Influence of Age and Breed on Bovine Ovarian Capillary Blood Supply, Ovarian Mitochondria and Telomere Length
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blood Collection and Further Processing
2.3. Ovary Sample Collection
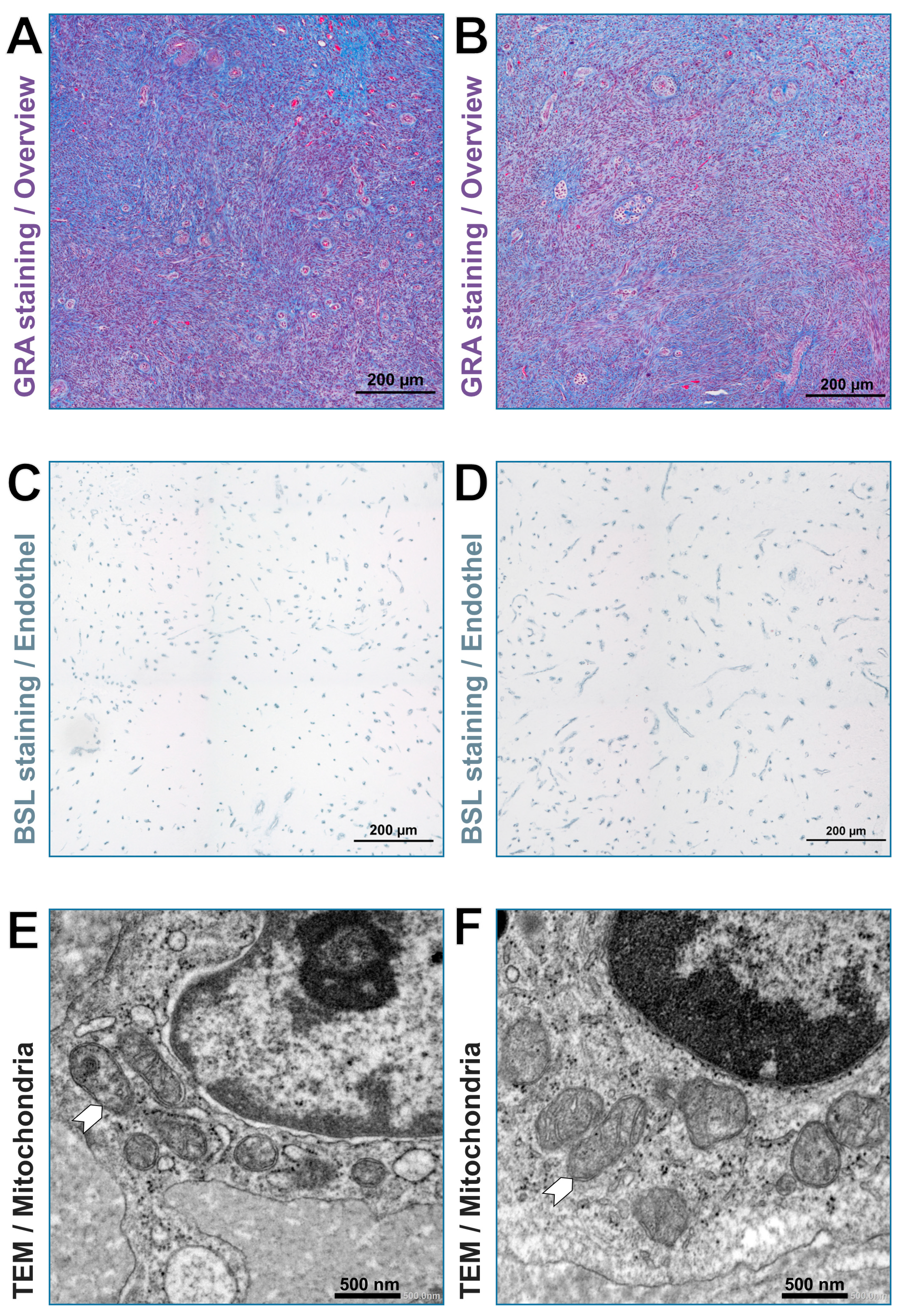
2.4. Sample Preparation for Light and Transmission Electron Microscopy
2.5. Capillary Measurement
2.6. Mitochondria Measurement
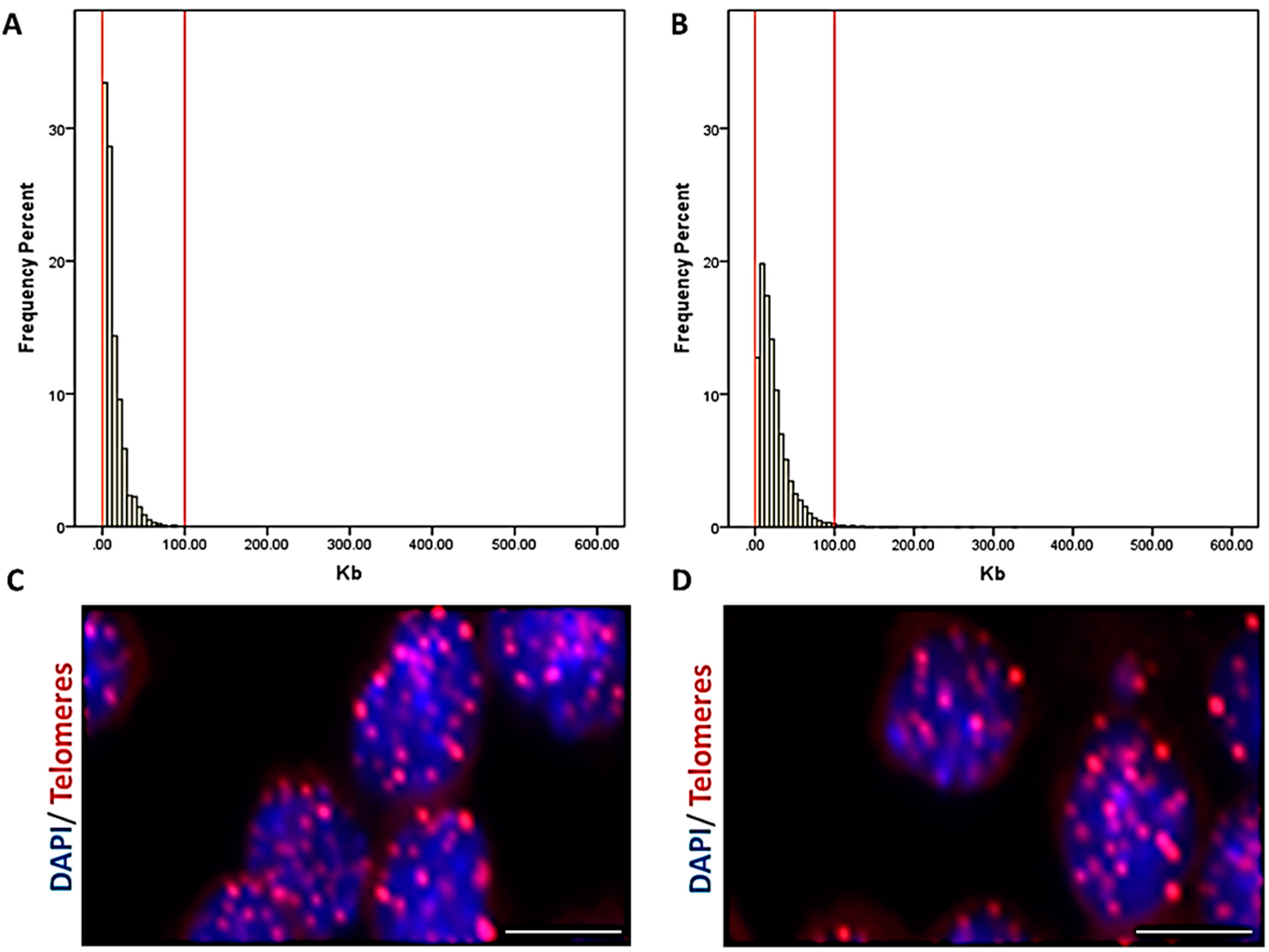
2.7. High-Throughput Q-FISH for Telomere Length Measurement
2.8. Statistics
- Influence of the size (area) of the tertiary follicle on the number of capillaries per area
- Influence of age on the average distance between two capillaries in the tertiary follicle, as well as on the average area per capillary, the average diameter per capillary and on the proportion of the lumen area of the capillaries in relation to the total measuring area in areas without functional structures
3. Results
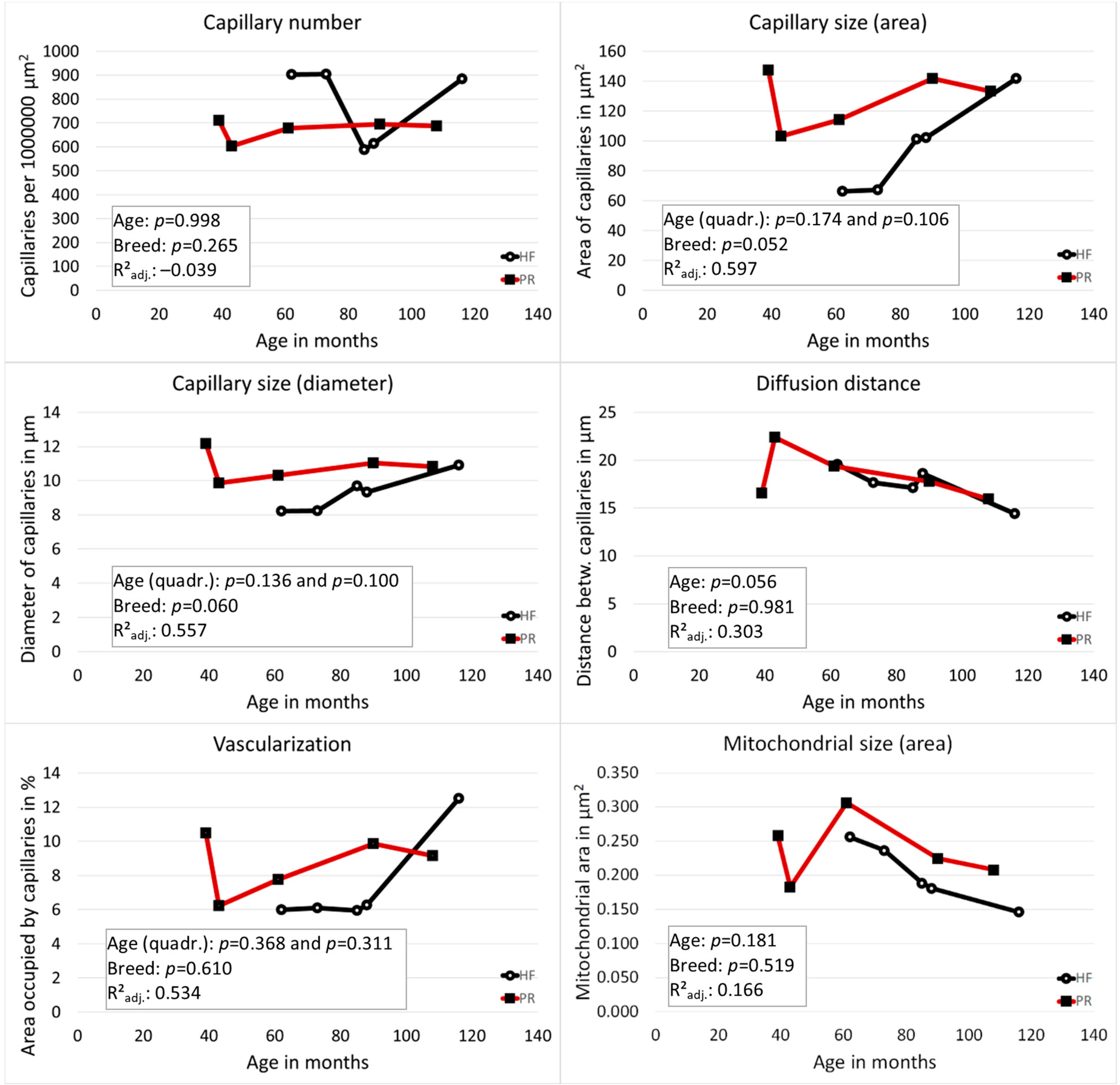
3.1. Vascularization of the Tertiary Follicles Was Strongly Dependent on the Follicle Size
3.2. Vascularization of the Zona Parenchymatosa without Functional Structures: PR Had Larger Capillaries Than HF and the Distance between Blood Capillaries Became Smaller with Increasing Age in Both Breeds
3.3. No Correlation between the Vascularization Measurements of the Two Areas
3.4. The Size of Mitochondria Decreased with Increasing Age
3.5. The Length of Telomeres Decreased with Advancing Age
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobson, H.; Smith, R.; Royal, M.D.; Knight, C.; Sheldon, I.M. The High-producing Dairy Cow and its Reproductive Performance. Reprod. Domest. Anim. 2007, 42, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Raboisson, D.; Mounié, M.; Maigné, E. Diseases, reproductive performance, and changes in milk production associated with subclinical ketosis in dairy cows: A meta-analysis and review. J. Dairy Sci. 2014, 97, 7547–7563. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.; Jensen, C.; Ostergaard, S.; Weisbjerg, M.; Aaes, O.; Nielsen, N. Feeding, production, and efficiency of Holstein-Friesian, Jersey, and mixed-breed lactating dairy cows in commercial Danish herds. J. Dairy Sci. 2015, 98, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, M.; Llewellyn, S.; Fitzpatrick, R.; Kenny, D.; Murphy, J.J.; Patton, J.; Wathes, D.C. Negative energy balance in dairy cows is associated with specific changes in IGF-binding protein expression in the oviduct. REPRODUCTION 2007, 135, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Martens, H. Leistung und Gesundheit von Milchkühen: Bedeutung von Genetik (Ursache) und Management (Wirkung). Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2016, 44, 253–258. [Google Scholar] [CrossRef]
- Ansari-Lari, M.; Mohebbi-Fani, M.; Rowshan-Ghasrodashti, A. Causes of culling in dairy cows and its relation to age at culling and interval from calving in Shiraz, Southern Iran. Vet. Res. Forum: Int. Q. J. 2012, 3, 233–237. [Google Scholar]
- Goto, A.; Nakada, K.; Katamoto, H. The association of culling and death rate within 30 days after calving with productivity or reproductive performance in dairy herds in Fukuoka, Southern Japan. J. Veter- Med. Sci. 2016, 78, 587–592. [Google Scholar] [CrossRef][Green Version]
- Kaessmeyer, S.; Plendl, J. Angiogenesis and vasculogenesis in the corpus luteum in vitro. Clin. Hemorheol. Microcirc. 2009, 41, 83–101. [Google Scholar] [CrossRef]
- Safayi, S.; Theil, P.; Hou, L.; Engbæk, M.; Nørgaard, J.V.; Sejrsen, K.; Nielsen, M. Continuous lactation effects on mammary remodeling during late gestation and lactation in dairy goats. J. Dairy Sci. 2010, 93, 203–217. [Google Scholar] [CrossRef]
- Schoen, K.; Hirschberg, R.M.; Plendl, J.; Kaessmeyer, S. Identification of CD133-, CD34- and KDR-positive cells in the bovine ovary: A new site of vascular wall resident endothelial progenitor cells. Clin. Hemorheol. Microcirc. 2012, 52, 67–84. [Google Scholar] [CrossRef]
- Kaessmeyer, S.; Huenigen, H.; Al Masri, S.; Dieckhoefer, P.; Richardson, K.; Plendl, J. Corpus luteal angiogenesis in a high milk production dairy breed differs from that of cattle with lower milk production levels. Vet. Med. 2016, 61, 497–503. [Google Scholar] [CrossRef]
- Shimada, T.; Takeshita, Y.; Murohara, T.; Sasaki, K.-I.; Egami, K.; Shintani, S.; Katsuda, Y.; Ikeda, H.; Nabeshima, Y.-I.; Imaizumi, T. Angiogenesis and Vasculogenesis Are Impaired in the Precocious-Aging klotho Mouse. Circulation 2004, 110, 1148–1155. [Google Scholar] [CrossRef]
- Seo, B.J.; Yoon, S.H.; Do, J.T. Mitochondrial Dynamics in Stem Cells and Differentiation. Int. J. Mol. Sci. 2018, 19, 3893. [Google Scholar] [CrossRef]
- Zamponi, N.; Zamponi, E.; Cannas, S.A.; Billoni, O.V.; Helguera, P.R.; Chialvo, D. Mitochondrial network complexity emerges from fission/fusion dynamics. Sci. Rep. 2018, 8, 363. [Google Scholar] [CrossRef]
- Morsci, N.; Hall, D.H.; Driscoll, M.; Sheng, Z.-H. Age-Related Phasic Patterns of Mitochondrial Maintenance in Adult Caenorhabditis elegans Neurons. J. Neurosci. 2016, 36, 1373–1385. [Google Scholar] [CrossRef]
- Kordowitzki, P.; Hamdi, M.; Derevyanko, A.; Rizos, D.; Blasco, M.A. The effect of rapamycin on bovine oocyte maturation success and metaphase telomere length maintenance. Aging 2020, 12, 7576–7584. [Google Scholar] [CrossRef]
- Fulle, S.; Centurione, L.; Mancinelli, R.; Sancilio, S.; Manzoli, F.A.; Di Pietro, R. Stem Cell Ageing and Apoptosis. Curr. Pharm. Des. 2012, 18, 1694–1717. [Google Scholar] [CrossRef]
- In, M.K.; Richardson, K.C.; Loewa, A.; Hedtrich, S.; Kaessmeyer, S.; Plendl, J. Histological and functional comparisons of four anatomical regions of porcine skin with human abdominal skin. Anat. Histol. Embryol. 2019, 48, 207–217. [Google Scholar] [CrossRef]
- Kordowitzki, P.; De Silanes, I.L.; Guío-Carrión, A.; Blasco, M.A. Dynamics of telomeric repeat-containing RNA expression in early embryonic cleavage stages with regards to maternal age. Aging 2020, 12, 15906–15917. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Okuda, K. Multiple roles of hypoxia in bovine corpus luteum. J. Reprod. Dev. 2020, 66, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Mattar, D.; Samir, M.; Laird, M.; Knight, P.G. Modulatory effects of TGF-β1 and BMP6 on thecal angiogenesis and steroidogenesis in the bovine ovary. Reproduction 2020, 159, 397–408. [Google Scholar] [CrossRef]
- Chen, J.; Lippo, L.; Labella, R.; Tan, S.L.; Marsden, B.D.; Dustin, M.L.; Ramasamy, S.K.; Kusumbe, A.P. Decreased blood vessel density and endothelial cell subset dynamics during ageing of the endocrine system. EMBO J. 2020, 40, e105242. [Google Scholar] [CrossRef] [PubMed]
- McFee, R.M.; Rozell, T.G.; Cupp, A.S. The balance of proangiogenic and antiangiogenic VEGFA isoforms regulate follicle development. Cell Tissue Res. 2012, 349, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.M. Regulation of the ovarian follicular vasculature. Reprod. Biol. Endocrinol. 2006, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Braw-Tal, R.; Pen, S.; Roth, Z. Ovarian cysts in high-yielding dairy cows. Theriogenology 2009, 72, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Kiss, T.; Wren, J.D.; Giles, C.B.; Griffin, C.T.; Murfee, W.L.; Pacher, P.; Csiszar, A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018, 15, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Rosas, F.; Gaytan, M.; Morales, C.; Gomez, R.; Gaytan, F. Superficial ovarian cortex vascularization is inversely related to the follicle reserve in normal cycling ovaries and is increased in polycystic ovary syndrome. Hum. Reprod. 2009, 24, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, T.; Leroy, J.; Dewulf, J.; Duchateau, L.; Coryn, M.; de Kruif, A.; Opsomer, G. Hormonal and Metabolic Profiles of High-yielding Dairy Cows Prior to Ovarian Cyst formation or First Ovulation Post Partum. Reprod. Domest. Anim. 2005, 40, 460–467. [Google Scholar] [CrossRef]
- Mimoune, N.; Kaidi, R.; Azzouz, M.Y.; Zenia, S.; Benaissa, M.H.; England, G. Sütçü İneklerde Ovaryum Kistlerinin Tanısı ve Metabolik Profili Üzerine Bir Çalışma. Kafkas Univ. Veter- Fak. Derg. 2017. [Google Scholar] [CrossRef]
- Sharma, A.; Smith, H.; Yao, P.; Mair, W.B. Causal roles of mitochondrial dynamics in longevity and healthy aging. EMBO Rep. 2019, 20, e48395. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, P.; Lu, X.; Hsueh, B.; Billig, F.M.; Yanez, L.Z.; Tomer, R.; Boerboom, D.; Carmeliet, P.; Deisseroth, K.; et al. CLARITY reveals dynamics of ovarian follicular architecture and vasculature in three-dimensions. Sci. Rep. 2017, 7, srep44810. [Google Scholar] [CrossRef] [PubMed]
- Biswas, L.; Chen, J.; De Angelis, J.; Chatzis, A.; Nanchahal, J.; Dustin, M.L.; Ramasamy, S.K.; Kusumbe, A.P. SUMIC: A Simple Ultrafast Multicolor Immunolabelling and Clearing Approach for Whole-Organ and Large Tissue 3D Imaging. bioRxiv 2021. [Google Scholar] [CrossRef]
- Macchiarelli, G.A.; Nottola, S.; Palmerini, M.G.; Bianchi, S.; Maione, M.; Lorenzo, C.; Stifano, G.; Di Marco, E.; Correr, S. Morphological expression of angiogenesis in the mammalian ovary as seen by SEM of corrosion casts. Ital. J. Anat. Embryol. Arch. Ital. Anat. Embriol. 2010, 115. [Google Scholar]
- Seo, A.Y.; Joseph, A.-M.; Dutta, D.; Hwang, J.C.Y.; Aris, J.P.; Leeuwenburgh, C. New insights into the role of mitochondria in aging: Mitochondrial dynamics and more. J. Cell Sci. 2010, 123, 2533–2542. [Google Scholar] [CrossRef]
- Hammadah, M.; Al Mheid, I.; Wilmot, K.; Ramadan, R.; Abdelhadi, N.; Alkhoder, A.; Obideen, M.; Pimple, P.M.; Levantsevych, O.; Kelli, H.M.; et al. Telomere Shortening, Regenerative Capacity, and Cardiovascular Outcomes. Circ. Res. 2017, 120, 1130–1138. [Google Scholar] [CrossRef]
- Balk, B.; Maicher, A.; Dees, M.; Klermund, J.; Luke-Glaser, S.; Bender, K.; Luke, B. Telomeric RNA-DNA hybrids affect telomere-length dynamics and senescence. Nat. Struct. Mol. Biol. 2013, 20, 1199–1205. [Google Scholar] [CrossRef]
- Arora, R.; Lee, Y.; Wischnewski, H.; Brun, C.M.; Schwarz, T.; Azzalin, C.M. RNaseH1 regulates TERRA-telomeric DNA hybrids and telomere maintenance in ALT tumour cells. Nat. Commun. 2014, 5, 5220. [Google Scholar] [CrossRef]
- Flynn, R.L.; Cox, K.E.; Jeitany, M.; Wakimoto, H.; Bryll, A.R.; Ganem, N.; Bersani, F.; Pineda, J.R.; Suvà, M.L.; Benes, C.H.; et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science 2015, 347, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Mossa, F.; Ireland, J.J. Physiology and Endocrinology Symposium:: Anti-Müllerian hormone: A biomarker for the ovarian reserve, ovarian function, and fertility in dairy cows. J. Anim. Sci. 2019, 97, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
| Breed | Age in Months | Capillaries per mm2 | Intercapillary Distance in µm | Capillary Diameter in µm | Capillary Lumen in µm2 | Area Occupied by Capillaries in % | Area of the Theca Interna Folliculi in mm2 |
|---|---|---|---|---|---|---|---|
| HF | 62 | 812 | 18.32 | 6.25 | 36.81 | 2.99 | 0.29 |
| HF | 73 | 1361 | 12.93 | 6.97 | 49.98 | 6.80 | 0.11 |
| HF | 85 | 1115 | 12.29 | 10.61 | 166.87 | 18.61 | 1.68 |
| HF | 88 | - | - | - | - | - | - |
| HF | 116 | 861 | 14.60 | 9.91 | 134.81 | 11.61 | 0.85 |
| PR | 39 | 1030 | 16.07 | 7.67 | 74.69 | 6.10 | 0.24 |
| PR | 43 | 817 | 15.71 | 9.51 | 166.70 | 13.62 | 0.32 |
| PR | 61 | 1174 | 12.40 | 8.95 | 91.60 | 10.75 | 0.34 |
| PR | 90 | 1200 | 11.45 | 9.58 | 104.29 | 12.51 | 0.40 |
| PR | 108 | 1662 | 10.99 | 8.29 | 74.96 | 12.45 | 0.10 |
| Breed | Age in Months | Capillaries per mm2 | Inter-Capillary Distance in µm | Capillary Diameter in µm | Capillary Lumen in µm2 | Area Occupied by Capillaries in % | Mito-Chondrial Size (Area in µm2) | Telomere Length in kb | Percentile of Short Telomeres |
|---|---|---|---|---|---|---|---|---|---|
| HF | 62 | 903 | 19.57 | 8.22 | 66.36 | 5.99 | 0.26 | 29.75 | 0.125 |
| HF | 73 | 905 | 17.64 | 8.23 | 67.33 | 6.09 | 0.24 | 23.33 | 0.147 |
| HF | 85 | 588 | 17.12 | 9.70 | 101.24 | 5.96 | 0.19 | 23.65 | 0.158 |
| HF | 88 | 614 | 18.59 | 9.33 | 102.19 | 6.27 | 0.18 | 22.78 | 0.147 |
| HF | 116 | 884 | 14.40 | 10.90 | 141.67 | 12.52 | 0.15 | 12.48 | 0.692 |
| PR | 39 | 711 | 16.55 | 12.17 | 147.34 | 10.48 | 0.26 | 34.46 | 0.073 |
| PR | 43 | 604 | 22.40 | 9.87 | 103.19 | 6.23 | 0.18 | 34.78 | 0.082 |
| PR | 61 | 679 | 19.37 | 10.31 | 114.25 | 7.76 | 0.31 | 27.30 | 0.088 |
| PR | 90 | 695 | 17.76 | 11.04 | 141.84 | 9.86 | 0.23 | 16.53 | 0.213 |
| PR | 108 | 687 | 15.95 | 10.82 | 133.30 | 9.16 | 0.21 | 16.54 | 0.373 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kordowitzki, P.; Merle, R.; Hass, P.-K.; Plendl, J.; Rieger, J.; Kaessmeyer, S. Influence of Age and Breed on Bovine Ovarian Capillary Blood Supply, Ovarian Mitochondria and Telomere Length. Cells 2021, 10, 2661. https://doi.org/10.3390/cells10102661
Kordowitzki P, Merle R, Hass P-K, Plendl J, Rieger J, Kaessmeyer S. Influence of Age and Breed on Bovine Ovarian Capillary Blood Supply, Ovarian Mitochondria and Telomere Length. Cells. 2021; 10(10):2661. https://doi.org/10.3390/cells10102661
Chicago/Turabian StyleKordowitzki, Paweł, Roswitha Merle, Pascal-Kolja Hass, Johanna Plendl, Juliane Rieger, and Sabine Kaessmeyer. 2021. "Influence of Age and Breed on Bovine Ovarian Capillary Blood Supply, Ovarian Mitochondria and Telomere Length" Cells 10, no. 10: 2661. https://doi.org/10.3390/cells10102661
APA StyleKordowitzki, P., Merle, R., Hass, P.-K., Plendl, J., Rieger, J., & Kaessmeyer, S. (2021). Influence of Age and Breed on Bovine Ovarian Capillary Blood Supply, Ovarian Mitochondria and Telomere Length. Cells, 10(10), 2661. https://doi.org/10.3390/cells10102661








