The Review of Bioeffects of Static Magnetic Fields on the Oral Tissue-Derived Cells and Its Application in Regenerative Medicine
Abstract
:1. Introduction
2. Characteristics of Magnets and Their Flux Extent
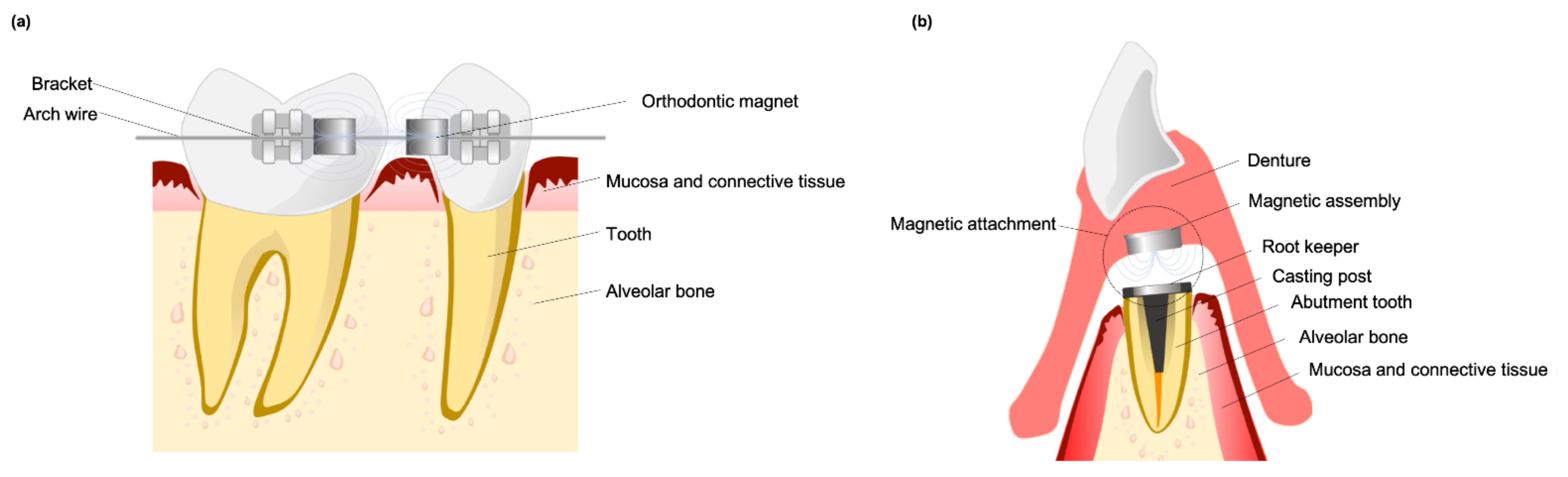
2.1. Development and Features of Dental Magnets
2.2. The Characteristics of SMFs—Flux Intensity and Gradient
3. Biocompatibility of Magnets and Surrounding Tissue
3.1. In Vitro Studies Evaluated the Risk of Hazard from Dental Magnets
3.2. In Vivo Studies Evaluated the Deleterious Effects of Magnets on Surrounding Tissue
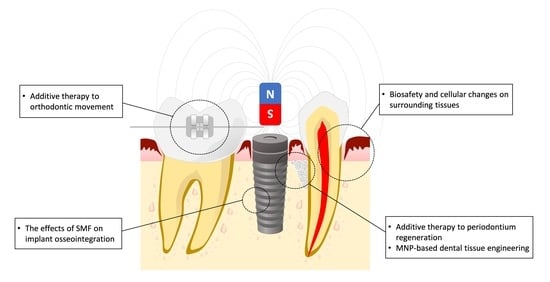
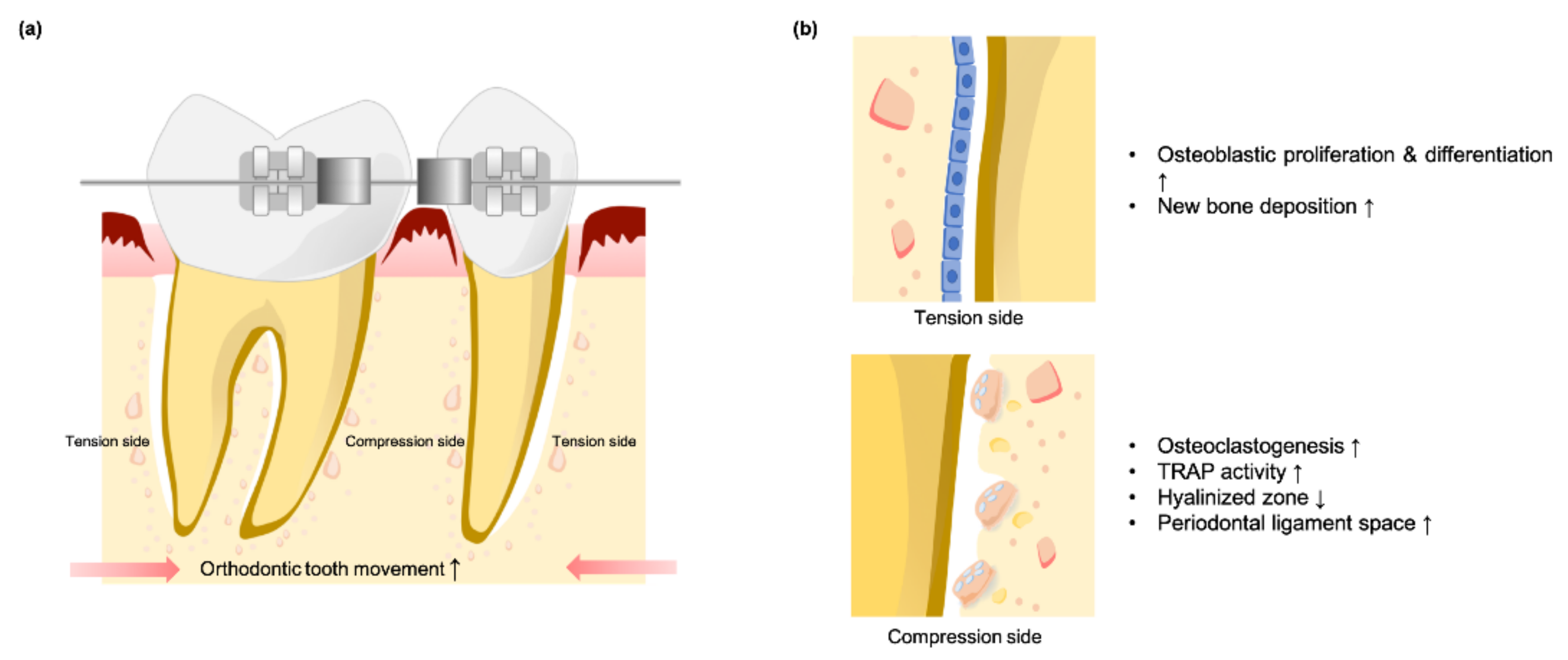
4. SMFs May Recruit and Enhance Osteoclastic Activity during Orthodontic Tooth Movement
5. Biological Effects of Oral Tissue-Derived Cell Interactions with SMF
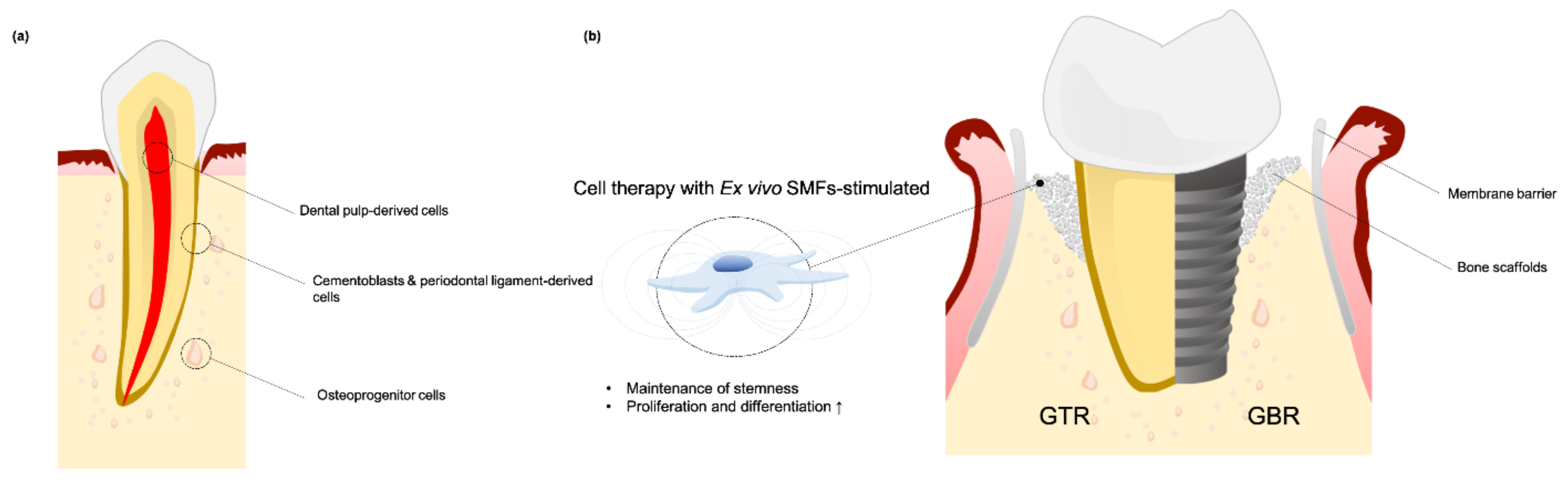
5.1. SMFs Regulate Cell Fate for GBR and GTR
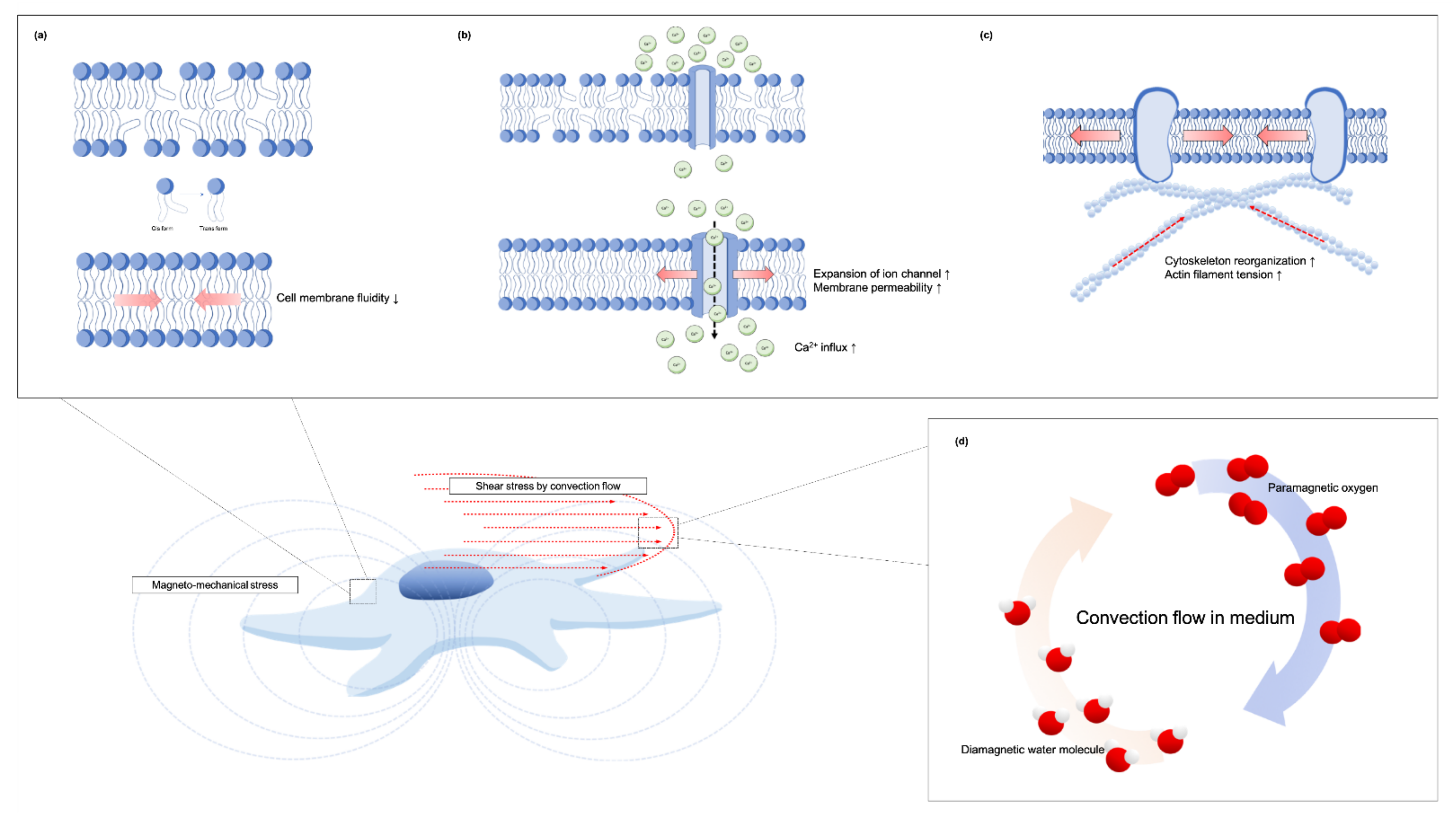
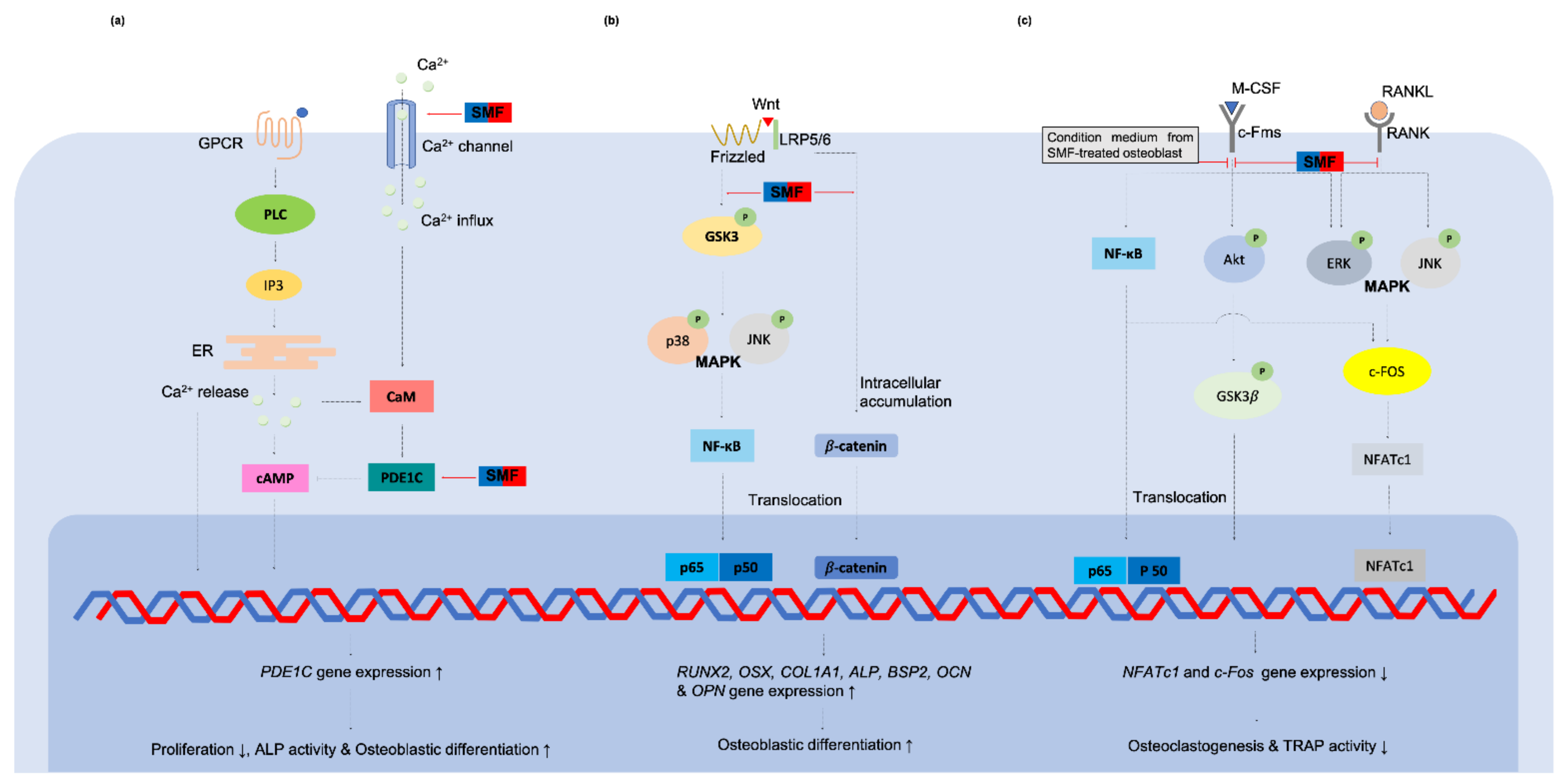
The Subcellular changed and the Putative Mechanisms of SMFs to the Oral Tissue-Derived Cells
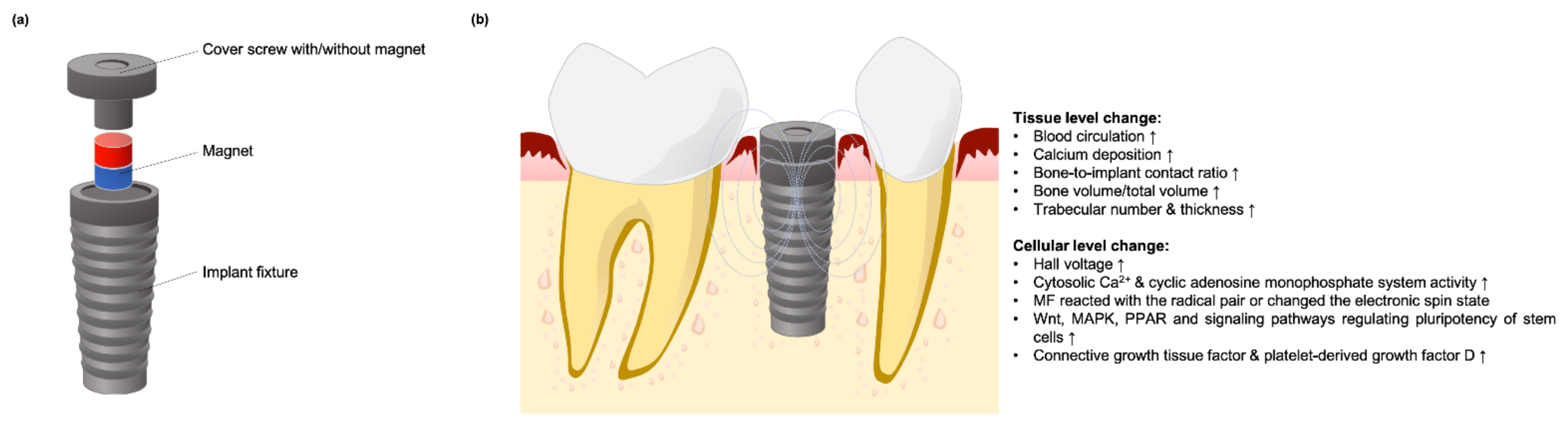
5.2. Using SMF to Enhance Dental Implant Osteointegration
Mechanisms of SMF Stimulation on Osseointegration
6. Synergistic Effects of SPIONs and SMFs Substantially Enhance Tissue Regeneration
6.1. Strategies for Using SMFs on Labeled Cells
6.2. Magnetic Biomaterials for Oral Tissue Engineering
6.3. Strategies of SPIONs-Incorporated Materials in Implant Dentistry
6.4. Putative Mechanism of Cellular Bioeffect through Interactions between SPIONs and Externally Applied Magnetic Field
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bondemark, L.; Kurol, J.; Wisten, Å. Extent and flux density of static magnetic fields generated by orthodontic samarium-cobalt magnets. Am. J. Orthod. Dentofac. Orthop. 1995, 107, 488–496. [Google Scholar] [CrossRef]
- Riley, M.A.; Walmsley, A.D.; Harris, I.R. Magnets in prosthetic dentistry. J. Prosthet. Dent. 2001, 86, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Bondemark, L.; Hurol, J.; Wennberg, A. Biocompatibility of new, clinically used, and recycled orthodontic samarium-cobalt magnets. Am. J. Ortho. Dentofac. Orthop. 1994, 105, 568–574. [Google Scholar] [CrossRef]
- Jena, A.K.; Duggal, R.; Batra, P. Magnet as a dental material—an overview. Trends Biomater. Artif. Organs. 2003, 16, 73–115. [Google Scholar]
- International Commission on Non-Ionizing Radiation Protection. Guidelines on limits of exposure to static magnetic fields. Health Phys. 2009, 96, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Dini, L.; Abbro, L. Bioeffects of moderate-intensity static magnetic fields on cell cultures. Micron 2005, 36, 195–217. [Google Scholar] [CrossRef]
- Miyakoshi, J. Effects of static magnetic fields at the cellular level. Prog. Biophys. Mol. Biol. 2005, 87, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Darendeliler, M.A.; Darendeliler, A.; Sinclair, P.M. Effects of static magnetic and pulsed electromagnetic fields on bone healing. Int. J. Adult Orthodon. Orthognath. Surg. 1997, 12, 43–53. [Google Scholar] [PubMed]
- Xu, C.; Fan, Z.; Chao, Y.-L.; Du, L.; Zhang, F.-Q. Magnetic fields of 10mT and 120mT change cell shape and structure of F-actins of periodontal ligament cells. Bioelectrochemistry 2008, 72, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Bondemark, L.; Kurol, J.; Wennberg, A. Orthodontic rare earth magnets—In vitro assessment of cytotoxicity. Br. J. Orthod. 1994, 21, 335–341. [Google Scholar] [CrossRef]
- Marycz, K.; Kornicka, K.; Röcken, M. Static magnetic field (SMF) as a regulator of stem cell fate—New perspectives in regenerative medicine arising from an underestimated tool. Stem Cell Rev. Rep. 2018, 14, 785–792. [Google Scholar] [CrossRef]
- Trubiani, O.; Marconi, G.D.; Pierdomenico, S.D.; Piattelli, A.; Diomede, F.; Pizzicannella, J. Human oral stem cells, biomaterials and extracellular vesicles: A promising tool in bone tissue repair. Int. J. Mol. Sci. 2019, 20, 4987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.S.; Marconi, G.D.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral bone tissue regeneration: Mesenchymal stem cells, secretome, and biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.T.; Kim, J.H.; Seonwoo, H.; Son, H.M.; Baik, S.J.; Park, J.Y.; Jeon, S.H.; Kim, E.S.; Choung, Y.H.; Cho, C.S.; et al. In vitro effects of electromagnetic field stimulation on cells in tissue engineering. J. Tissue Eng. Regen. Med. 2009, 6, 675–684. [Google Scholar]
- Marędziak, M.; Marycz, K.; Lewandowski, D.; Siudzińska, D.; Śmieszek, A. Static magnetic field enhances synthesis and secretion of membrane-derived microvesicles (MVs) rich in VEGF and BMP-2 in equine adipose-derived stromal cells (EqASCs)—A new approach in veterinary regenerative medicine. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Martino, C.F.; Perea, H.; Hopfner, U.; Ferguson, V.L.; Wintermantel, E. Effects of weak static magnetic fields on endothelial cells. Bioelectromagnetics 2010, 31, 296–301. [Google Scholar] [CrossRef]
- Tenuzzo, B.; Chionna, A.; Panzarini, E.; Lanubile, R.; Tarantino, P.; Jeso, B.D.; Dwikat, M.; Dini, L. Biological effects of 6 mT static magnetic fields: A comparative study in different cell types. Bioelectromagnetics 2006, 27, 560–577. [Google Scholar] [CrossRef]
- Kim, E.-C.; Leesungbok, R.; Lee, S.-W.; Lee, H.-W.; Park, S.H.; Mah, S.-J.; Ahn, S.-J. Effects of moderate intensity static magnetic fields on human bone marrow-derived mesenchymal stem cells. Bioelectromagnetics 2015, 36, 267–276. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, X.; Yang, X.; Zhang, X. Cell type- and density-dependent effect of 1 T static magnetic field on cell proliferation. Oncotarget 2017, 8, 13126–13141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, Y.; Kawasumi, M.; Saito, M. Effect of static magnetic field on cell migration. IEEJ Trans. Electron. Inf. Syst. 2004, 124, 1719–1724. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, L.; Chen, L.; Jiang, J.; Zhou, X.; Wang, M.; Fan, Y. Static magnetic field regulates proliferation, migration, differentiation, and YAP/TAZ activation of human dental pulp stem cells. J. Tissue Eng. Regen. Med. 2018, 12, 2029–2040. [Google Scholar] [CrossRef] [PubMed]
- Kotani, H.; Kawaguchi, H.; Shimoaka, T.; Iwasaka, M.; Ueno, S.; Ozawa, H.; Nakamura, K.; Hoshi, K. Strong static magnetic field stimulates bone formation to a definite orientation in vitro and in vivo. J. Bone Miner. Res. 2002, 17, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.-H.; Ou, K.-L.; Lee, S.-Y.; Lin, C.-T.; Chang, W.-J.; Chen, C.-C.; Huang, H.-M. Static magnetic fields promote osteoblast-like cells differentiation via increasing the membrane rigidity. Ann. Biomd. Eng. 2007, 35, 1932–1939. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Chang, J.-C. The static magnetic field accelerates the osteogenic differentiation and mineralization of dental pulp cells. Cytotechnology 2010, 62, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Marędziak, M.; Śmieszek, A.; Tomaszewski, K.A.; Lewandowski, D.; Marycz, K. The effect of low static magnetic field on osteogenic and adipogenic differentiation potential of human adipose stromal/stem cells. J. Magn. Magn. Mater. 2016, 398, 235–245. [Google Scholar] [CrossRef]
- Cheng, K.; Li, T.-S.; Malliaras, K.; Davis, D.R.; Zhang, Y.; Marbán, E. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ. Res. 2010, 106, 1570–1581. [Google Scholar] [CrossRef] [Green Version]
- Vandergriff, A.C.; Hensley, T.M.; Henry, E.T.; Shen, D.; Anthony, S.; Zhang, J.; Cheng, K. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials 2014, 35, 8528–8539. [Google Scholar] [CrossRef]
- Tukmachev, D.; Lunov, O.; Zablotskii, V.; Dejneka, A.; Babic, M.; Syková, E.; Kubinová, Š. An effective strategy of magnetic stem cell delivery for spinal cord injury therapy. Nanoscale 2015, 7, 3954–3958. [Google Scholar] [CrossRef]
- Fayol, D.; Frasca, G.; Visage, C.L.; Gazeau, F.; Luciani, N.; Wilhelm, C. Use of magnetic forces to promote stem cell aggregation during differentiation, and cartilage tissue modeling. Adv. Mater. 2013, 25, 2611–2616. [Google Scholar] [CrossRef]
- Cai, Q.; Shi, Y.; Shan, D.; Jia, W.; Duan, S.; Deng, X.; Yang, X. Osteogenic differentiation of MC3T3-E1 cells on poly(L-lactide)/Fe3O4 nanofibers with static magnetic field exposure. Mater. Sci. Eng. C. Mater. Biol. Appl. 2015, 55, 166–173. [Google Scholar] [CrossRef]
- Goranov, V.; Shelyakova, T.; Santis, R.D.; Haranava, Y.; Makhaniok, A.; Gloria, A.; Tampieri, A.; Russo, A.; Kon, E.; Marcacci, M.; et al. 3D patterning of cells in magnetic scaffolds for tissue engineering. Sci. Rep. 2020, 10, 2289. [Google Scholar] [CrossRef] [Green Version]
- Bondemark, L.; Kurol, J.; Larsson, Å. Human dental pulp and gingival tissue after static magnetic field exposure. Eur. J. Orthod. 1995, 17, 85–91. [Google Scholar] [CrossRef]
- Noar, J.H.; Evans, R.D. Rare earth magnets in orthodontics: An overview. Br. J. Orth. 1999, 26, 29–37. [Google Scholar] [CrossRef]
- Nishida, M.; Tegawa, Y.; Kinouchi, Y. Comparison and evaluation of leakage flux on various types of dental magnetic attachment. In Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008. [Google Scholar] [CrossRef]
- Nishida, M.; Tegawa, Y.; Kinouchi, Y. Evaluation of leakage flux out of a dental magnetic attachment. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007. [Google Scholar] [CrossRef]
- Yagci, F.; Kesim, B. Cytotoxic and genotoxic effects on gingival fibroblasts from static magnetic fields produced by dental magnetic attachments. Gerodontology 2016, 33, 421–427. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.-J.; Guo, Y.; Weir, M.D.; Raynolds, M.A.; Gu, N.; Xu, H.H.K. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T.; Lunov, O.; Dejneka, A. How a high-gradient magnetic field could affect cell life. Sci. Rep. 2016, 6, 37407. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Zhao, Y.; Zhang, F.; Li, X.; Wang, L.; Weir, M.D.; Ma, J.; Reynolds, M.A.; Gu, N.; et al. Novel magnetic calcium phosphate-stem cell construct with magnetic field enhances osteogenic differentiation and bone tissue engineering. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 98, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zou, Q.; Man, Y.; Li, W. Synergistic effects of novel superparamagnetic/upconversion HA material and Ti/magnet implant on biological performance and long-term in vivo tracking. Small 2019, 15, e1901617. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T.; Dejneka, A. Cells in the non-uniform magnetic world: How cells respond to high-gradient magnetic fields. Bioessays 2018, 40, e1800017. [Google Scholar] [CrossRef]
- Guttal, S.S.; Nadiger, R.K.; Shetty, P. Cytotoxic effect of indigenously fabricated dental magnets for application in prosthodontics. J. Indian. Prosthodont. Soc. 2018, 18, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Donohue, V.E.; McDonald, F.; Evans, R. In vitro cytotoxicity testing of neodymium-iron-boron magnets. J. Appl. Biomater. 1995, 6, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yamaguchi, H.; Miyamoto, H.; Kinouchi, Y. Growth of human cultured cells exposed to a non-homogenous static magnetic field generated by Sm-Co magnets. Biochim. Biophys. Acta. 1992, 1136, 231–238. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hosokawa, K.; Soda, A.; Miyamoto, H.; Kinouchi, Y. Effects of seven months’ exposure to a static 0.2 T magnetic field on growth and glycolytic activity of human gingival fibroblasts. Biochim. Biophys. Acta. 1993, 1156, 302–306. [Google Scholar] [CrossRef]
- McDonald, F. Effect of static magnetic fields on osteoblasts and fibroblasts in vitro. Bioelectromagnetics 1993, 14, 187–196. [Google Scholar] [CrossRef]
- Cerny, R. The reaction of dental tissues to magnetic fields. Aust. Dent. J. 1980, 25, 264–268. [Google Scholar] [CrossRef]
- Linder-Aronson, A.; Lindskog, S.; Rygh, P. Orthodontic magnets: Effects on gingival epithelium and alveolar bone in monkeys. Eur. J. Orthod. 1992, 14, 255–263. [Google Scholar] [CrossRef]
- Linder-Aronson, A.; Forsberg, C.-M.; Rygh, P.; Lindskog, S. Tissue response to space closure in monkeys: A comparison of orthodontic magnets and superelastic coil springs. Eur. J. Orthod. 1996, 18, 581–588. [Google Scholar] [CrossRef]
- Bondemark, L.; Kurol, J.; Larsson, Å. Long-term effects of orthodontic magnets on human buccal mucosa—A clinical, histological and immunohistochemical study. Eur. J. Orthod. 1998, 20, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Shan, Y.; Han, H.; Zhu, J.; Yan, X.; Zhang, X.; Long, H.; Jian, F.; Li, X.; Wang, Y.; Lai, W. The effects of static magnetic field on orthodontic tooth movement in mice. Bioelectromagnetics 2021, 42, 398–406. [Google Scholar] [CrossRef]
- Darendeliler, M.A.; Sinclair, P.M.; Kusy, R.P. The effects of samarium-cobalt magnets and pulsed electromagnetic fields on tooth movement. Am. J. Orthod. Dentofacial. Orthop. 1995, 107, 578–588. [Google Scholar] [CrossRef]
- Sakata, M.; Yamamoto, Y.; Imamura, N.; Nakata, S.; Nakasima, A. The effects of static magnetic field on orthodontic tooth movement. J. Orthod. 2008, 35, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Tengku, B.S.; Joseph, B.K.; Harbrow, D.; Taverne, A.A.R.; Symons, A.L. Effect of a static magnetic field on orthodontic tooth movement in the rat. Eur. J. Orthod. 2000, 22, 475–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, G.; Pagni, G.; Rasperini, G. Surgical approaches based on biological objectives: GTR versus GBR techniques. Int. J. Dent. 2013, 2013, 521547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, Y.; Ohsaki, Y.; Goto, T.; Nakasima, A.; Iijima, T. Effects of static magnetic fields on bone formation in rat osteoblast cultures. J. Dent. Res. 2003, 82, 962–966. [Google Scholar] [CrossRef]
- Feng, S.-W.; Lo, Y.-J.; Chang, W.-J.; Lin, C.-T.; Lee, S.-Y.; Abiko, Y.; Huang, H.-M. Static magnetic field exposure promotes differentiation of osteoblastic cells grown on the surface of a poly-L-lactide substrate. Med. Biol. Eng. Comput. 2010, 48, 793–798. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Lew, W.-Z.; Feng, S.-W.; Wu, C.-L.; Wang, H.-H.; Hsieh, S.-C.; Huang, H.-M. Static magnetic field-enhanced osteogenic differentiation of human umbilical cord-derived mesenchymal stem cells via matrix vesicle secretion. Int. J. Radiat. Biol. 2020, 96, 1207–1217. [Google Scholar] [CrossRef]
- Kim, E.-C.; Park, J.; Kwon, I.K.; Lee, S.-W.; Park, S.-W.; Ahn, S.-J. Static magnetic fields promote osteoblastic/cementoblastic differentiation in osteoblasts, cementoblasts, and periodontal ligament cells. J. Periodontal. Implant. Sci. 2017, 47, 273–291. [Google Scholar] [CrossRef] [Green Version]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 9 (Suppl. S1), 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.-C.; Park, J.; Noh, G.; Park, S.-J.; Noh, K.; Kwon, I.-K.; Ahn, S.-J. Effects of moderate intensity static magnetic fields on osteoclastic differentiation in mouse bone marrow cells. Bioelectromagnetics 2018, 39, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chao, Y.-L.; Du, L. Study of biologic effects of simulating static magnetic field of magnetic attachment on human periodontal ligament fibroblasts. West China J. Stomatol. 2007, 25, 316–319. [Google Scholar]
- Lew, W.-Z.; Huang, Y.-C.; Huang, K.-Y.; Lin, C.-T.; Tsai, M.-T.; Huang, H.-M. Static magnetic fields enhance dental pulp stem cell proliferation by activating the p38 mitogen-activated protein kinase pathway as its putative mechanism. J. Tissue Eng. Regen Med. 2018, 12, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Lew, W.-Z.; Feng, S.-W.; Lin, C.-T.; Huang, H.-M. Use of 0.4-Tesla static magnetic field to promote reparative dentine formation of dental pulp stem cells through activation of p38 MAPK signaling pathway. Int. Endod. J. 2019, 52, 28–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, A.D. Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell Biochem. Biophys. 2003, 39, 163–173. [Google Scholar] [CrossRef]
- Rosen, A.D. Studies on the effect of static magnetic fields on biological systems. PIERS Online 2010, 6, 133–136. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Sarje, A.; Che, P.-L.; Yarema, K.J. Moderate strength (0.23-0.28 T) static magnetic fields (SMF) modulate signaling and differentiation in human embryonic cells. BMC Genomics. 2009, 10, 356. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Che, P.-L.; Du, J.; Ha, B.; Yarema, K.J. Static magnetic field exposure reproduces cellular effects of the Parkinson’s disease drug candidate ZM241385. PLoS ONE 2010, 5, e13883. [Google Scholar] [CrossRef]
- Poinapen, D.; Toppozini, L.; Dies, H.; Brown, D.C.W.; Rheinstädter, M.C. Static magnetic fields enhance lipid order in native plant plasma membrane. Soft Matter. 2013, 29, 6804–6813. [Google Scholar] [CrossRef]
- Fanelli, C.; Coppola, S.; Barone, R.; Colussi, C.; Gualandi, G.; Volpe, P.; Ghibelli, L. Magnetic fields increase cell survival by inhibiting apoptosis via modulation of Ca2+ influx. FASEB J. 1999, 13, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yang, W.; Peng, S.; Gao, C.; Guo, W.; Lai, Y.; Feng, P. Physical stimulations and their osteogenesis-inducing mechanisms. Int. J. Bioprint. 2018, 4, 138. [Google Scholar] [CrossRef] [PubMed]
- Chionna, A.; Dwikat, M.; Panzarini, E.; Tenuzzo, B.; Carlà, E.C.; Verri, T.; Pagliara, P.; Abbro, L.; Dini, L. Cell shape and plasma membrane alterations after static magnetic fields exposure. Eur. J. Histochem. 2003, 47, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-C.; Lee, S.-Y.; Chen, C.-A.; Ling, C.-T.; Chen, C.-C.; Huang, H.-M. The role of the calmodulin-dependent pathway in static magnetic field-induced mechanotransduction. Bioelectromagnetics 2010, 31, 255–261. [Google Scholar] [CrossRef]
- Zablotskii, V.; Lunov, O.; Polyakova, T.; Kubinova, S.; Dejneka, A. Cell electrophysiology and mechanics in high-gradient magnetic fields. In Proceedings of the 2017 IEEE International Magnetics Conference (INTERMAG), Dublin, Ireland, 24–28 April 2017. [Google Scholar] [CrossRef]
- Iwasaka, W.; Yamamoto, K.; Ando, J.; Ueno, S. Verification of magnetic field gradient effects on medium convection and cell adhesion. J. Appl. Phys. 2003, 93, 6715. [Google Scholar] [CrossRef]
- Morarka, A. Detection and optical imaging of induced convection under the action of static gradient magnetic field in a non-conducting diamagnetic fluid. arXiv 2016, arXiv:1608.03973. [Google Scholar]
- Barak, S.; Matalon, S.; Dolkart, O.; Zavan, B.; Mortellaro, C.; Piattelli, A. Miniaturized electromagnetic device abutment improves stability of the dental implants. J. Craniofac. Surg. 2019, 30, 1055–1057. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Zhang, S.; Zhang, M.; Zhou, Z.; Zhang, X.; Li, W.; Cai, H.; Zhao, B.C.; Lee, E.-S.; Jiang, H.B. Effects of physical stimulation in the field of oral health. Scanning 2021, 2021, 5517567. [Google Scholar] [CrossRef]
- Leesungbok, R.; Ahn, S.-J.; Lee, S.-W.; Park, G.-H.; Kang, J.-S.; Choi, J.-J. The effects of a static magnetic field on bone formation around a sandblasted, large-grit, acid-etched-treated titanium implant. J. Oral. Implantol. 2013, 39, 248–255. [Google Scholar] [CrossRef]
- Kim, E.-C.; Leesungbok, R.; Lee, S.-W.; Hong, J.-Y.; Ko, E.-J.; Ahn, S.-J. Effects of static magnetic fields on bone regeneration of implants in the rabbit: Micro-CT, histologic, microarray, and real-time PCR analyses. Clin. Oral. Implants Res. 2017, 28, 396–405. [Google Scholar] [CrossRef]
- Naito, Y.; Yamada, S.; Jinno, Y.; Arai, K.; Galli, S.; Ichikawa, T.; Jimbo, R. Bone-forming effect of a static magnetic field in rabbit femurs. Int. J. Periodontics Restor. Dent. 2019, 39, 259–264. [Google Scholar] [CrossRef]
- Gujjalapudi, M.; Anam, C.; Mamidi, P.; Chiluka, R.; Kumar, A.G.; Bibinagar, R. Effect of magnetic field on bone healing around endosseous implants—an In Vitro study. J. Clin. Diagn. Res. 2016, 10, ZF01–ZF04. [Google Scholar] [CrossRef] [PubMed]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic iron oxide nanoparticles—Current and prospective medical applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Chen, H.; Zhang, F.; Wang, L.; Chen, B.; Reynolds, M.A.; Ma, J.; Schneider, A.; Gu, N.; Xu, H.H.K. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Zhao, Y.; Zhang, F.; Chen, B.; Hu, X.; Weir, M.D.; Schneider, A.; Jia, L.; Gu, N.; Xu, H.H.K. Iron oxide nanoparticles in liquid or powder form enhanced osteogenesis via stem cells on injectable calcium phosphate scaffold. Nanomedicine 2019, 21, 102069. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, G.; Wang, X.; Jiang, L.; Jiang, F.; Li, G.; Zhang, Z.; Jiang, X. Magnetically controlled growth-factor-immobilized multilayer cell sheets for complex tissue regeneration. Adv. Mater. 2017, 29, 1703795. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-H.; Lee, Y.-C.; Hung, C.-Y.; Yang, P.-J.; Lai, P.-C.; Feng, S.-W. Three-dimensional spheroid culture enhances multipotent differentiation and stemness capacities of human dental pulp-derived mesenchymal stem cells by modulating MAPK and NF-ĸB signaling pathways. Stem Cell Rev. Rep. 2021, 1–17, Online ahead of print. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Q.; Liu, Y. Polarization of stem cells directed by magnetic field-manipulated supramolecular polymeric nanofibers. ACS Appl. Mater. Interfaces 2021, 13, 9580–9588. [Google Scholar] [CrossRef]
- Xia, Y.; Guo, Y.; Yang, Z.; Chen, H.; Ren, K.; Weir, M.D.; Chow, L.C.; Raynolds, M.A.; Zhang, F.; Gu, N.; et al. Iron oxide nanoparticle-calcium phosphate cement enhanced the osteogenic activities of stem cells through WNT/β-catenin signaling. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 104, 109955. [Google Scholar] [CrossRef]
- Yun, H.-M.; Lee, E.-S.; Kim, M.-J.; Kim, J.-J.; Lee, J.-H.; Lee, H.-H.; Park, K.-R.; Yi, J.-K.; Kim, H.-W.; Kim, E.-C. Magnetic nanocomposite scaffold-induced stimulation of migration and odontogenesis of human dental pulp cells through integrin signaling pathways. PLoS ONE 2015, 10, e0138614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Ren, S.; Zhang, X.; Yu, Y.; Liu, C.; Yang, J.; Miao, L. Safety and efficacy of PLGA(Ag-Fe3O4)-coated dental implants in inhibiting bacteria adherence and osteogenic inducement under a magnetic field. Int. J. Nanomedicine 2018, 13, 3751–3762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wu, J.; Li, D.; Zou, Q.; Man, Y.; Zou, L.; Li, W. Pro-osteogenesis and in vivo tracking investigation of a dental implantation system comprising novel mTi implant and HYH-Fe particles. Bioact. Mater. 2021, 6, 2658–2666. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, C.; Wang, P.; Song, T. Mechanism of cellular effect directly induced by magnetic nanoparticles under magnetic fields. J. Nanomater. 2017, 2017, 1564634. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lew, W.-Z.; Feng, S.-W.; Lee, S.-Y.; Huang, H.-M. The Review of Bioeffects of Static Magnetic Fields on the Oral Tissue-Derived Cells and Its Application in Regenerative Medicine. Cells 2021, 10, 2662. https://doi.org/10.3390/cells10102662
Lew W-Z, Feng S-W, Lee S-Y, Huang H-M. The Review of Bioeffects of Static Magnetic Fields on the Oral Tissue-Derived Cells and Its Application in Regenerative Medicine. Cells. 2021; 10(10):2662. https://doi.org/10.3390/cells10102662
Chicago/Turabian StyleLew, Wei-Zhen, Sheng-Wei Feng, Sheng-Yang Lee, and Haw-Ming Huang. 2021. "The Review of Bioeffects of Static Magnetic Fields on the Oral Tissue-Derived Cells and Its Application in Regenerative Medicine" Cells 10, no. 10: 2662. https://doi.org/10.3390/cells10102662
APA StyleLew, W.-Z., Feng, S.-W., Lee, S.-Y., & Huang, H.-M. (2021). The Review of Bioeffects of Static Magnetic Fields on the Oral Tissue-Derived Cells and Its Application in Regenerative Medicine. Cells, 10(10), 2662. https://doi.org/10.3390/cells10102662