J.D.; et al. In-depth analysis of laboratory parameters reveals the interplay between sex, age, and systemic inflammation in individuals with COVID-19. Int. J. Infect. Dis. 2021, 105, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Alnor, A.; Sandberg, M.B.; Toftanes, B.E.; Vinholt, P.J. Platelet parameters and leukocyte morphology is altered in COVID-19 patients compared to non-COVID-19 patients with similar symptomatology. Scand. J. Clin. Lab. Investig. 2021, 81, 213–217. [Google Scholar] [CrossRef]
- Kazancioglu, S.; Bastug, A.; Ozbay, B.O.; Kemirtlek, N.; Bodur, H. The role of haematological parameters in patients with COVID-19 and influenza virus infection. Epidemiology Infect. 2020, 148, 1–16. [Google Scholar] [CrossRef]
- Vitte, J.; Diallo, A.B.; Boumaza, A.; Lopez, A.; Michel, M.; Allardet-Servent, J.; Mezouar, S.; Sereme, Y.; Busnel, J.-M.; Miloud, T.; et al. A Granulocytic Signature Identifies COVID-19 and Its Severity. J. Infect. Dis. 2020, 222. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Sang, L.; Jiang, M.; Yang, Z.; Jia, N.; Fu, W.; Xie, J.; Guan, W.; Liang, W.; Ni, Z.; et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J. Allergy Clin. Immunol. 2020, 146, 89–100. [Google Scholar] [CrossRef]
- Laing, A.G.; Lorenc, A.; del Molino Del Barrio, I.; Das, A.; Fish, M.; Monin, L.; Muñoz-Ruiz, M.; McKenzie, D.R.; Hayday, T.S.; Francos-Quijorna, I.; et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 2020, 26, 1623–1635. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, J.; Ye, K. White Blood Cells and Severe COVID-19: A Mendelian Randomization Study. J. Pers. Med. 2021, 11, 195. [Google Scholar] [CrossRef]
- Junior, J.D.S.M.; Miggiolaro, A.F.R.D.S.; Nagashima, S.; De Paula, C.B.V.; Baena, C.P.; Scharfstein, J.; De Noronha, L. Mast Cells in Alveolar Septa of COVID-19 Patients: A Pathogenic Pathway That May Link Interstitial Edema to Immunothrombosis. Front. Immunol. 2020, 11, 2369. [Google Scholar] [CrossRef]
- Wang, J.; Lindholt, J.S.; Sukhova, G.K.; Shi, M.A.; Xia, M.; Chen, H.; Xiang, M.; He, A.; Wang, Y.; Xiong, N.; et al. IgE actions on CD4+ T cells, mast cells, and mac-rophages participate in the pathogenesis of experimental abdominal aortic aneurysms. EMBO Mol. Med. 2014, 6, 952–969. [Google Scholar] [CrossRef]
- Kempuraj, D.; Selvakumar, G.P.; Ahmed, M.E.; Raikwar, S.P.; Thangavel, R.; Khan, A.; Zaheer, S.A.; Iyer, S.S.; Burton, C.; James, D.; et al. COVID-19, Mast Cells, Cyto-kine Storm, Psychological Stress, and Neuroinflammation. Neuroscientist 2020, 26, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Arac, A.; Grimbaldeston, M.A.; Galli, S.J.; Bliss, T.M.; Steinberg, G.K. Meningeal Mast Cells as Key Effectors of Stroke Pathol-ogy. Front. Cell Neurosci. 2019, 13, 126. [Google Scholar] [CrossRef] [Green Version]
- Varricchi, G.; Rossi, F.W.; Galdiero, M.R.; Granata, F.; Criscuolo, G.; Spadaro, G.; de Paulis, A.; Marone, G. Physiological Roles of Mast Cells: Col-legium Internationale Allergologicum Update 2019. Int. Arch. Allergy Immunol. 2019, 179, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, A.M.; Austin, S.J.; Metcalfe, D.D. Mast Cell Biology: Introduction and Overview. Mast Cell Biol. 2011, 716, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Okayama, Y.; Kawakami, T. Development, Migration, and Survival of Mast Cells. Immunol. Res. 2006, 34, 97–116. [Google Scholar] [CrossRef]
- Conti, P.; Caraffa, A.; Tetè, G.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Di Emidio, P.; Ronconi, G. Mast cells activated by SARS-CoV-2 release hista-mine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19. J. Biol. Regul. Homeost. Agents 2020, 34, 1629–1632. [Google Scholar]
- Sun, X.; Wang, T.; Cai, D.; Hu, Z.; Chen, J.; Liao, H.; Zhi, L.; Wei, H.; Zhang, Z.; Qiu, Y.; et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020, 53, 38–42. [Google Scholar] [CrossRef]
- Theoharides, T.C. COVID -19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin. BioFactors 2020, 46, 306–308. [Google Scholar] [CrossRef]
- Gigante, A.; Aquili, A.; Farinelli, L.; Caraffa, A.; Ronconi, G.; Gallenga, E.C.; Tetè, G.; Kritas, S.K.; Conti, P. Sodium chromo-glycate and palmitoyleth-anolamide: A possible strategy to treat mast cell-induced lung inflammation in COVID-19. Med. Hypotheses 2020, 143, 109856. [Google Scholar] [CrossRef]
- Karamloo, F.; Konig, R. SARS-CoV-2 immunogenicity at the crossroads. Allergy 2020, 75, 1822–1824. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Goplen, N.P.; Li, C.; Cheon, I.S.; Dai, Q.; Huang, S.; Shan, J.; Ma, C.; Ye, Z.; et al. PD-1(hi) CD8(+) resident memory T cells balance immuni-ty and fibrotic sequelae. Sci Immunol. 2019, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Kahan, S.M.; Zajac, A.J. Immune Exhaustion: Past Lessons and New Insights from Lymphocytic Choriomeningitis Virus. Viruses 2019, 11, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, D.J.; Busse, W.W.; Bacharier, L.B.; Kattan, M.; O’Connor, G.T.; Wood, R.A.; Visness, C.M.; Durham, S.R.; Larson, D.; Esnault, S.; et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J. Allergy Clin. Immunol. 2020, 146, 203–206.e3. [Google Scholar] [CrossRef] [PubMed]
- Carli, G.; Cecchi, L.; Stebbing, J.; Parronchi, P.; Farsi, A. Is asthma protective against COVID-19? Allergy 2021, 76, 866–868. [Google Scholar] [CrossRef]
- Dufour, J.H.; Dziejman, M.; Liu, M.T.; Leung, J.H.; Lane, T.E.; Luster, A.D. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 2002, 168, 3195–3204. [Google Scholar] [CrossRef] [Green Version]
- Angiolillo, A.L.; Sgadari, C.; Taub, D.D.; Liao, F.; Farber, J.M.; Maheshwari, S.; Kleinman, H.K.; Reaman, G.H.; Tosato, G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J. Exp. Med. 1995, 182, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M.; et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect. Dis. 2020, 20, 1135–1140. [Google Scholar] [CrossRef]
- Crivellato, E.; Travan, L.; Ribatti, D. Mast cells and basophils: A potential link in promoting angiogenesis during allergic in-flammation. Int. Arch. Allergy Immunol. 2010, 151, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Swystun, L.L.; Liaw, P.C. The role of leukocytes in thrombosis. Blood 2016, 128, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Merle, N.S.; Noé, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological mis-firing in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Voehringer, D. Protective and pathological roles of mast cells and basophils. Nat. Rev. Immunol. 2013, 13, 362–375. [Google Scholar] [CrossRef]
- Karasuyama, H.; Mukai, K.; Obata-Ninomiya, K.; Tsujimura, Y.; Wada, T. Nonredundant Roles of Basophils in Immunity. Annu. Rev. Immunol. 2011, 29, 45–69. [Google Scholar] [CrossRef]
- Wedemeyer, J.; Tsai, M.; Galli, S.J. Roles of mast cells and basophils in innate and acquired immunity. Curr. Opin. Immunol. 2000, 12, 624–631. [Google Scholar] [CrossRef]
- Lett-Brown, M.A.; Aelvoet, M.A.; Hooks, J.J.; Georgiades, J.A.; Thueson, D.O.; Grant, J.A. Enhancement of basophil chemotaxis in vitro by virus-induced interferon. J. Clin. Investig. 1981, 67, 547–552. [Google Scholar] [CrossRef]
- Marone, G.; Florio, G.; Petraroli, A.; Triggiani, M.; de Paulis, A. Human mast cells and basophils in HIV-1 infection. Trends Immunol. 2001, 22, 229–232. [Google Scholar] [CrossRef]
- Kumamoto, Y.; Linehan, M.; Weinstein, J.S.; Laidlaw, B.J.; Craft, J.E.; Iwasaki, A. CD301b+ Dermal Dendritic Cells Drive T Helper 2 Cell-Mediated Immunity. Immunity 2013, 39, 733–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, A.; Nakajima, S.; Kubo, M.; Egawa, G.; Honda, T.; Kitoh, A.; Nomura, T.; Hanakawa, S.; Moniaga, C.S.; Kim, B.; et al. Basophils are required for the induction of Th2 immunity to haptens and peptide antigens. Nat. Commun. 2013, 4, 1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Cao, W.; Kasturi, S.P.; Ravindran, R.; Nakaya, H.I.; Kundu, K.; Murthy, N.; Kepler, T.B.; Malissen, B.; Pulendran, B. The T helper type 2 response to cysteine pro-teases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat. Immunol. 2010, 11, 608–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Li, Y. Mechanisms Controlling Mast Cell and Basophil Lineage Decisions. Curr. Allergy Asthma Rep. 2014, 14, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Paula, C.B.V.; De Azevedo, M.L.V.; Nagashima, S.; Martins, A.P.C.; Malaquias, M.A.S.; Miggiolaro, A.F.R.D.S.; Júnior, J.D.S.M.; Avelino, G.; Carmo, L.A.P.D.; Carstens, L.B.; et al. IL-4/IL-13 remodeling pathway of COVID-19 lung injury. Sci. Rep. 2020, 10, 18689. [Google Scholar] [CrossRef]
- Skaria, T.; Burgener, J.; Bachli, E.; Schoedon, G. IL-4 Causes Hyperpermeability of Vascular Endothelial Cells through Wnt5A Signaling. PLoS ONE 2016, 11, e0156002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oschatz, C.; Maas, C.; Lecher, B.; Jansen, T.; Bjorkqvist, J.; Tradler, T.; Sedlmeier, R.; Burfeind, P.; Cichon, S.; Hammerschmidt, S.; et al. Mast cells increase vascular permeability by hep-arin-initiated bradykinin formation in vivo. Immunity 2011, 34, 258–268. [Google Scholar] [CrossRef]
- Moreno-Sanchez, D.; Hernández, L.; Ruiz, F.A.; Docampo, R. Polyphosphate Is a Novel Pro-inflammatory Regulator of Mast Cells and Is Located in Acidocalcisomes. J. Biol. Chem. 2012, 287, 28435–28444. [Google Scholar] [CrossRef] [Green Version]
- Ponomaryov, T.; Payne, H.; Fabritz, L.; Wagner, D.D.; Brill, A. Mast Cells Granular Contents Are Crucial for Deep Vein Thrombosis in Mice. Circ. Res. 2017, 121, 941–950. [Google Scholar] [CrossRef]
| Reference and Type of Study | No. of Patients | Main Results |
|---|---|---|
| Rodriguez et al. [42] Systems-level blood immune-monitoring study | 37 patients | Basophils were depleted during acute disease but increase during recovery. Moreover, levels of basophils were significantly correlated with the titers of IgG antibodies to SARS-CoV-2. |
| Mao et al. [43] Observational study | 127 patients admitted at Wuhan No. 1 Hospital | Basophil count was low in 17 (13.39%) patients during the first three days of hospitalization and returned to normal levels shortly after. |
| Fátima Conceição-Silva et al. [44] Review discusses the presence of ETs | ETs produced by neutrophils were associated with severity in SARS-CoV-2 infection favoring thrombosis. ET produced by basophils, which have bacteral killing and antifungal activity, could have a protective role during COVID-19 infection. | |
| Ten-Caten et al. [45] A multidimensional analysis | 178,887 Brazilian individuals, of which there were 33,266 positives for SARS-CoV-2 | Lower counts of platelets, basophils, lymphocytes, and eosinophils were observed in COVID-19 cases compared with controls in both males and females. |
| Alnor et al. [46] Case–control study | 74 COVID-19-positive and 228 COVID-19-negative patients. | COVID-19 patients presented significant lower values for all white blood cells, including basophil count, in comparison with non-COVID-19 patients. |
| Kazancioglu et al. [47] Retrospective case–control study | 120 COVID-19 patients, 100 influenza patients, and 61 healthy controls | Lower basophil count was found both in COVID-19 and influenza patients compared to healthy controls. |
| Vitte et al. [48] Observational, prospective, multicentric, case–control study | 26 patients | Low number of basophils were detected in COVID-19 patients as compared with healthy donors. Both CRTH2 (CD294), a receptor for prostaglandin D2, and CD11b expression were decreased at the surface of basophils. SARS-CoV-2 infection was associated with inhibition of T helper 2 polarized immune responses and a decreased chemotaxis of CRTH2+ cells, through reduction of basophils, eosinophils, and CRTH2 itself. |
| Chen et al. [49] Retrospective study | 548 patients with COVID-19 | A difference of basophil levels between admission and end-hospitalization greater than 0.02 × 109/L (HR, 2.73; 95% CI, 1.5–6.47) resulted a risk factor for fatal outcome. |
| Laing et al. [50] Observational study | 63 patients | Basophils and dendritic cells are reduced in severe COVID-19. Basophil reduction correlate with elevated interferon-inducible protein-10. |
| Qin et al. [51] Observational study | 452 patients admitted at Tongji Hospital | Severe COVID-19 patients had a lower percentage of basophils than non-severe ones (0.1 vs. 0.2%; p = 0.015). |
| Sun et al. [52] Mendelian randomization study | 408,112 and 562,132 European subjectsfrom two different GWAS | Negative association between basophil count and basophil percentage of WBC are present in severe and hospitalized COVID-19 disease. |
| Motta et al. [53] Histopathological comparative control study | 6 post-mortem covid patients, 10 post-mortem H1N1-infected patients, 10 patients who died for different reasons. | COVID-19 patients samples presented a significantly higher number of mast cells as compared con H1N1 patients and controls. In the context of a neutrophilic endothelitis, mast cells were more frequently localized in the perivascular spaces between the alveolar sacs and terminal bronchioles and in the alveolar septa, close to the alveolar capillaries. |
| Bahareh Hafezi et al. [54] cytokine response by mast cells during COVID-19. | MCs can respond to SARS-CoV-2 and accumulate in the lungs of patients with COVID-19, where they correlate with pulmonary edema, inflammation, and thrombosis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murdaca, G.; Di Gioacchino, M.; Greco, M.; Borro, M.; Paladin, F.; Petrarca, C.; Gangemi, S. Basophils and Mast Cells in COVID-19 Pathogenesis. Cells 2021, 10, 2754. https://doi.org/10.3390/cells10102754
Murdaca G, Di Gioacchino M, Greco M, Borro M, Paladin F, Petrarca C, Gangemi S. Basophils and Mast Cells in COVID-19 Pathogenesis. Cells. 2021; 10(10):2754. https://doi.org/10.3390/cells10102754
Chicago/Turabian StyleMurdaca, Giuseppe, Mario Di Gioacchino, Monica Greco, Matteo Borro, Francesca Paladin, Claudia Petrarca, and Sebastiano Gangemi. 2021. "Basophils and Mast Cells in COVID-19 Pathogenesis" Cells 10, no. 10: 2754. https://doi.org/10.3390/cells10102754
APA StyleMurdaca, G., Di Gioacchino, M., Greco, M., Borro, M., Paladin, F., Petrarca, C., & Gangemi, S. (2021). Basophils and Mast Cells in COVID-19 Pathogenesis. Cells, 10(10), 2754. https://doi.org/10.3390/cells10102754










