Neuron-Specific IMP2 Overexpression by Synapsin Promoter-Driven AAV9: A Tool to Study Its Role in Axon Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Sciatic Nerve Crush, Injury-Conditioned DRG Culture and Viral Transduction
2.2. Immunofluorescence
2.3. Data Analysis
2.4. N2A Cell Culture and DNA Transfection
2.5. Western Blot Analysis
2.6. Immunoprecipitation and Quantitative Measurement of β-actin mRNA
3. Results
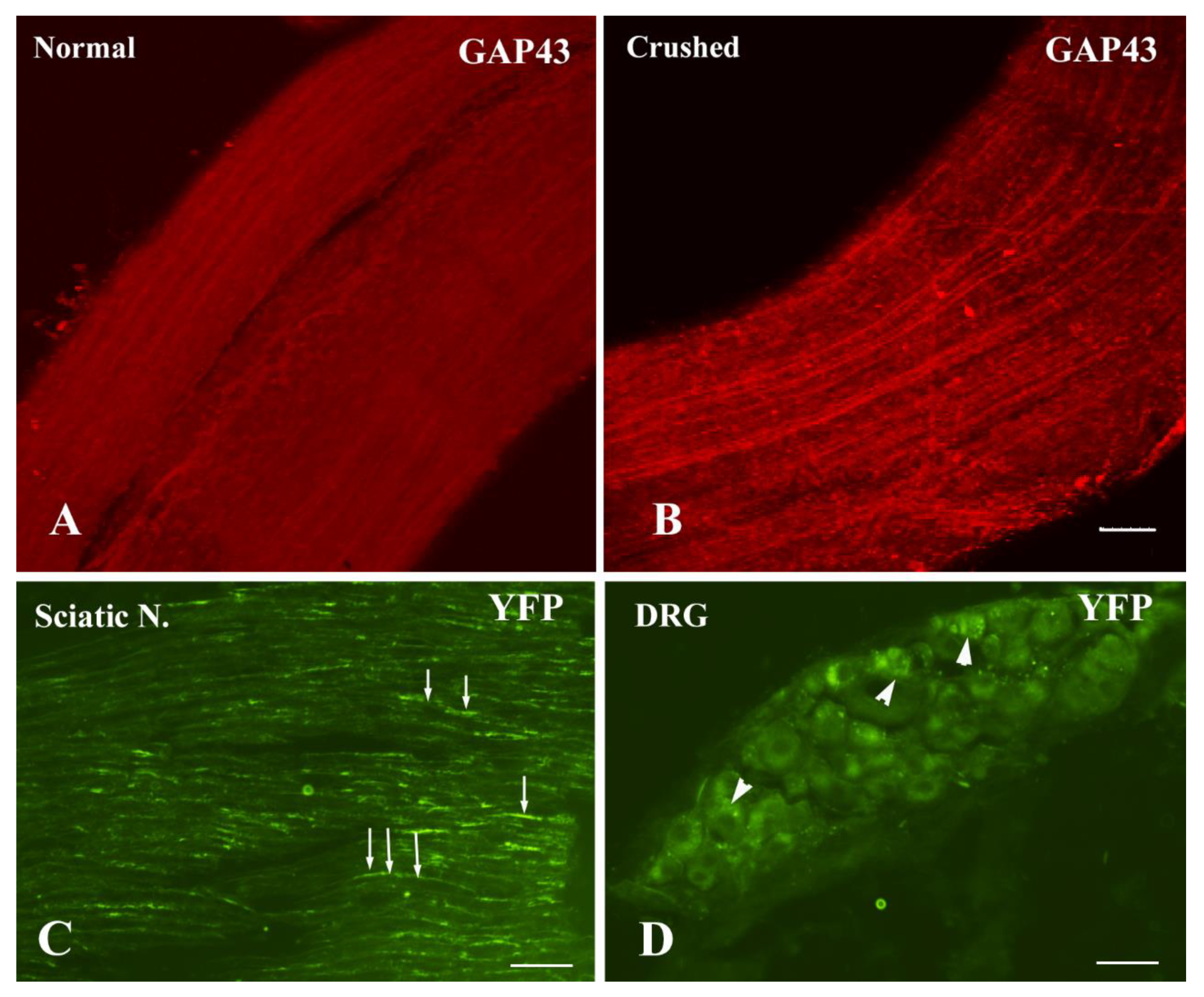
3.1. Neuron-Specific Transgene Expression Driven by AAV9.hSyn.YFP
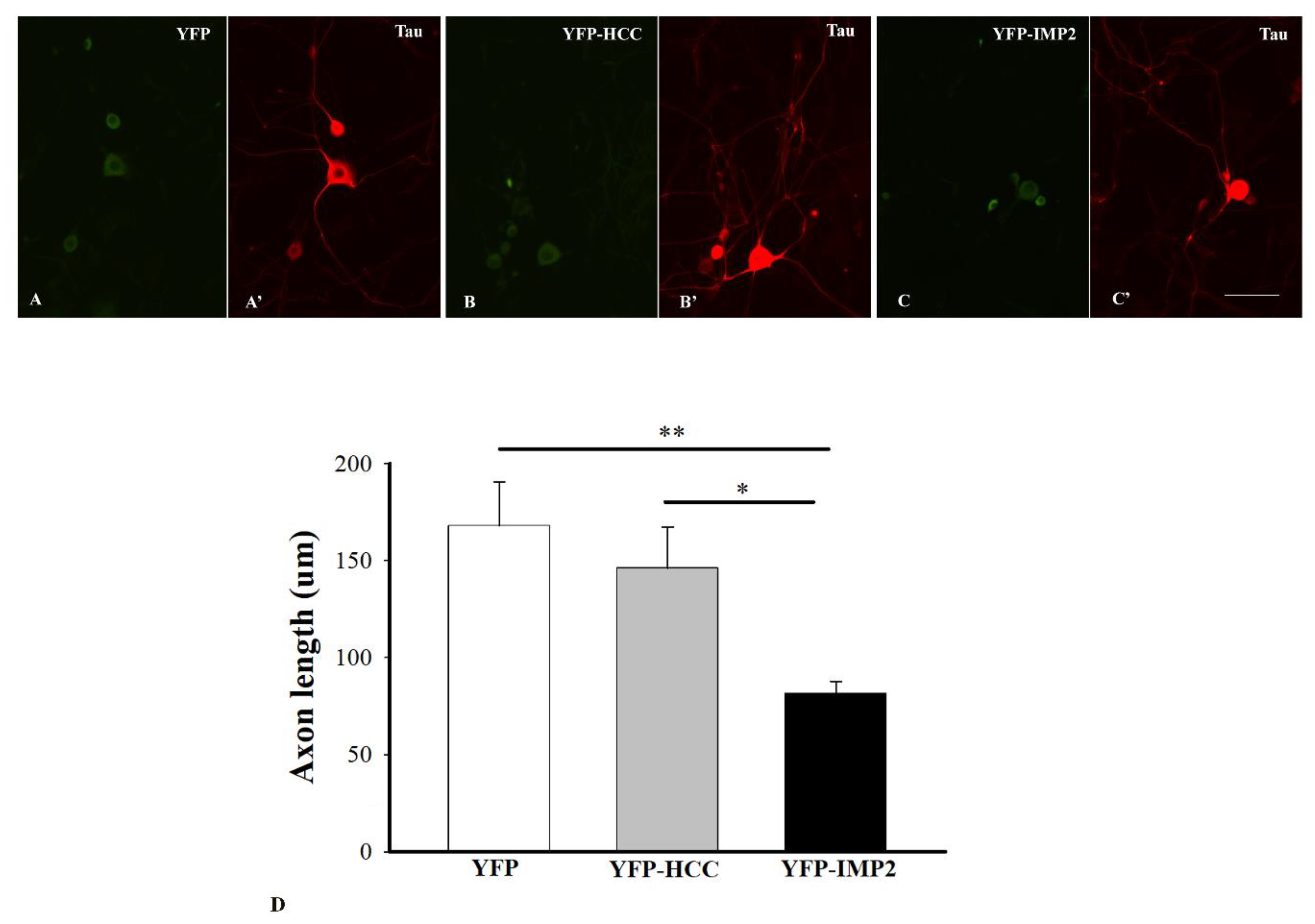
3.2. Different Effects of Overexpression of IMP2 and HCC on Axon Outgrowth
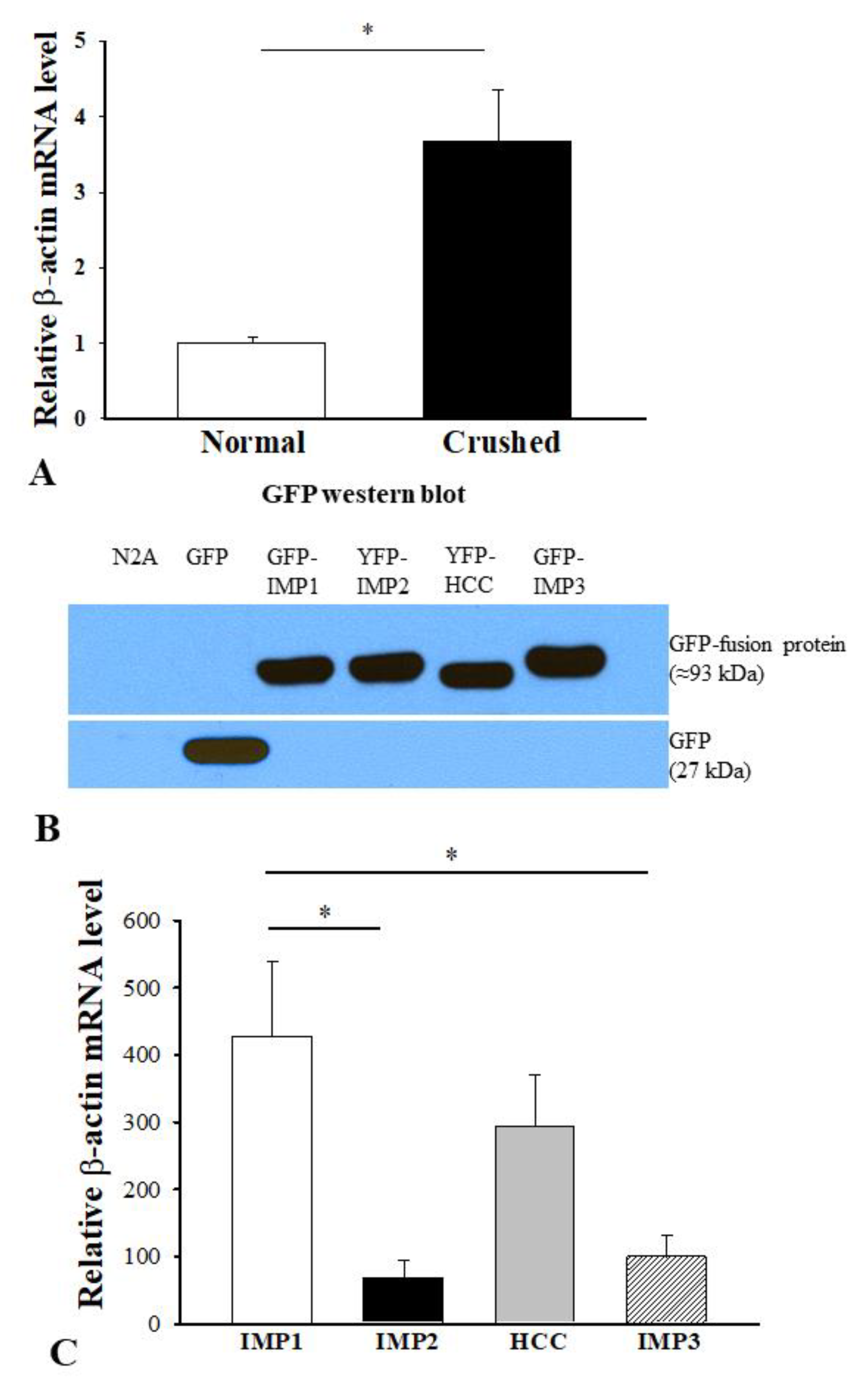
3.3. Association of Diverse Functions of IMPs in Axon Regeneration with their mRNA Binding Preferences
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Available Statement
Acknowledgments
Conflicts of Interest
References
- Nielsen, F.C.; Nielsen, J.; Christiansen, J. A family of IGF-II mRNA binding proteins (IMP) involved in RNA trafficking. Scand. J. Clin. Lab. Invest. Suppl. 2001, 234, 93–99. [Google Scholar] [CrossRef]
- Yaniv, K.; Yisraeli, J.K. The involvement of a conserved family of RNA binding proteins in embryonic development and carcinogenesis. Gene 2002, 287, 49–54. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Chan, E.K.; Peng, X.X.; Tan, E.M. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J. Exp. Med. 1999, 189, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Pillasch, F.; Pohl, B.; Wilda, M.; Lacher, U.; Beil, M.; Wallrapp, C.; Hameister, H.; Knochel, W.; Adler, G.; Gress, T.M. Expression of the highly conserved RNA binding protein KOC in embryogenesis. Mech. Dev. 1999, 88, 95–99. [Google Scholar] [CrossRef]
- Nielsen, J.; Christiansen, J.; Lykke-Andersen, J.; Johnsen, A.H.; Wewer, U.M.; Nielsen, F.C. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol. Cell. Biol. 1999, 19, 1262–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, C.J.; Willis, D.E.; Xu, M.; Tep, C.; Jiang, C.; Yoo, S.; Schanen, N.C.; Kirn-Safran, C.B.; van Minnen, J.; English, A.; et al. Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity. EMBO J. 2011, 30, 4665–4677. [Google Scholar] [CrossRef] [PubMed]
- Hammer, N.A.; Hansen, T.; Byskov, A.G.; Rajpert-De Meyts, E.; Grondahl, M.L.; Bredkjaer, H.E.; Wewer, U.M.; Christiansen, J.; Nielsen, F.C. Expression of IGF-II mRNA-binding proteins (IMPs) in gonads and testicular cancer. Reproduction 2005, 130, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, Y.; Kishi, Y.; Gotoh, Y. IMP2 regulates differentiation potentials of mouse neocortical neural precursor cells. Genes Cells 2013, 18, 79–89. [Google Scholar] [CrossRef]
- Ross, A.F.; Oleynikov, Y.; Kislauskis, E.H.; Taneja, K.L.; Singer, R.H. Characterization of a beta-actin mRNA zipcode-binding protein. Mol. Cell. Biol. 1997, 17, 2158–2165. [Google Scholar] [CrossRef] [Green Version]
- Kislauskis, E.H.; Zhu, X.; Singer, R.H. Sequences responsible for intracellular localization of beta-actin messenger RNA also affect cell phenotype. J Cell. Biol. 1994, 127, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Preitner, N.; Quan, J.; Li, X.; Nielsen, F.C.; Flanagan, J.G. IMP2 axonal localization, RNA interactome, and function in the development of axon trajectories. Development 2016, 143, 2753–2759. [Google Scholar] [CrossRef] [Green Version]
- Saxena, R.; Voight, B.F.; Lyssenko, V.; Burtt, N.P.; de Bakker, P.I.; Chen, H.; Roix, J.J.; Kathiresan, S.; Hirschhorn, J.N.; Daly, M.J.; et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007, 316, 1331–1336. [Google Scholar] [CrossRef]
- Scott, L.J.; Mohlke, K.L.; Bonnycastle, L.L.; Willer, C.J.; Li, Y.; Duren, W.L.; Erdos, M.R.; Stringham, H.M.; Chines, P.S.; Jackson, A.U.; et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007, 316, 1341–1345. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, J.; Kolte, A.M.; Hansen, T.; Nielsen, F.C. IGF2 mRNA-binding protein 2: Biological function and putative role in type 2 diabetes. J. Mol. Endocrinol. 2009, 43, 187–195. [Google Scholar] [CrossRef]
- Dai, N. The Diverse Functions of IMP2/IGF2BP2 in Metabolism. Trends Endocrinol. Metab. 2020, 31, 670–679. [Google Scholar] [CrossRef]
- Barghash, A.; Helms, V.; Kessler, S.M. Overexpression of IGF2 mRNA-Binding Protein 2 (IMP2/p62) as a Feature of Basal-like Breast Cancer Correlates with Short Survival. Scand. J. Immunol. 2015, 82, 142–143. [Google Scholar] [CrossRef]
- Dai, N.; Ji, F.; Wright, J.; Minichiello, L.; Sadreyev, R.; Avruch, J. IGF2 mRNA binding protein-2 is a tumor promoter that drives cancer proliferation through its client mRNAs IGF2 and HMGA1. Elife 2017, 6. [Google Scholar] [CrossRef]
- Kessler, S.M.; Lederer, E.; Laggai, S.; Golob-Schwarzl, N.; Hosseini, K.; Petzold, J.; Schweiger, C.; Reihs, R.; Keil, M.; Hoffmann, J.; et al. IMP2/IGF2BP2 expression, but not IMP1 and IMP3, predicts poor outcome in patients and high tumor growth rate in xenograft models of gallbladder cancer. Oncotarget 2017, 8, 89736–89745. [Google Scholar]
- Zheng, J.Q.; Kelly, T.K.; Chang, B.; Ryazantsev, S.; Rajasekaran, A.K.; Martin, K.C.; Twiss, J.L. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J. Neurosci. 2001, 21, 9291–9303. [Google Scholar] [CrossRef]
- Willis, D.; Li, K.W.; Zheng, J.Q.; Chang, J.H.; Smit, A.B.; Kelly, T.; Merianda, T.T.; Sylvester, J.; van Minnen, J.; Twiss, J.L. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J. Neurosci. 2005, 25, 778–791. [Google Scholar] [CrossRef] [Green Version]
- Willis, D.E.; Twiss, J.L. The evolving roles of axonally synthesized proteins in regeneration. Curr. Opin. Neurobiol. 2006, 16, 111–118. [Google Scholar] [CrossRef]
- Hanz, S.; Perlson, E.; Willis, D.; Zheng, J.Q.; Massarwa, R.; Huerta, J.J.; Koltzenburg, M.; Kohler, M.; van-Minnen, J.; Twiss, J.L.; et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 2003, 40, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- Verma, P.; Chierzi, S.; Codd, A.M.; Campbell, D.S.; Meyer, R.L.; Holt, C.E.; Fawcett, J.W. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J. Neurosci. 2005, 25, 331–342. [Google Scholar] [CrossRef]
- Yudin, D.; Hanz, S.; Yoo, S.; Iavnilovitch, E.; Willis, D.; Gradus, T.; Vuppalanchi, D.; Segal-Ruder, Y.; Ben-Yaakov, K.; Hieda, M.; et al. Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron 2008, 59, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Terenzio, M.; Koley, S.; Samra, N.; Rishal, I.; Zhao, Q.; Sahoo, P.K.; Urisman, A.; Marvaldi, L.; Oses-Prieto, J.A.; Forester, C.; et al. Locally translated mTOR controls axonal local translation in nerve injury. Science 2018, 359, 1416–1421. [Google Scholar] [CrossRef] [Green Version]
- Koley, S.; Rozenbaum, M.; Fainzilber, M.; Terenzio, M. Translating regeneration: Local protein synthesis in the neuronal injury response. Neurosci. Res. 2019, 139, 26–36. [Google Scholar] [CrossRef]
- Shigeoka, T.; Jung, H.; Jung, J.; Turner-Bridger, B.; Ohk, J.; Lin, J.Q.; Amieux, P.S.; Holt, C.E. Dynamic Axonal Translation in Developing and Mature Visual Circuits. Cell 2016, 166, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Lentz, T.B.; Gray, S.J.; Samulski, R.J. Viral vectors for gene delivery to the central nervous system. Neurobiol. Dis. 2012, 48, 179–188. [Google Scholar] [CrossRef] [Green Version]
- McCown, T.J.; Xiao, X.; Li, J.; Breese, G.R.; Samulski, R.J. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996, 713, 99–107. [Google Scholar] [CrossRef]
- Gray, S.J.; Matagne, V.; Bachaboina, L.; Yadav, S.; Ojeda, S.R.; Samulski, R.J. Preclinical differences of intravascular AAV9 delivery to neurons and glia: A comparative study of adult mice and nonhuman primates. Mol. Ther. 2011, 19, 1058–1069. [Google Scholar] [CrossRef]
- Agbandje-McKenna, M.; Kleinschmidt, J. AAV capsid structure and cell interactions. Methods Mol. Biol. 2011, 807, 47–92. [Google Scholar] [CrossRef]
- McLean, J.R.; Smith, G.A.; Rocha, E.M.; Hayes, M.A.; Beagan, J.A.; Hallett, P.J.; Isacson, O. Widespread neuron-specific transgene expression in brain and spinal cord following synapsin promoter-driven AAV9 neonatal intracerebroventricular injection. Neurosci. Lett. 2014, 576, 73–78. [Google Scholar] [CrossRef]
- Schuster, D.J.; Dykstra, J.A.; Riedl, M.S.; Kitto, K.F.; Belur, L.R.; McIvor, R.S.; Elde, R.P.; Fairbanks, C.A.; Vulchanova, L. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front. Neuroanat. 2014, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Foust, K.D.; Nurre, E.; Montgomery, C.L.; Hernandez, A.; Chan, C.M.; Kaspar, B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009, 27, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo-Hernandez, M.; Tadokoro, T.; Navarro, M.R.; Platoshyn, O.; Kobayashi, Y.; Marsala, S.; Miyanohara, A.; Juhas, S.; Juhasova, J.; Skalnikova, H.; et al. Spinal subpial delivery of AAV9 enables widespread gene silencing and blocks motoneuron degeneration in ALS. Nat. Med. 2020, 26, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.M.; Armao, D.; Nagabhushan Kalburgi, S.; Gray, S.J. Development of Intrathecal AAV9 Gene Therapy for Giant Axonal Neuropathy. Mol. Ther. Methods Clin. Dev. 2018, 9, 160–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kugler, S.; Kilic, E.; Bahr, M. Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Ther. 2003, 10, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.S.; Skene, J.H. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J. Neurosci. 1997, 17, 646–658. [Google Scholar] [CrossRef]
- Willis, D.E.; Xu, M.; Donnelly, C.J.; Tep, C.; Kendall, M.; Erenstheyn, M.; English, A.W.; Schanen, N.C.; Kirn-Safran, C.B.; Yoon, S.O. Axonal Localization of transgene mRNA in mature PNS and CNS neurons. J. Neurosci. 2011, 31, 14481–14487. [Google Scholar] [CrossRef] [Green Version]
- Lykken, E.A.; Shyng, C.; Edwards, R.J.; Rozenberg, A.; Gray, S.J. Recent progress and considerations for AAV gene therapies targeting the central nervous system. J. Neurodev. Disord. 2018, 10, 16. [Google Scholar] [CrossRef]
- Zhang, H.L.; Eom, T.; Oleynikov, Y.; Shenoy, S.M.; Liebelt, D.A.; Dictenberg, J.B.; Singer, R.H.; Bassell, G.J. Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron 2001, 31, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Huttelmaier, S.; Zenklusen, D.; Lederer, M.; Dictenberg, J.; Lorenz, M.; Meng, X.; Bassell, G.J.; Condeelis, J.; Singer, R.H. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature 2005, 438, 512–515. [Google Scholar] [CrossRef]
- Murlidharan, G.; Samulski, R.J.; Asokan, A. Biology of adeno-associated viral vectors in the central nervous system. Front. Mol. Neurosci. 2014, 7, 76. [Google Scholar] [CrossRef] [Green Version]
- Massaro, G.; Hughes, M.P.; Whaler, S.M.; Wallom, K.L.; Priestman, D.A.; Platt, F.M.; Waddington, S.N.; Rahim, A.A. Systemic AAV9 gene therapy using the synapsin I promoter rescues a mouse model of neuronopathic Gaucher disease but with limited cross-correction potential to astrocytes. Hum. Mol. Genet. 2020, 29, 1933–1949. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.; Pan, M.; Ge, L.; Liu, J.; Deng, J.; Wang, X.; Li, L.; Wen, J.; Tan, D.; Zhang, H.; et al. NeuroD1 overexpression in spinal neurons accelerates axonal regeneration after sciatic nerve injury. Exp. Neurol. 2020, 327, 113215. [Google Scholar] [CrossRef]
- Knöferle, J.; Koch, J.C.; Ostendorf, T.; Michel, U.; Planchamp, V.; Vutova, P.; Tönges, L.; Stadelmann, C.; Brück, W.; Bähr, M.; et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. PNAS 2010, 107, 6064–6069. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.P.; Sahoo, P.K.; Kar, A.N.; Twiss, J.L. Intra-axonal mechanisms driving axon regeneration. Brain Res. 2020, 1740, 146864. [Google Scholar] [CrossRef]
- Biswas, J.; Patel, V.L.; Bhaskar, V.; Chao, J.A.; Singer, R.H.; Eliscovich, C. The structural basis for RNA selectivity by the IMP family of RNA-binding proteins. Nat. Commun. 2019, 10, 4440. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, Y.; Welshhans, K.; Wen, Z.; Yao, J.; Xu, M.; Goshima, Y.; Zheng, J.Q.; Bassell, G.J. Phosphorylation of zipcode binding protein 1 is required for brain-derived neurotrophic factor signaling of local beta-actin synthesis and growth cone turning. J. Neurosci. 2010, 30, 9349–9358. [Google Scholar] [CrossRef]
- Farina, K.L.; Huttelmaier, S.; Musunuru, K.; Darnell, R.; Singer, R.H. Two ZBP1 KH domains facilitate beta-actin mRNA localization, granule formation, and cytoskeletal attachment. J. Cell. Biol. 2003, 160, 77–87. [Google Scholar] [CrossRef]
- Biswas, J.; Nunez, L.; Das, S.; Yoon, Y.J.; Eliscovich, C.; Singer, R.H. Zipcode Binding Protein 1 (ZBP1; IGF2BP1): A Model for Sequence-Specific RNA Regulation. Cold Spring Harb. Symp. Quant. Biol. 2019, 84, 1–10. [Google Scholar] [CrossRef]
- Hirokawa, N.; Niwa, S.; Tanaka, Y. Molecular motors in neurons: Transport mechanisms and roles in brain function, development, and disease. Neuron 2010, 68, 610–638. [Google Scholar] [CrossRef] [Green Version]
- Costa, I.D.; Buchanan, C.N.; Zdradzinski, M.D.; Sahoo, P.K.; Smith, T.P.; Thames, E.; Kar, A.N.; Twiss, J.L. The functional organization of axonal mRNA transport and translation. Nat. Rev. Neurosci. 2021, 22, 77–91. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blizard, S.; Park, D.; O’Toole, N.; Norooz, S.; Dela Torre, M.; Son, Y.; Holstein, A.; Austin, S.; Harman, J.; Haraszti, S.; et al. Neuron-Specific IMP2 Overexpression by Synapsin Promoter-Driven AAV9: A Tool to Study Its Role in Axon Regeneration. Cells 2021, 10, 2654. https://doi.org/10.3390/cells10102654
Blizard S, Park D, O’Toole N, Norooz S, Dela Torre M, Son Y, Holstein A, Austin S, Harman J, Haraszti S, et al. Neuron-Specific IMP2 Overexpression by Synapsin Promoter-Driven AAV9: A Tool to Study Its Role in Axon Regeneration. Cells. 2021; 10(10):2654. https://doi.org/10.3390/cells10102654
Chicago/Turabian StyleBlizard, Sarah, Danielle Park, Natalie O’Toole, Sheeva Norooz, Martin Dela Torre, Young Son, Adam Holstein, Scarlett Austin, Joshua Harman, Samantha Haraszti, and et al. 2021. "Neuron-Specific IMP2 Overexpression by Synapsin Promoter-Driven AAV9: A Tool to Study Its Role in Axon Regeneration" Cells 10, no. 10: 2654. https://doi.org/10.3390/cells10102654
APA StyleBlizard, S., Park, D., O’Toole, N., Norooz, S., Dela Torre, M., Son, Y., Holstein, A., Austin, S., Harman, J., Haraszti, S., Fared, D., & Xu, M. (2021). Neuron-Specific IMP2 Overexpression by Synapsin Promoter-Driven AAV9: A Tool to Study Its Role in Axon Regeneration. Cells, 10(10), 2654. https://doi.org/10.3390/cells10102654





