Regulation of T Cells in Cancer by Nitric Oxide
Abstract
:1. Introduction
2. The Immune System
2.1. Overview of the Immune System
2.2. CD4 and CD8 T Cells
3. Regulation of T Cells by .NO
3.1. Nitric Oxide
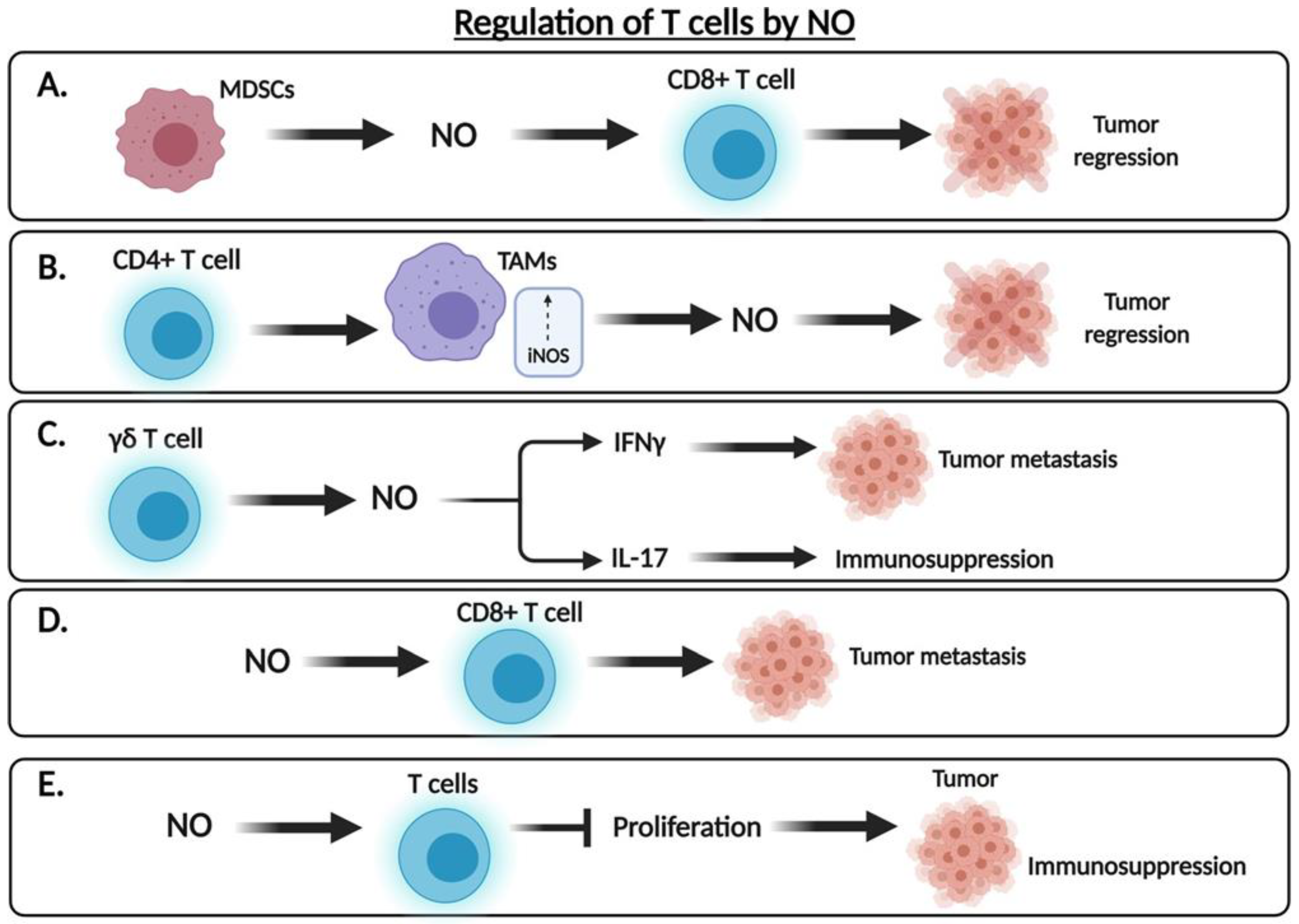
3.2. The Dual Role of .NO in the Regulation of Tumor Cell Cytotoxicity
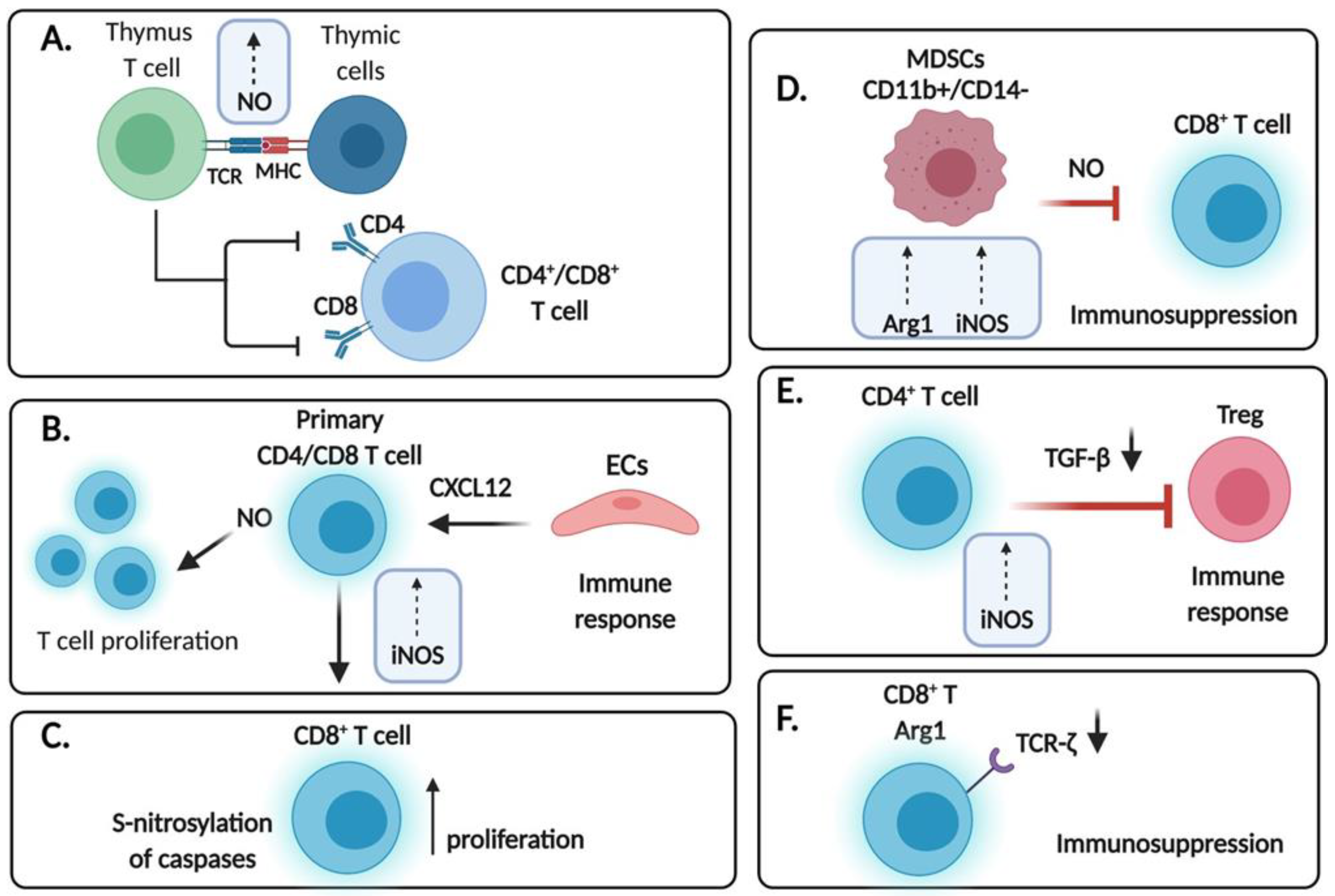
4. Expression of .NO Synthases in T Cells
5. Role of Exogenous .NO Donors on T Cells
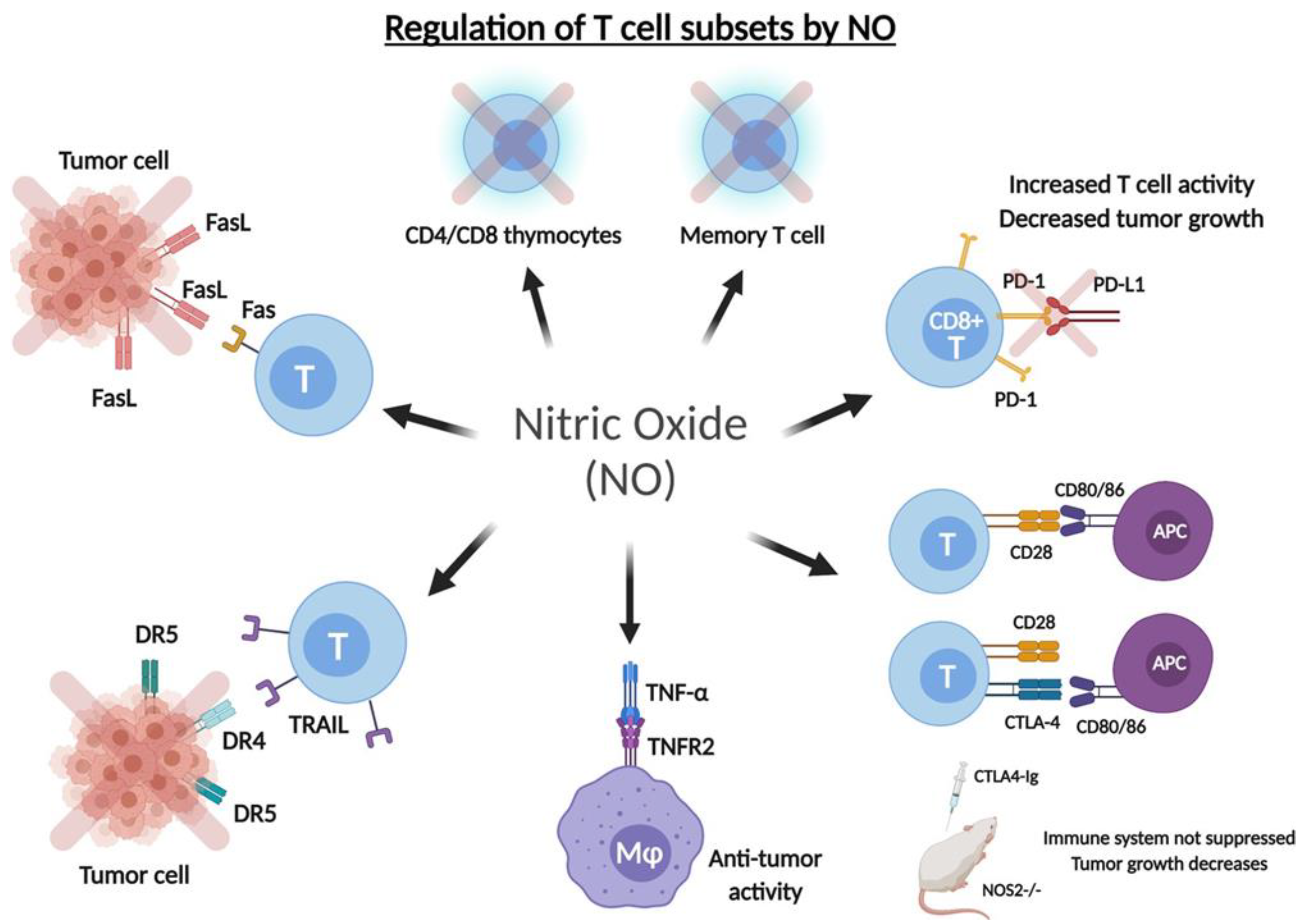
5.1. Role on the Anti-Tumor T Cell Response
5.2. Role of .NO in the Regulation of Checkpoint Inhibitory Receptors
5.3. Role of .NO in the Expression of TNF-Alpha, Fas and TRAIL on T Cells
5.3.1. Tumor Necrosis Factor-α (TNF-α)
5.3.2. Fas/FasL
5.3.3. TRAIL
6. Role of .NO in the Activation of Induced Cell Death of T Cells
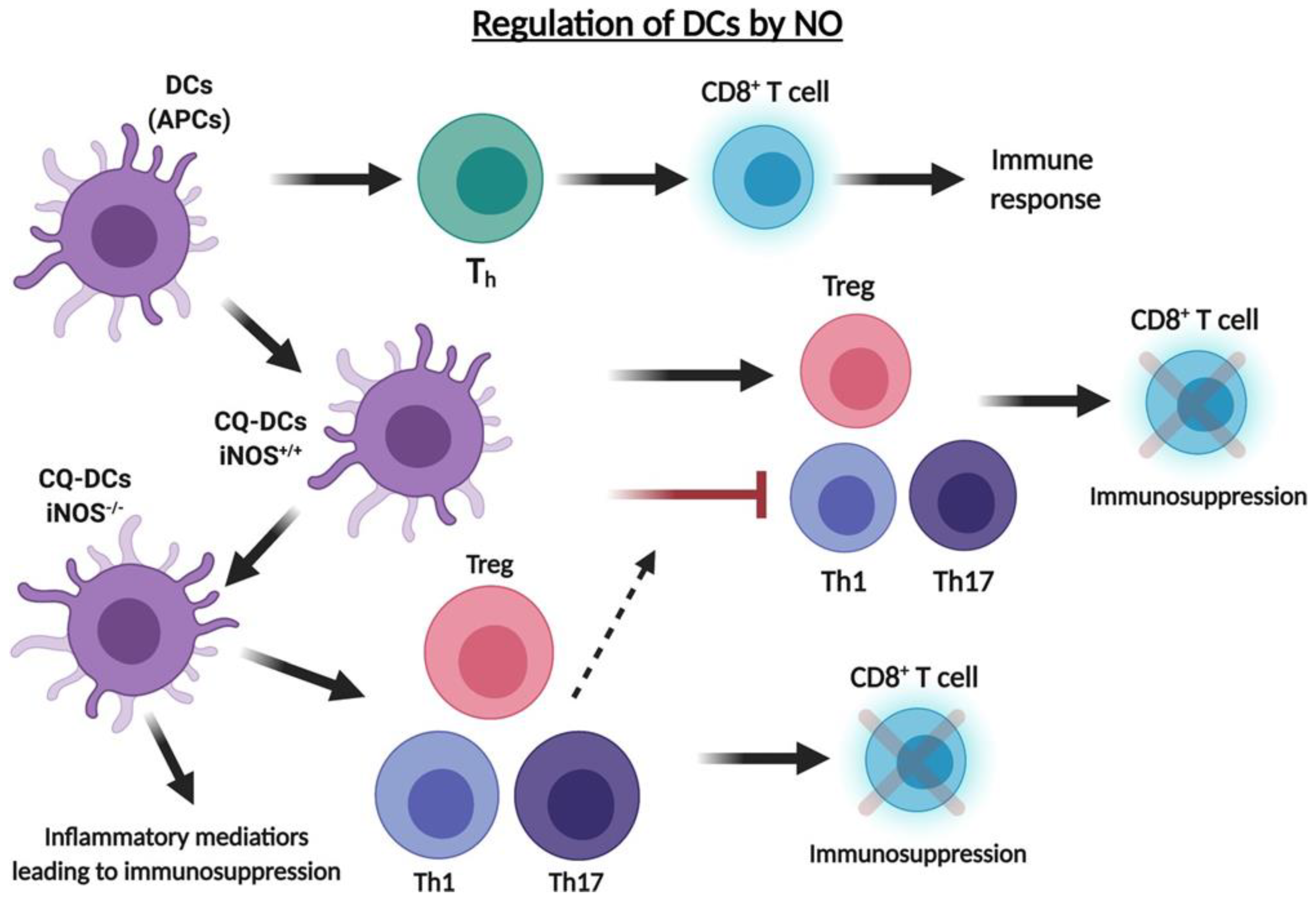
7. Role of .NO in the Regulation of T Regulatory Cells (Tregs) and Dendritic Cells (DCs) in the TME
8. Role of .NO in the Regulation of Myeloid Derived Suppressor Cells (MDSCs) in the TME
9. Future Perspectives and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting cell |
| Arg1 | Arginase 1 |
| CAR-T | Chimeric antigen receptor T cell |
| CTL | Cytotoxic T lymphocyte |
| DC | Dendritic cell |
| EC | Endothelial cell |
| eNOS | Endothelial NOS |
| IFN-γ | Interferon-γ |
| iNOS | Inducible NOS |
| MDSC | Myeloid derived suppressor cell |
| nNOS | Neuronal NOS |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| PD-1 | Programmed cell death protein-1 |
| TAM | Tumor-associated macrophage |
| TCR | T cell receptor |
| TGF-β | Transforming growth factor beta |
| Th | T helper |
| TME | Tumor microenvironment |
| TNF-α | Tumor necrosis factor-α |
| TNFR1 | TNF receptor 1 |
| TNFR2 | TNF receptor 2 |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| Treg | T regulatory |
| YY1 | Yin-yang 1 |
References
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Niedbala, W.; Alves-Filho, J.C.; Fukada, S.Y.; Vieira, S.M.; Mitani, A.; Sonego, F.; Mirchandani, A.; Nascimento, D.C.; Cunha, F.Q.; Liew, F.Y. Regulation of type 17 helper T-cell function by nitric oxide during in-flammation. Proc. Natl. Acad. Sci USA 2011, 108, 9220–9225. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Choi, H.; Eun, S.Y.; Fukuyama, S.; Croft, M. Nitric oxide modulates TGF-beta-directive signals to suppress Foxp3+ regulatory T cell differentiation and potentiate Th1 development. J. Immunol. 2011, 186, 6972–6980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedbala, W.; Besnard, A.G.; Jiang, H.R.; Alves-Filho, J.C.; Fukada, S.Y.; Nascimento, D.; Mitani, A.; Pushparaj, P.; Alqahtani, M.H.; Liew, F.Y. Nitric oxide-induced regulatory T cells inhibit Th17 but not Th1 cell differen-tiation and function. J. Immunol. 2013, 191, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on Immune Cells and Its Role in Diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, L.B. The immune system. Essays Biochem. 2016, 60, 275–301. [Google Scholar] [CrossRef] [Green Version]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef] [Green Version]
- Kaymak, I.; Williams, K.S.; Cantor, J.R.; Jones, R.G. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell 2020, 39, 28–37. [Google Scholar] [CrossRef]
- Wan, Y.Y. Multi-tasking of helper T cells. Immunology 2010, 130, 166–171. [Google Scholar] [CrossRef]
- Richardson, J.R.; Schöllhorn, A.; Gouttefangeas, C.; Schuhmacher, J.C. CD4+ T Cells: Multitasking Cells in the Duty of Cancer Immuno-therapy. Cancers 2021, 13, 596. [Google Scholar] [CrossRef]
- Jacobson, N.G.; Szabo, S.J.; Weber-Nordt, R.M.; Zhong, Z.; Schreiber, R.D.; E Darnell, J.; Murphy, K.M. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J. Exp. Med. 1995, 181, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Bradley, L.M.; Dalton, D.K.; Croft, M. A direct role for IFN-gamma in regulation of Th1 cell development. J. Immunol. 1996, 157, 1350–1358. [Google Scholar]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Hatton, R.D. TGF-β in Th17 Cell Development: The Truth Is Out There. Immunity 2011, 34, 288–290. [Google Scholar] [CrossRef] [Green Version]
- Plitas, G.; Rudensky, A.Y. Regulatory T Cells: Differentiation and Function. Cancer Immunol. Res. 2016, 4, 721–725. [Google Scholar] [CrossRef] [Green Version]
- Cinier, J.; Hubert, M.; Besson, L. Recruitment and Expansion of Tregs Cells in the Tumor Environment-How to Target Them? Cancers 2021, 13, 1850. [Google Scholar] [CrossRef]
- Zhang, N.; Bevan, M.J. CD8+ T Cells: Foot Soldiers of the Immune System. Immunity 2011, 35, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Guisier, F.; Filho, M.B.; Rock, L.D.; Strachan-Whaley, M.; Marshall, E.A.; Dellaire, G.; Lam, W.L. Janus or Hydra: The Many Faces of T Helper Cells in the Human Tumour Microenvironment. Tumor Microenviron. 2020, 1224, 35–51. [Google Scholar] [CrossRef]
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schietinger, A.; Greenberg, P.D. Tolerance and exhaustion: Defining mechanisms of T cell dysfunction. Trends Immunol. 2013, 35, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; He, Y.; He, W.; Wu, G.; Zhou, X.; Sheng, Q.; Zhong, W.; Lu, Y.; Ding, Y.; Lu, Q.; et al. Exhausted CD8+T Cells in the Tumor Immune Microenvironment: New Pathways to Therapy. Front. Immunol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Bonavida, B.; Baritaki, S.; Huerta-Yepez, S.; Vega, M.I.; Chatterjee, D.; Yeung, K. Novel therapeutic applications of nitric oxide donors in cancer: Roles in chemo- and immunosensitization to apoptosis and inhibition of metastases. Nitric Oxide 2008, 19, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Hays, E.; Bonavida, B. Nitric Oxide-Mediated Enhancement and Reversal of Resistance of Anticancer Therapies. Antioxidants 2019, 8, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonavida, B. Sensitizing activities of nitric oxide donors for cancer resistance to anticancer therapeutic drugs. Biochem. Pharmacol. 2020, 176, 113913. [Google Scholar] [CrossRef]
- Xu, W.; Liu, L.Z.; Loizidou, M.; Ahmed, M.; Charles, I.G. The role of nitric oxide in cancer. Cell Res. 2002, 12, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Förstermann, U.; Sessa, W. Nitric oxide synthases: Regulation and function. Eur. Hear. J. 2011, 33, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Ye, T.; Liu, H. Prognostic Value of Inducible Nitric Oxide Synthase (iNOS) in Human Cancer: A Systematic Review and Meta-Analysis. Biomed Res. Int. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Schlossmann, J.; Feil, R.; Hofmann, F. Signaling through .NO and cGMP-dependent protein kinases. Ann. Med. 2003, 35, 21–27. [Google Scholar] [CrossRef]
- Friebe, A.; Sandner, P.; Schmidtko, A. cGMP: A unique 2nd messenger molecule–Recent developments in cGMP research and development. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 393, 287–302. [Google Scholar] [CrossRef] [Green Version]
- Brüne, B.; Mohr, S.; Messmer, U.K. Protein thiol modification and apoptotic cell death as cGMP-independent nitric oxide (.NO) signaling pathways. Rev. Physiol. Biochem. Pharmacol. 1996, 127, 1–30. [Google Scholar]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, K.; Ghassemi, F.; Sotolongo, A.; Siu, A.; Shauger, L.; Kots, A.; Murad, F. NOS-2 signaling and cancer therapy. Iubmb Life 2012, 64, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, S.K.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vannini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibbs, J.B., Jr.; Taintor, R.R.; Vavrin, Z.; Rachlin, E.M. Nitric oxide: A cytotoxic activated macrophage effector molecule. Biochem Biophys Res. Commun. 1988, 157, 87–94. [Google Scholar] [CrossRef]
- Li, L.M.; Kilbourn, R.G.; Adams, J.; Fidler, I.J. Role of nitric oxide in lysis of tumor cells by cytokine-activated endothelial cells. Cancer Res. 1991, 51, 2531–2535. [Google Scholar]
- Lamrani, M.; Sassi, N.; Paul, C.; Yousfi, N.; Boucher, J.-L.; Gauthier, N.; Labbé, J.; Seignez, C.; Racoeur, C.; Athias, A.; et al. TLR4/IFNγ pathways induce tumor regression via NOS II-dependent.NO and ROS production in murine breast cancer models. Oncoimmunology 2015, 5, e1123369. [Google Scholar] [CrossRef] [Green Version]
- Marigo, I.; Zilio, S.; Desantis, G.; Mlecnik, B.; Agnellini, A.H.R.; Ugel, S.; Zanovello, S.M.; Molon, B.; Ries, C.H.; Runza, V.; et al. T Cell Cancer Therapy Requires CD40-CD40L Activation of Tumor Necrosis Factor and Inducible Nitric-Oxide-Synthase-Producing Dendritic Cells. Cancer Cell. 2016, 30, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Fauskanger, M.; Haabeth, O.A.W.; Skjeldal, F.M.; Bogen, B.; Tveita, A. Tumor Killing by CD4+ T Cells Is Mediated via Induction of Inducible Nitric Oxide Synthase-Dependent Macrophage Cytotoxicity. Front. Immunol. 2018, 9, 1684. [Google Scholar] [CrossRef] [Green Version]
- Douguet, L.; Bod, L.; Lengagne, R.; Labarthe, L.; Kato, M.; Avril, M.-F.; Prévost-Blondel, A. Nitric oxide synthase 2 is involved in the pro-tumorigenic potential of γδ17 T cells in melanoma. OncoImmunology 2016, 5, e1208878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegert, A.; Rosenberg, C.; Schmitt, W.D.; Denkert, C.; Hauptmann, S. Nitric oxide of human colorectal adenocarcinoma cell lines promotes tumour cell invasion. Br. J. Cancer 2002, 86, 1310–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.M.; Xu, Q. Metastatic melanoma cells escape from immunosurveillance through the novel mechanism of releasing nitric oxide to induce dysfunction of immunocytes. Melanoma Res. 2001, 11, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Jadeski, L.C.; Chakraborty, C.; Lala, P.K. Nitric oxide-mediated promotion of mammary tumour cell migration requires sequential activation of nitric oxide synthase, guanylate cyclase and mitogen-activated protein kinase. Int. J. Cancer 2003, 106, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Ambs, S.; Merriam, W.G.; Ogunfusika, M.O.; Bennett, W.P.; Ishibe, N.; Hussain, S.P.; Tzeng, E.E.; Geller, D.A.; Billiar, T.R.; Harris, C.C. p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nat. Med. 1998, 4, 1371–1376. [Google Scholar] [CrossRef]
- Arcos, M.L.B.; Gorelik, G.; Klecha, A.; Goren, N.; Cerquetti, C.; A Cremaschi, G. Inducible nitric oxide synthase-mediated proliferation of a T lymphoma cell line. Nitric Oxide 2003, 8, 111–118. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Papi, S.; Ahmadizar, F.; Hasanvand, A. The role of nitric oxide in inflammation and oxidative stress. Immunopathol. Persa 2019, 5, e08. [Google Scholar] [CrossRef]
- Bogdan, C.; Rollinghoff, M.; Diefenbach, A. The role of nitric oxide in innate immunity. Immunol. Rev. 2000, 173, 17–26. [Google Scholar] [CrossRef]
- Xiong, H.; Zhu, C.; Li, F.; Hegazi, R. Inhibition of interleukin-12 p40 transcription and NF-kappaB activation by nitric oxide in murine macrophages and dendritic cells. J. Biol Chem. 2004, 279, 10776–10783. [Google Scholar] [CrossRef] [Green Version]
- Tai, X.G.; Toyooka, K.; Yamamoto, N.; Yashiro, Y.; Mu, J.; Hamaoka, T.; Fujiwara, H. Expression of an inducible type of nitric oxide (NO) synthase in the thymus and involvement of NO in deletion of TCR-stimulated double-positive thymocytes. J. Immunol. 1997, 158, 4696–4703. [Google Scholar]
- Jayaraman, P.; Alfarano, M.G.; Svider, P.F.; Parikh, F.; Lu, G.; Kidwai, S.; Xiong, H.; Sikora, A.G. iNOS Expression in CD4+ T Cells Limits Treg Induction by Repressing TGFβ1: Combined iNOS Inhibition and Treg Depletion Unmask Endogenous Antitumor Immunity. Clin. Cancer Res. 2014, 20, 6439–6451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choy, J.; Wang, Y.; Tellides, G.; Pober, J.S. Induction of inducible NO synthase in bystander human T cells increases allogeneic responses in the vasculature. Proc. Natl. Acad. Sci. USA 2007, 104, 1313–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esaki, T.; Hayashi, T.; Asai, Y.; Kumar, T.N.; Kano, H.; Muto, E.; Iguchi, A. Expression of inducible nitric oxide synthase in T lymphocytes and macrophages in vessels with advanced atherosclerosis. Heart Vessel. 1997, 12, 89–92. [Google Scholar]
- Koh, K.P.; Wang, Y.; Yi, T.; Shiao, S.L.; Lorber, M.I.; Sessa, W.C.; Tellides, G.; Pober, J.S. T cell-mediated vascular dysfunction of human allografts results from IFN-gamma dysreg-ulation of .NO synthase. J. Clin. Invest. 2004, 114, 846–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choy, J.; Yi, T.; Rao, D.A.; Tellides, G.; Fox-Talbot, K.; Baldwin, W.M.; Pober, J.S.; K, F.-T. CXCL12 Induction of Inducible Nitric Oxide Synthase in Human CD8 T Cells. J. Hear. Lung Transplant. 2008, 27, 1333–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Enis, D.R.; Koh, K.P.; Shiao, S.L.; Pober, J.S.T. lymphocyte-endothelial cell interactions. Annu Rev. Immunol. 2004, 22, 683–709. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Hirota, S.; Tachibana, K.; Takakura, N.; Nishikawa, S.-I.; Kitamura, Y.; Yoshida, N.; Kikutani, H.; Kishimoto, T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996, 382, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Colamussi, M.L.; Secchiero, P.; Gonelli, A.; Marchisio, M.; Zauli, G.; Capitani, S. Stromal derived factor-1 alpha (SDF-1 alpha) induces CD4+ T cell apoptosis via the functional up-regulation of the Fas (CD95)/Fas ligand (CD95L) pathway. J. Leukoc. Biol. 2001, 69, 263–270. [Google Scholar]
- Suzuki, Y.; Rahman, M.; Mitsuya, H. Diverse Transcriptional Response of CD4+T Cells to Stromal Cell-Derived Factor (SDF)-1: Cell Survival Promotion and Priming Effects of SDF-1 on CD4+T Cells. J. Immunol. 2001, 167, 3064–3073. [Google Scholar] [CrossRef] [Green Version]
- Vandervelde, S.; Vanluyn, M.; Tio, R.; Harmsen, M. Signaling factors in stem cell-mediated repair of infarcted myocardium. J. Mol. Cell. Cardiol. 2005, 39, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Choy, J.C.; Pober, J.S. Generation of .NO by bystander human CD8 T cells augments allogeneic responses by inhibiting cy-tokine deprivation-induced cell death. Am. J. Transplant. 2009, 9, 2281–2291. [Google Scholar]
- Hughes, P.D.; Belz, G.; Fortner, K.A.; Budd, R.C.; Strasser, A.; Bouillet, P. Apoptosis Regulators Fas and Bim Cooperate in Shutdown of Chronic Immune Responses and Prevention of Autoimmunity. Immunity 2008, 28, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weant, A.E.; Michalek, R.D.; Khan, I.U.; Holbrook, B.C.; Willingham, M.C.; Grayson, J.M. Apoptosis Regulators Bim and Fas Function Concurrently to Control Autoimmunity and CD8+ T Cell Contraction. Immunity 2008, 28, 218–230. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-Y.; Wang, Y.-M.; Wang, C.-L.; Feng, P.-H.; Ko, H.-W.; Liu, Y.-H.; Wu, Y.-C.; Chu, Y.; Chung, F.-T.; Kuo, C.-H.; et al. Population alterations of l-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14−/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2009, 136, 35–45. [Google Scholar] [CrossRef]
- Bronte, V.; Serafini, P.; De Santo, C.; Marigo, I.; Tosello, V.; Mazzoni, A.; Segal, D.M.; Staib, C.; Lowel, M.; Sutter, G.; et al. IL-4-Induced Arginase 1 Suppresses Alloreactive T Cells in Tumor-Bearing Mice. J. Immunol. 2003, 170, 270–278. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Quiceno, D.G.; Zabaleta, J.; Ortiz, B.; Zea, A.H.; Piazuelo, M.B.; Delgado, A.; Correa, P.; Brayer, J.; Sotomayor, E.M.; et al. Arginase I Production in the Tumor Microenvironment by Mature Myeloid Cells Inhibits T-Cell Receptor Expression and Antigen-Specific T-Cell Responses. Cancer Res. 2004, 64, 5839–5849. [Google Scholar] [CrossRef] [Green Version]
- Parker, K.H.; Beury, D.W.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Sup-pression in the Tumor Microenvironment. Adv Cancer Res. 2015, 128, 95–139. [Google Scholar]
- Remick, D.; Villarete, L. Regulation of cytokine gene expression by reactive oxygen and reactive nitrogen intermediates. J. Leukoc. Biol. 1996, 59, 471–475. [Google Scholar] [CrossRef] [Green Version]
- Hildeman, D.; Mitchell, T.; Teague, K.; Henson, P.; Day, B.J.; Kappler, J.; Marrack, P.C. Reactive Oxygen Species Regulate Activation-Induced T Cell Apoptosis. Immunity 1999, 10, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Legorreta-Herrera, M.; Rivas-Contreras, S.; Ventura-Gallegos, J.; Zentella-Dehesa, A. Nitric oxide is involved in the upregulation of IFN-γ and IL-10 mRNA expression by CD8+ T cells during the blood stages of P.chabaudi AS infection in CBA/Ca mice. Int. J. Biol. Sci. 2011, 7, 1401–1411. [Google Scholar] [PubMed]
- Jayaraman, P.; Parikh, F.; Lopez-Rivera, E.; Hailemichael, Y.; Clark, A.; Ma, G.; Cannan, D.; Ramacher, M.; Kato, M.; Overwijk, W.W.; et al. Tumor-Expressed Inducible Nitric Oxide Synthase Controls Induction of Functional Myeloid-Derived Suppressor Cells through Modulation of Vascular Endothelial Growth Factor Release. J. Immunol. 2012, 188, 5365–5376. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, R.; Lu, G.; Shen, Y.; Peng, L.; Zhu, C.; Cui, M.; Wang, W.; Arnaboldi, P.; Tang, M.; et al. T cell–derived inducible nitric oxide synthase switches off TH17 cell differentiation. J. Exp. Med. 2013, 210, 1447–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Deng, J.; Wu, F.; Lu, X.; Huang, L.; Zhao, M. Expression of arginase I and inducible nitric oxide synthase in the peripheral blood and lymph nodes of HIV-positive patients. Mol. Med. Rep. 2015, 13, 731–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloke, T.E.; Garvey, L.; Choi, B.-S.; Abebe, T.; Hailu, A.; Hancock, M.; Kadolsky, U.; Bangham, C.R.M.; Munder, M.; Müller, I.; et al. Increased Level of Arginase Activity Correlates with Disease Severity in HIV-Seropositive Patients. J. Infect. Dis. 2010, 202, 374–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairoli, E.; Scott-Algara, D.; Pritsch, O.; Dighiero, G.; Cayota, A. HIV-1 induced decrease of nitric oxide production and inducible nitric oxide synthase expression during in vivo and in vitro infection. Clin. Immunol. 2008, 127, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, K.A.P.; Hoeksema, M.A.; Meesters, D.M.; Akker, N.M.S.V.D.; Molin, D.G.M.; Briedé, J.J.; Ghosh, M.; Köhler, S.E.; Van Zandvoort, M.A.M.J.; De Winther, M.P.J.; et al. Arginase-1 Deficiency Regulates Arginine Concentrations and NOS2-Mediated NO Production during Endotoxemia. PLoS ONE 2014, 9, e86135. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, R.; Asim, M.; Lewis, N.; Algood, H.M.S.; Cover, T.L.; Kim, P.Y.; Wilson, K.T. l -Arginine Availability Regulates Inducible Nitric Oxide Synthase-Dependent Host Defense against Helicobacter pylori. Infect. Immun. 2007, 75, 4305–4315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roozendaal, R.; Vellenga, E.; Postma, D.S.; De Monchy, J.G.; Kauffman, H.F. Nitric oxide selectively decreases interferon-gamma expression by activated human T lymphocytes via a cGMP-independent mechanism. Immunology 1999, 98, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Aguilera, J.V.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Sys-tematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Ha, S.J.; Kaech, S.M.; Haining, W.N.; Sarkar, S.; Kalia, V.; Subramaniam, S.; Blattman, J.N.; Barber, D.L.; Ahmed, R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007, 27, 670–684. [Google Scholar] [CrossRef] [Green Version]
- Ahn, E.; Araki, K.; Hashimoto, M.; Li, W.; Riley, J.; Cheung, J.; Sharpe, A.H.; Freeman, G.J.; Irving, B.A.; Ahmed, R. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. USA 2018, 115, 4749–4754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.; Inui, N.; Karayama, M.; Imokawa, S.; Yamada, T.; Yokomura, K.; Asada, K.; Kusagaya, H.; Kaida, Y.; Matsuda, H.; et al. Effect of PD-1 inhibitor on exhaled nitric oxide and pulmonary function in non-small cell lung cancer patients with and without COPD. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1867–1877. [Google Scholar] [CrossRef] [Green Version]
- Pedicord, V.A.; Montalvo, W.; Leiner, I.M.; Allison, J.P. Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc. Natl Acad Sci. USA 2011, 108, 266–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deppong, C.M.; Parulekar, A.; Boomer, J.S.; Bricker, T.L.; Green, J.M. CTLA4-Ig inhibits allergic airway inflammation by a novel CD28-independent, nitric oxide synthase-dependent mechanism. Eur. J. Immunol. 2010, 40, 1985–1994. [Google Scholar] [CrossRef]
- Ye, L.-L.; Wei, X.-S.; Zhang, M.; Niu, Y.-R.; Zhou, Q. The Significance of Tumor Necrosis Factor Receptor Type II in CD8+ Regulatory T Cells and CD8+ Effector T Cells. Front. Immunol. 2018, 9, 583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Mohaupt, M.; Jiang, J.; Liu, S.; Li, B.; Qin, Z. Tumor Necrosis Factor Receptor 2–Mediated Tumor Suppression Is Nitric Oxide Dependent and Involves Angiostasis. Cancer Res. 2007, 67, 4443–4450. [Google Scholar] [CrossRef] [Green Version]
- Matthews, J.R.; Botting, C.H.; Panico, M.; Morris, H.R.; Hay, R.T. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res. 1996, 24, 2236–2242. [Google Scholar] [CrossRef]
- Nam, N.H. Naturally occurring NF-kappaB inhibitors. Mini Rev. Med. Chem. 2006, 6, 945–951. [Google Scholar] [CrossRef]
- Garban, H.; Bonavida, B. Nitric Oxide Sensitizes Ovarian Tumor Cells to Fas-Induced Apoptosis. Gynecol. Oncol. 1999, 73, 257–264. [Google Scholar] [CrossRef]
- Gordon, S.; Akopyan, G.; Garban, H.; Bonavida, B. Transcription factor YY1: Structure, function, and therapeutic implications in cancer biology. Oncogene 2005, 25, 1125–1142. [Google Scholar] [CrossRef] [Green Version]
- Bonavida, B.; Garban, H. Nitric oxide-mediated sensitization of resistant tumor cells to apoptosis by chemo-immunotherapeutics. Redox Biol. 2015, 6, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; El-Deiry, W.S. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003, 22, 8628–8633. [Google Scholar] [CrossRef] [Green Version]
- Zauli, G.; Pandolfi, A.; Gonelli, A.; Di Pietro, R.; Guarnieri, S.; Ciabattoni, G.; Rana, R.; Vitale, M.; Secchiero, P. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) sequentially up-regulates nitric oxide and prostanoid production in primary human endothelial cells. Circ. Res. 2003, 92, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Yepez, S.; Vega, M.; Jazirehi, A.; Garban, H.; Hongo, F.; Cheng, G.; Bonavida, B. Nitric oxide sensitizes prostate carcinoma cell lines to TRAIL-mediated apoptosis via inactivation of NF-kappa B and inhibition of Bcl-xl expression. Oncogene 2004, 23, 4993–5003. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Yepez, S.; Vega, M.; Escoto-Chavez, S.E.; Murdock, B.; Sakai, T.; Baritaki, S.; Bonavida, B. Nitric oxide sensitizes tumor cells to TRAIL-induced apoptosis via inhibition of the DR5 transcription repressor Yin Yang 1. Nitric Oxide 2009, 20, 39–52. [Google Scholar] [CrossRef]
- Chyuan, I.-T.; Tsai, H.-F.; Wu, C.-S.; Sung, C.-C.; Hsu, P.-N. TRAIL-Mediated Suppression of T Cell Receptor Signaling Inhibits T Cell Activation and Inflammation in Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2018, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fehsel, K.; Kröncke, K.D.; Meyer, K.L.; Huber, H.; Wahn, V.; Kolb-Bachofen, V. Nitric oxide induces apoptosis in mouse thymocytes. J. Immunol. 1995, 155, 2858–2865. [Google Scholar] [PubMed]
- Williams, M.S.; Noguchi, S.; Henkart, P.A.; Osawa, Y. Nitric oxide synthase plays a signaling role in TCR-triggered apoptotic death. J. Immunol. 1998, 161, 6526–6531. [Google Scholar]
- Saio, M.; Radoja, S.; Marino, M.; Frey, A. Tumor-Infiltrating Macrophages Induce Apoptosis in Activated CD8+T Cells by a Mechanism Requiring Cell Contact and Mediated by Both the Cell-Associated Form of TNF and Nitric Oxide. J. Immunol. 2001, 167, 5583–5593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vig, M.; Srivastava, S.; Kandpal, U.; Sade, H.; Lewis, V.; Sarin, A.; George, A.; Bal, V.; Durdik, J.M.; Rath, S. Inducible nitric oxide synthase in T cells regulates T cell death and immune memory. J. Clin. Investig. 2004, 113, 1734–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef] [Green Version]
- Farc, O.; Cristea, V. An overview of the tumor microenvironment, from cells to complex networks (Review). Exp. Ther. Med. 2020, 21, 96. [Google Scholar] [CrossRef]
- Feng, G.; Gao, W.; Strom, T.B.; Oukka, M.; Francis, R.S.; Wood, K.J.; Bushell, A. Exogenous IFN-gamma ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3+ regulatory T cells. Eur J. Immunol. 2008, 38, 2512–2527. [Google Scholar] [CrossRef] [Green Version]
- Niedbala, W.; Cai, B.; Liu, H.; Pitman, N.; Chang, L.; Liew, F.Y. Nitric oxide induces CD4+CD25+ Foxp3 regulatory T cells from CD4+CD25 T cells via p53, IL-2, and OX40. Proc. Natl. Acad. Sci. USA 2007, 104, 15478–15483. [Google Scholar] [CrossRef] [Green Version]
- Asadzadeh, Z.; Mohammadi, H.; Safarzadeh, E.; Hemmatzadeh, M.; Mahdian-Shakib, A.; Jadidi-Niaragh, F.; Azizi, G.; Baradaran, B. The paradox of Th17 cell functions in tumor immunity. Cell. Immunol. 2017, 322, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Dobrzanski, M.J. Expanding Roles for CD4 T Cells and Their Subpopulations in Tumor Immunity and Therapy. Front. Oncol. 2013, 3, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verinaud, L.; Issayama, L.K.; Zanucoli, F.; De Carvalho, A.C.; da Costa, T.A.; Di Gangi, R.; Bonfanti, A.; Ferreira, I.T.; Oliveira, A.; Machado, D.R.S.; et al. Nitric oxide plays a key role in the suppressive activity of tolerogenic dendritic cells. Cell. Mol. Immunol. 2014, 12, 384–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raber, P.L.; Thevenot, P.; Sierra, R.; Wyczechowska, D.; Halle, D.; Ramirez, M.E.; Ochoa, A.C.; Fletcher, M.; Velasco, C.; Wilk, A.; et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int. J. Cancer 2013, 134, 2853–2864. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, M.; Gerhardt, L.; Vareki, S.M. Immunosuppressive Effects of Myeloid-Derived Suppressor Cells in Cancer and Immunotherapy. Cells 2021, 10, 1170. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Dhodapkar, M.V.; Dhodapkar, K.M.; Palucka, A.K. Interactions of tumor cells with dendritic cells: Balancing immunity and tolerance. Cell Death Differ. 2007, 15, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Markowitz, J.; Wang, J.; Vangundy, Z.; You, J.; Yildiz, V.; Yu, L.; Foote, I.P.; Branson, O.E.; Stiff, A.R.; Brooks, T.R.; et al. Nitric oxide mediated inhibition of antigen presentation from DCs to CD4+ T cells in cancer and measurement of STAT1 nitration. Sci Rep. 2017, 7, 15424. [Google Scholar] [CrossRef] [Green Version]
- Cartwright, A.N.; Suo, S.; Badrinath, S.; Kumar, S.; Melms, J.; Luoma, A.; Bagati, A.; Saadatpour, A.; Izar, B.; Yuan, G.-C.; et al. Immunosuppressive Myeloid Cells Induce Nitric Oxide–Dependent DNA Damage and p53 Pathway Activation in CD8+ T Cells. Cancer Immunol. Res. 2021, 9, 470–485. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Zhou, W.-L.; Huang, Y.; Liang, X.; Jiang, L.; Yang, X.; Sun, J.; Li, Z.; Han, W.-D.; et al. Genetically engineered T cells for cancer immunotherapy. Signal Transduct. Target. Ther. 2019, 4, 1–17. [Google Scholar] [CrossRef]
- Perrotta, C.; Cervia, D.; Di Renzo, I.; Moscheni, C.; Bassi, M.T.; Campana, L.; Martelli, C.; Catalani, E.; Giovarelli, M.; Zecchini, S.; et al. Nitric Oxide Generated by Tumor-Associated Macrophages Is Responsible for Cancer Resistance to Cisplatin and Correlated with Syntaxin 4 and Acid Sphingomyelinase Inhibition. Front. Immunol. 2018, 9, 1186. [Google Scholar] [CrossRef] [Green Version]
- Giavridis, T.; van der Stegen, S.J.C.; Eyquem, J.; Hamieh, M.; Piersigilli, A.; Sadelain, M. CAR T cell-induced cytokine release syndrome is mediated by mac-rophages and abated by IL-1 blockade. Nat. Med. 2018, 24, 731–738. [Google Scholar] [CrossRef]
- Saini, A.S.; Shenoy, G.; Rath, S.; Bal, V.; George, A. Inducible nitric oxide synthase is a major intermediate in signaling pathways for the survival of plasma cells. Nat. Immunol. 2014, 15, 275–282. [Google Scholar] [CrossRef]
- Dinarello, C.A. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- García-Ortiz, A.; Serrador, J.M. Nitric Oxide Signaling in T Cell-Mediated Immunity. Trends Mol. Med. 2018, 24, 412–427. [Google Scholar] [CrossRef] [PubMed]
- PeÑarando, J.; Aranda, E.; RodrÍguez-Ariza, A. Immunomodulatory roles of nitric oxide in cancer: Tumor microenvironment says “.NO” to antitumor immune response. Transl. Res. 2019, 210, 99–108. [Google Scholar] [PubMed]
- Porrini, C.; Ramarao, N.; Tran, S.L. Dr. NO and Mr. Toxic-the versatile role of nitric oxide. Biol Chem. 2020, 401, 547–572. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Shi, J.; Zhang, J.; Wang, Y.; Guo, Y.; Zhang, Z. Progress and Prospects of Regulatory Functions Mediated by Nitric Oxide on Immunity and Immunotherapy. Adv. Ther. 2021, 4, 2100032. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navasardyan, I.; Bonavida, B. Regulation of T Cells in Cancer by Nitric Oxide. Cells 2021, 10, 2655. https://doi.org/10.3390/cells10102655
Navasardyan I, Bonavida B. Regulation of T Cells in Cancer by Nitric Oxide. Cells. 2021; 10(10):2655. https://doi.org/10.3390/cells10102655
Chicago/Turabian StyleNavasardyan, Inesa, and Benjamin Bonavida. 2021. "Regulation of T Cells in Cancer by Nitric Oxide" Cells 10, no. 10: 2655. https://doi.org/10.3390/cells10102655
APA StyleNavasardyan, I., & Bonavida, B. (2021). Regulation of T Cells in Cancer by Nitric Oxide. Cells, 10(10), 2655. https://doi.org/10.3390/cells10102655





