The PSA-NCAM-Positive “Immature” Neurons: An Old Discovery Providing New Vistas on Brain Structural Plasticity
Abstract
:1. Introduction
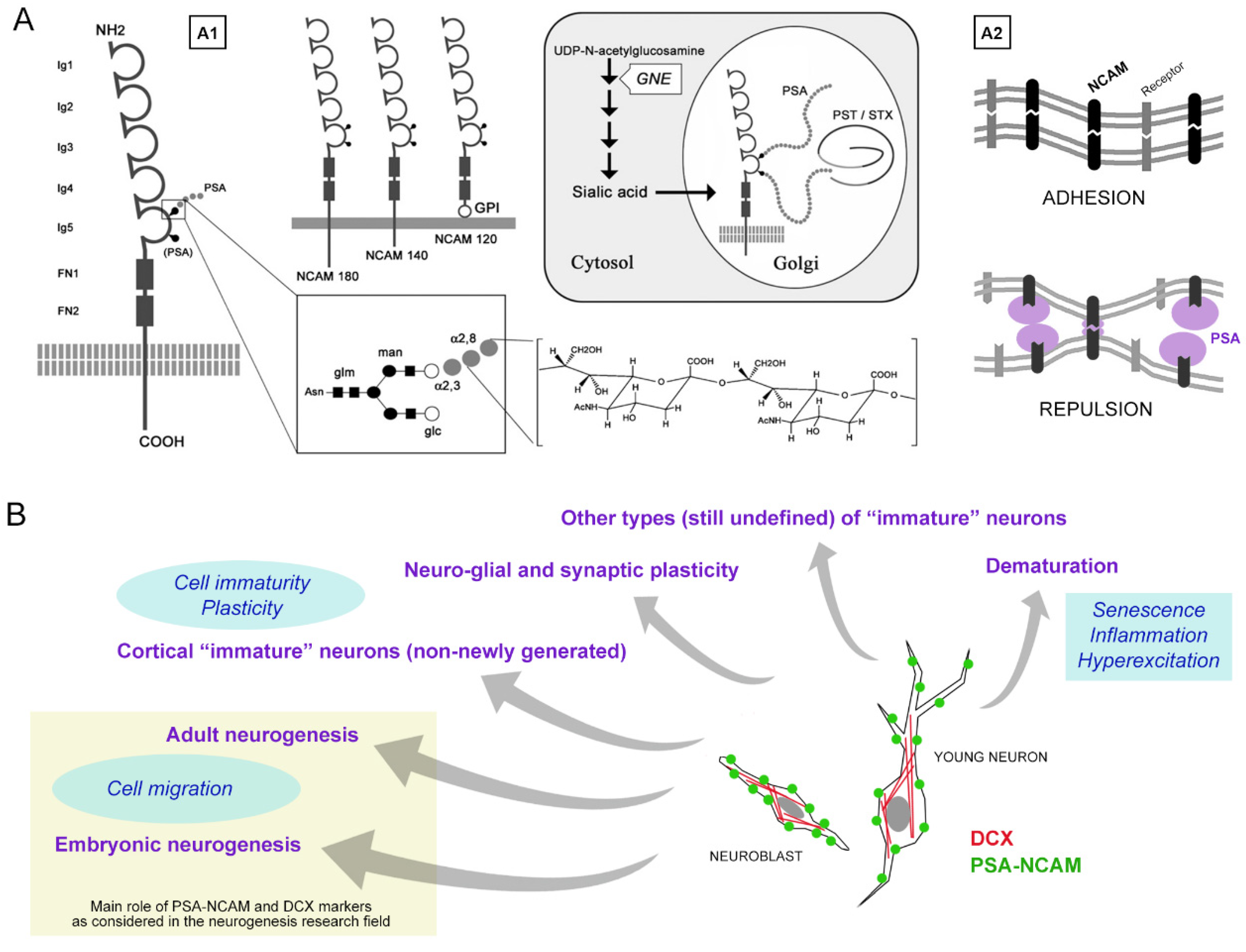
1.1. PSA-NCAM and Its Multifaceted Properties
1.2. Heterogeneity of Brain Plasticity and Related Ill-Defined Issues: Cell Markers and Neuronal Immaturity
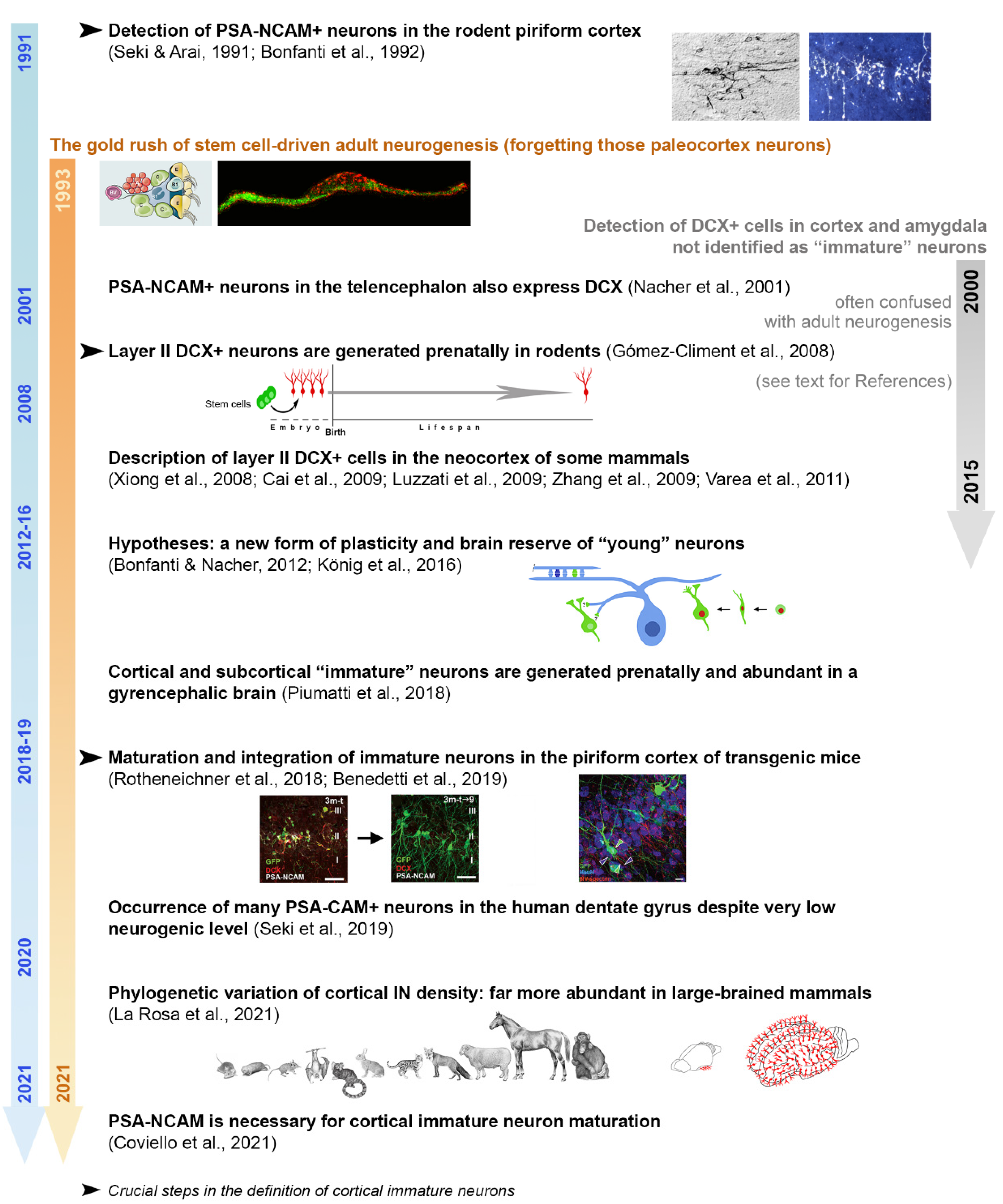
2. A Brief History of “Immature” Neurons
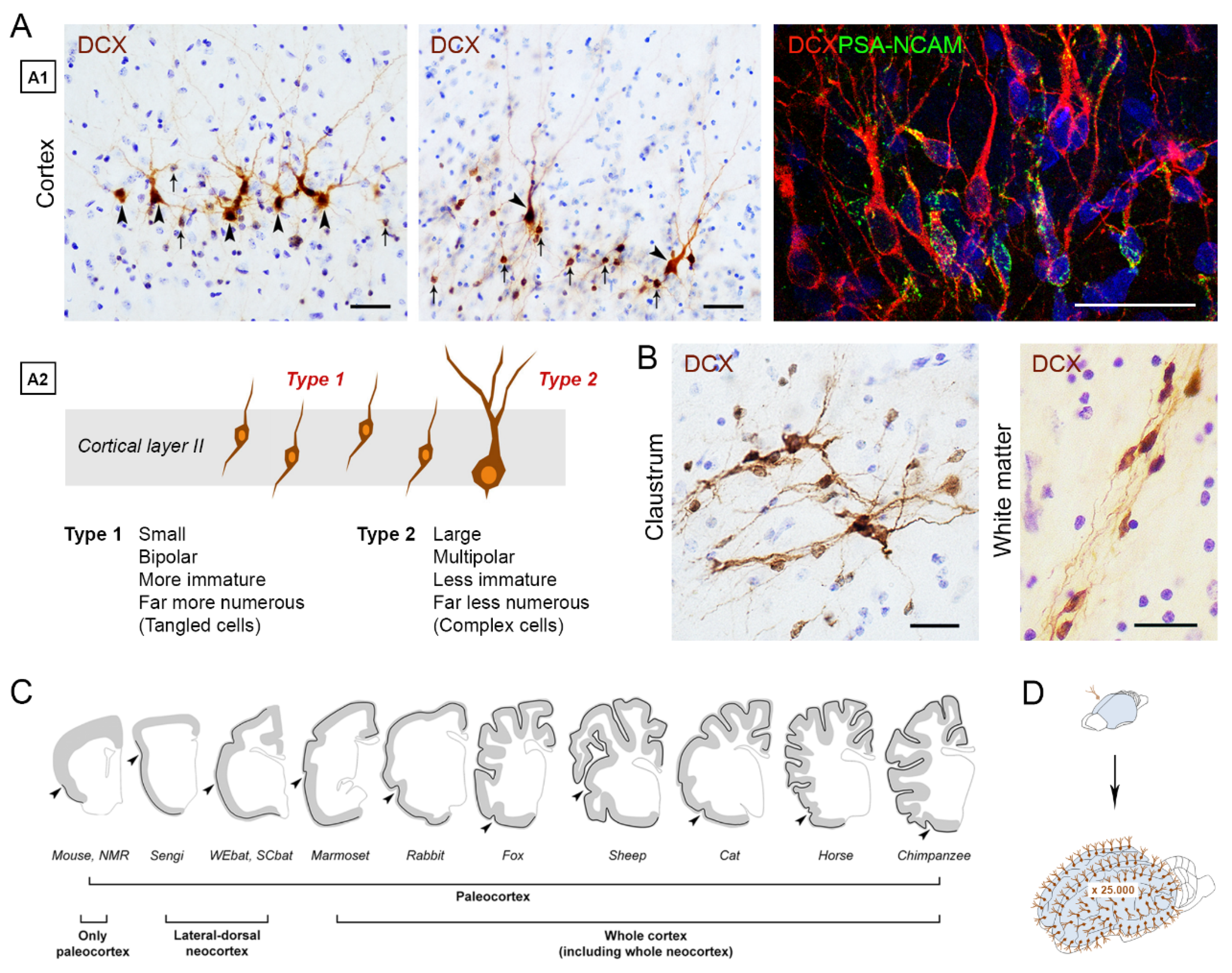
3. The “Immature” Neurons: Possible Heterogeneity and Problems for Their Definition
3.1. Immature Neurons and Neurogenesis
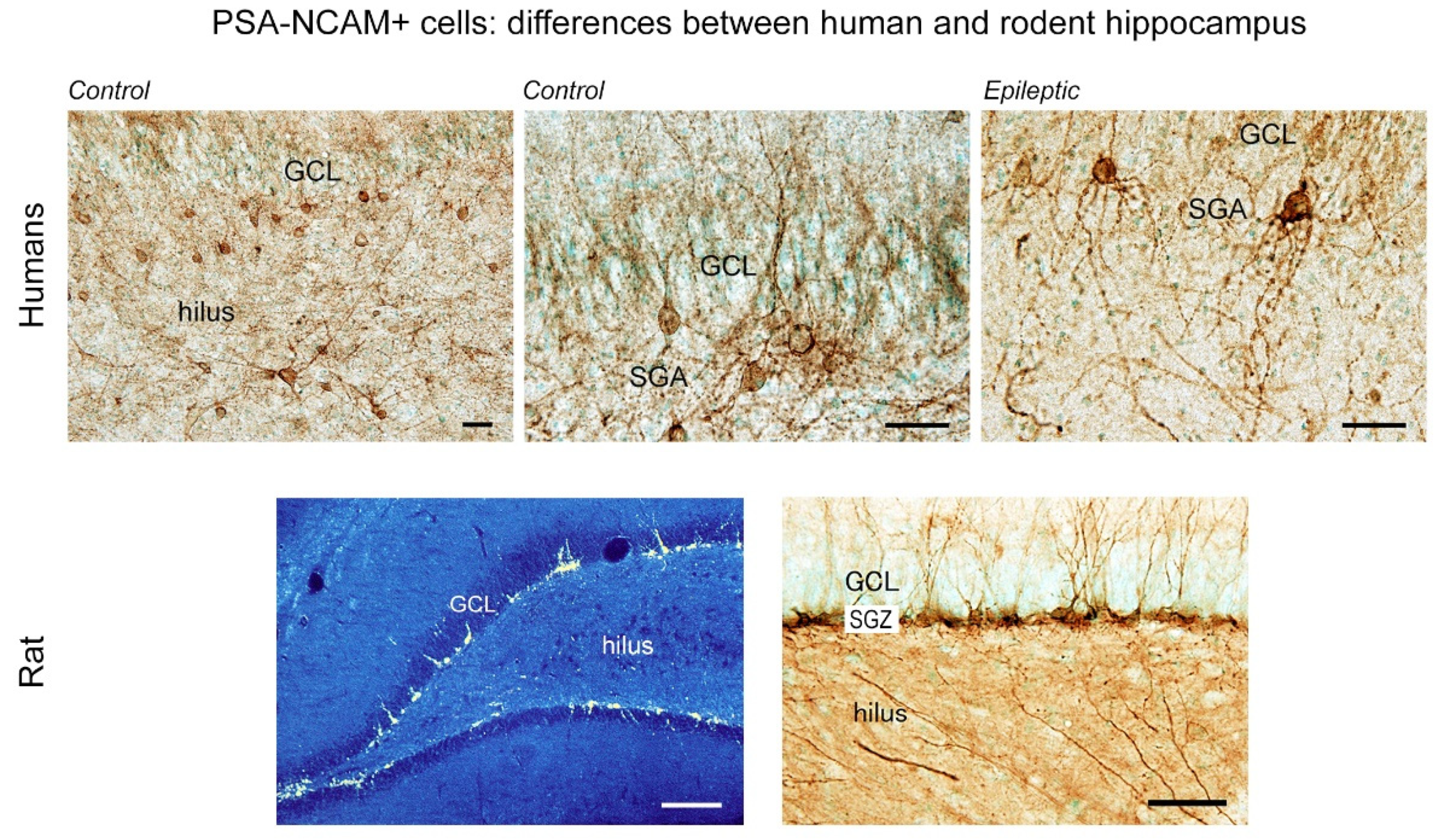
3.2. PSA-NCAM Expressing Neurons in the Human Hippocampus
4. Open Questions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hensch, T.K. Critical period regulation. Annu. Rev. Neurosci. 2004, 27, 549–579. [Google Scholar] [CrossRef] [Green Version]
- Semënov, M.V. Adult hippocampal neurogenesis is a developmental process involved in cognitive development. Front. Neurosci. 2019, 13, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cushman, J.D.; Drew, M.R.; Krasne, F.B. The environmental sculpting hypothesis of juvenile and adult hippocampal neurogenesis. Prog. Neurobiol. 2021, 199, 101961. [Google Scholar] [CrossRef] [PubMed]
- Holtmaat, A.; Svoboda, K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009, 10, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Sale, A.; Berardi, N.; Maffei, L. Enrich the environment to empower the brain. Trends Neurosci. 2009, 32, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Vivar, C.; van Praag, H. Running changes the brain: The long and the short of it. Physiology 2017, 32, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G. Environmental enrichment, new neurons and the neurobiology of individuality. Nat. Rev. Neurosci. 2019, 20, 235–245. [Google Scholar] [CrossRef]
- Zolochevska, O.; Taglialatela, G. Non-demented individuals with Alzheimer’s disease neuropathology: Resistance to cognitive decline may reveal new treatment strategies. Curr. Pharm. Des. 2016, 22, 4063–4068. [Google Scholar] [CrossRef]
- Stern, Y.; Arenaza-Urquijo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantilon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement. 2020, 16, 1305–1311. [Google Scholar] [CrossRef]
- McQuail, J.A.; Dunn, A.R.; Stern, Y.; Barnes, C.A.; Kempermann, G.; Rapp, P.R.; Kaczorowski, C.C.; Foster, T.C. Cognitive reserve in model systems for mechanistic discovery: The importance of longitudinal studies. Front. Aging Neurosci. 2021, 12, 607685. [Google Scholar] [CrossRef]
- Tooley, U.A.; Bassett, D.S.; Mackey, A.P. Environmental influences on the pace of brain development. Nat. Rev. Neurosci. 2021, 47, 100909. [Google Scholar]
- Martino, G.; Pluchino, S.; Bonfanti, L.; Schwartz, M. Brain regeneration in physiology and pathology: The immune signature driving therapeutic plasticity of neural stem cells. Phys. Rev. 2011, 91, 1281–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond, A.M.; Ming, G.; Song, H. Adult mammalian neural stem cells and neurogenesis: Five decades later. Cell Stem Cell 2015, 17, 385–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, H.; Song, J. Treating brain disorders by targeting adult neural stem cells. Trends Mol. Med. 2018, 24, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.A.; Götz, M.; Parmar, M. New approaches for brain repair-from rescue to reprogramming. Nature 2018, 557, 329–334. [Google Scholar] [CrossRef]
- Amrein, I. Adult hippocampal neurogenesis in natural populations of mammals. Cold Spring Harb. Perspect. Biol. 2015, 7, a021295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes, M.F.; Sorrells, S.F.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Brain size and limits to adult neurogenesis. J. Comp. Neurol. 2016, 524, 646–664. [Google Scholar]
- Parolisi, R.; Cozzi, B.; Bonfanti, L. Humans and dolphins: Decline and fall of adult neurogenesis. Front. Neurosci. 2018, 12, 497. [Google Scholar] [CrossRef] [Green Version]
- Palazzo, O.; La Rosa, C.; Piumatti, M.; Bonfanti, L. Do large brains of long-living mammals prefer non-newly generated, immature neurons? Neural Regen. Res. 2018, 13, 633–634. [Google Scholar]
- Bonfanti, L.; Charvet, C.J. Brain plasticity in humans and model systems: Advances, challenges, and future directions. Int. J. Mol. Sci. 2021, 22, 9358. [Google Scholar] [CrossRef]
- Charvet, C.J.; Finlay, B.L. Comparing adult hippocampal neurogenesis across species: Translating time to predict the tempo in humans. Front. Neurosci. 2018, 12, 706. [Google Scholar] [CrossRef]
- Seki, T.; Arai, Y. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J. Neurosci. 1993, 13, 2351–2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lois, C.; Alvarez-Buylla, A. Long-distance neuronal migration in the adult mammalian brain. Science 1994, 264, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.A.; Alvarez-Buylla, A. The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018820. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Von Bohlen Und Halbach, O. Immunohistological markers for staging neurogenesis in the adult hippocampus. Cell Tissue Res. 2007, 329, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Urbán, N.; Guillemot, F. Neurogenesis in the embryonic and adult brain: Same regulators, different roles. Front. Cell. Neurosci. 2014, 8, 396. [Google Scholar] [CrossRef] [Green Version]
- Seki, T.; Arai, Y. Expression of highly polysialylated NCAM in the neocortex and piriform cortex of the developing and the adult rat. Anat. Embryol. 1991, 184, 395–401. [Google Scholar] [CrossRef]
- Seki, T.; Arai, Y. The persistent expression of a highly polysialylated NCAM in the dentate gyrus of the adult rat. Neurosci. Res. 1991, 12, 503–513. [Google Scholar] [CrossRef]
- Theodosis, D.T.; Rougon, G.; Poulain, D.A. Retention of embryonic features by an adult neuronal system capable of plasticity: Polysialylated neural cell adhesion molecule in the hypothalamo-neurohypophysial system. Proc. Natl. Acad. Sci. USA 1991, 88, 5494–5498. [Google Scholar] [CrossRef] [Green Version]
- Rutishauser, U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 2008, 9, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, S.; Edelman, G.M. Kinetics of homophilic binding by embryonic and adult forms of the neural cell adhesion molecule. Proc. Natl. Acad. Sci. USA 1983, 80, 5762–5766. [Google Scholar] [CrossRef] [Green Version]
- Rutishauser, U.; Acheson, A.; Hall, A.K.; Mann, D.M.; Sunshine, J. The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science 1988, 240, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Acheson, A.; Sunshine, J.L.; Rutishauser, U. NCAM polysialic acid can regulate both cell~cell and cell-substrate interactions. J. Cell Biol. 1991, 114, 143–153. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef] [Green Version]
- Sato, C.; Kitajima, K. Polysialylation and disease. Mol. Asp. Med. 2021, 79, 100892. [Google Scholar] [CrossRef]
- Ono, K.; Tomasiewicz, H.; Magnuson, T.; Rutishauser, U. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron 1994, 13, 595–609. [Google Scholar] [CrossRef]
- Murakami, S.; Seki, T.; Rutishauser, U.; Arai, Y. Enzymatic removal of polysialic acid from neural cell adhesion molecule perturbs the migration route of luteinizing hormone-releasing hormone neurons in the developing chick forebrain. J. Comp. Neurol. 2000, 420, 171–181. [Google Scholar] [CrossRef]
- Doherty, P.; Cohen, J.; Walsh, F.S. Neurite outgrowth in response to transfected N-CAM changes during development and is modulated by polysialic acid. Neuron 1990, 5, 209–219. [Google Scholar] [CrossRef]
- Landmesser, L.; Dahm, L.; Tang, J.C.; Rutishauser, U. Polysialic acid as a regulator of intramuscular nerve branching during embryonic development. Neuron 1990, 4, 655–667. [Google Scholar] [CrossRef]
- Yamamoto, N.; Inui, K.; Matsuyama, Y.; Harada, A.; Hanamura, K.; Murakami, F.; Ruthazer, E.S.; Rutishauser, U.; Seki, T. Inhibitory mechanism by polysialic acid for lamina-specific branch formation of thalamocortical axons. J. Neurosci. 2000, 20, 9145–9151. [Google Scholar] [CrossRef] [Green Version]
- Di Cristo, G.; Chattopadhyaya, B.; Kuhlman, S.J.; Fu, Y.; Bélanger, M.C.; Wu, C.Z.; Rutishauser, U.; Maffei, L.; Huang, Z.J. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat. Neurosci. 2007, 10, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Dityatev, A.; Dityateva, G.; Sytnyk, V.; Delling, M.; Toni, N.; Nikonenko, I.; Muller, D.; Schachner, M. Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J. Neurosci. 2004, 24, 9372–9382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfanti, L.; Nacher, J. New scenarios for neuronal structural plasticity in non-neurogenic brain parenchyma: The case of cortical layer II immature neurons. Prog. Neurobiol. 2012, 98, 1–15. [Google Scholar] [CrossRef]
- König, R.; Benedetti, B.; Rotheneichner, P.; O′Sullivan, A.; Kreutzer, C.; Belles, M.; Nacher, J.; Weiger, T.M.; Aigner, L.; Couillard-Després, S. Distribution and fate of DCX/PSA-NCAM expressing cells in the adult mammalian cortex: A local reservoir for adult cortical neuroplasticity? Front. Biol. 2016, 11, 193–213. [Google Scholar] [CrossRef]
- Piumatti, M.; Palazzo, O.; La Rosa, C.; Crociara, P.; Parolisi, R.; Luzzati, F.; Lévy, F.; Bonfanti, L. Non-newly generated, “immature” neurons in the sheep brain are not restricted to cerebral cortex. J. Neurosci. 2018, 38, 826–842. [Google Scholar] [CrossRef] [Green Version]
- Sorrells, S.F.; Paredes, M.F.; Velmeshev, D.; Herranz-Pérez, V.; Sandoval, K.; Mayer, S.; Chang, E.F.; Insausti, R.; Kriegstein, A.R.; Rubenstein, J.L.; et al. Immature excitatory neurons develop during adolescence in the human amygdala. Nat. Commun. 2019, 10, 2748. [Google Scholar] [CrossRef] [Green Version]
- Chareyron, L.J.; Banta Lavenex, P.; Amaral, D.G.; Lavenex, P. Life and death of immature neurons in the juvenile and adult primate amygdala. Int. J. Mol. Sci. 2021, 22, 6691. [Google Scholar] [CrossRef]
- La Rosa, C.; Parolisi, R.; Bonfanti, L. Brain structural plasticity: From adult neurogenesis to immature neurons. Front. Neurosci. 2020, 14, 75. [Google Scholar] [CrossRef]
- Brown, J.P.; Couillard-Despres, S.; Cooper-Kuhn, C.M.; Winkler, J.; Aigner, L.; Kuhn, H.G. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003, 467, 1–10. [Google Scholar] [CrossRef]
- Marichal, N.; Garcìa, G.; Radmilovich, M.; Trujillo-Cenoz, O.; Russo, R.E. Enigmatic central canal contacting cells: Immature neurons in “standby mode”? J. Neurosci. 2009, 29, 10010–10024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfanti, L.; Theodosis, D.T. Polysialic acid and activity-dependent synaptic remodeling. Cell Adhes. Migr. 2009, 3, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Bonfanti, L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog. Neurobiol. 2006, 80, 129–164. [Google Scholar] [CrossRef] [PubMed]
- Gascon, E.; Vutskits, L.; Kiss, J.Z. The role of PSA-NCAM in adult neurogenesis. In Structure and Function of the Neural Cell Adhesion Molecule NCAM; Berezin, V., Ed.; Springer: New York, NY, USA, 2010; Volume 663, pp. 127–136. [Google Scholar]
- Theodosis, D.T.; Poulain, D.A.; Oliet, S.H. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol. Rev. 2008, 88, 983–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.X.; Eroglu, C. Cell adhesion molecules regulating astrocyte–neuron interactions. Curr. Opin. Neurobiol. 2021, 69, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Arai, Y. Distribution and possible roles of the highly polysialylated neural cell adhesion molecule (NCAM-H) in the developing and adult central nervous system. Neurosci. Res. 1993, 17, 265–290. [Google Scholar] [CrossRef]
- Bonfanti, L.; Olive, S.; Poulain, D.A.; Theodosis, D.T. Mapping of the distribution of polysialylated neural cell adhesion molecule throughout the central nervous system of the adult rat: An immunohistochemical study. Neuroscience 1992, 49, 419–436. [Google Scholar] [CrossRef]
- Luskin, M.B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 1993, 11, 173–189. [Google Scholar] [CrossRef]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef]
- Altman, J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol. 1969, 137, 433–457. [Google Scholar] [CrossRef]
- Gould, E.; Cameron, H.A.; Daniels, D.C.; Woolley, C.S.; McEwen, B.S. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J. Neurosci. 1992, 12, 3642–3650. [Google Scholar] [CrossRef]
- Cameron, H.A.; Woolley, C.S.; McEwen, B.S.; Gould, E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 1993, 56, 337–344. [Google Scholar] [CrossRef]
- Seki, T.; Arai, Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport 1995, 6, 2479–2482. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, L.; Theodosis, D.T. Expression of polysialylated neural cell adhesion molecule by proliferating cells in the subependymal layer of the adult rat, in its rostral extension and in the olfactory bulb. Neuroscience 1994, 62, 291–305. [Google Scholar] [CrossRef]
- Rousselot, P.; Lois, C.; Alvarez-Buylla, A. Embryonic (PSA) N-CAM reveals chains of migrating neuroblasts between the lateral ventricle and the olfactory bulb of adult mice. J. Comp. Neurol. 1995, 351, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempermann, G.; Kuhn, H.G.; Gage, F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature 1997, 386, 493–495. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.-M.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- Parent, J.M.; Yu, T.W.; Leibowitz, R.T.; Geschwind, D.H.; Sloviter, R.S.; Lowenstein, D.H. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997, 17, 3727–3738. [Google Scholar] [CrossRef]
- Doetsch, F.; Caille, I.; Lim, D.A.; Garcìa-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef] [Green Version]
- Burgess, A.; Wainwright, S.R.R.; Shihabuddin, L.S.S.; Rutishauser, U.; Seki, T.; Aubert, I. Polysialic acid regulates the clustering, migration, and neuronal differentiation of progenitor cells in the adult hippocampus. Dev. Neurobiol. 2008, 68, 1580–1590. [Google Scholar] [CrossRef]
- Weinhold, B.; Seidenfaden, R.; Röckle, I.; Mühlenhoff, M.; Schertzinger, F.; Conzelmann, S.; Marth, J.D.; Gerardy-Schahn, R.; Hildebrandt, H. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J. Biol. Chem. 2005, 280, 42971–42977. [Google Scholar] [CrossRef] [Green Version]
- Petridis, A.K.; El-Maarouf, A.; Rutishauser, U. Polysialic acid regulates cell contact-dependent neuronal differentiation of progenitor cells from the subventricular zone. Dev. Dyn. 2004, 230, 675–684. [Google Scholar] [CrossRef]
- Röckle, I.; Seidenfaden, R.; Weinhold, B.; Mühlenhoff, M.; Gerardy-Schahn, R.; Hildebrandt, H. Polysialic acid controls NCAM-induced differentiation of neuronal precursors into calretinin-positive olfactory bulb interneurons. Dev. Neurobiol. 2008, 68, 1170–1184. [Google Scholar] [CrossRef]
- Seki, T.; Rutishauser, U. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J. Neurosci. 1998, 18, 3757–3766. [Google Scholar] [CrossRef] [Green Version]
- Rotheneichner, P.; Belles, M.; Benedetti, B.; König, R.; Dannehl, D.; Kreutzer, C.; Zaunmair, P.; Engelhardt, M.; Aigner, L.; Nacher, J.; et al. Cellular plasticity in the adult murine piriform cortex: Continuous maturation of dormant precursors into excitatory neurons. Cereb. Cortex 2018, 28, 2610–2621. [Google Scholar] [CrossRef] [Green Version]
- La Rosa, C.; Cavallo, F.; Pecora, A.; Chincarini, M.; Ala, U.; Faulkes, C.G.; Nacher, J.; Cozzi, B.; Sherwood, C.C.; Amrein, I.; et al. Phylogenetic variation in cortical layer II immature neuron reservoir of mammals. ELife 2020, 9, e55456. [Google Scholar] [CrossRef] [PubMed]
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef] [Green Version]
- Oppenheim, R.W. Adult hippocampal neurogenesis in mammals (and humans): The death of a central dogma in neuroscience and its replacement by a new dogma. Dev. Neurobiol. 2019, 79, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.M.; Boonstra, R.; Wojtowicz, J.M. From pattern to purpose: How comparative studies contribute to understanding the function of adult neurogenesis. Eur. J. Neurosci. 2011, 34, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Climent, M.A.; Castillo-Gómez, E.; Varea, E.; Guirado, R.; Blasco-Ibáñez, J.M.; Crespo, C.; Martínez-Guijarro, F.J.; Nácher, J. A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb. Cortex 2008, 18, 2229–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedetti, B.; Dannehl, D.; König, R.; Coviello, S.; Kreutzer, C.; Zaunmair, P.; Jakubecova, D.; Weiger, T.M.; Aigner, L.; Nacher, J.; et al. Functional integration of neuronal precursors in the adult murine piriform cortex. Cereb. Cortex 2020, 30, 1499–1515. [Google Scholar] [CrossRef] [Green Version]
- Yuan, T.F.; Liang, Y.X.; So, K.F. Occurrence of new neurons in the piriform cortex. Front. Neuroanat. 2015, 8, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nacher, J.; Bonfanti, L. New neurons from old beliefs in the adult piriform cortex? A Commentary on: “Occurrence of new neurons in the piriform cortex” . Front. Neuroanat. 2015, 9, 62. [Google Scholar] [PubMed] [Green Version]
- Klempin, F.; Kronenberg, G.; Cheung, G.; Kettenmann, H.; Kempermann, G. Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice. PLoS ONE 2011, 6, e25760. [Google Scholar] [CrossRef] [Green Version]
- Varea, E.; Belles, M.; Vidueira, S.; Blasco-Ibáñez, J.M.; Crespo, C.; Pastor, Á.M.; Nacher, J. PSA-NCAM is expressed in immature, but not recently generated, neurons in the adult cat cerebral cortex layer II. Front. Neurosci. 2011, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Xiong, K.; Chu, Y.; Luo, D.W.; Luo, X.G.; Yuan, X.Y.; Struble, R.G.; Clough, R.W.; Spencer, D.D.; Williamson, A.; et al. Doublecortin expression in adult cat and primate cerebral cortex relates to immature neurons that develop into GABAergic subgroups. Exp. Neurol. 2009, 216, 342–356. [Google Scholar] [CrossRef] [Green Version]
- Luzzati, F.; Bonfanti, L.; Fasolo, A.; Peretto, P. DCX and PSA-NCAM expression identifies a population of neurons preferentially distributed in associative areas of different pallial derivatives and vertebrate species. Cereb. Cortex 2009, 19, 1028–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.M.; Cai, Y.; Chu, Y.; Chen, E.Y.; Feng, J.C.; Luo, X.G.; Xiong, K.; Struble, R.G.; Clough, R.W.; Patrylo, P.R.; et al. Doublecortin-expressing cells persist in the associative cerebral cortex and amygdala in aged nonhuman primates. Front. Neuroanat. 2009, 3, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloch, J.; Kaeser, M.; Sadeghi, Y.; Rouiller, E.M.; Redmond, D.E., Jr.; Brunet, J. Doublecortin-positive cells in the adult primate cerebral cortex and possible role in brain plasticity and development. J. Comp. Neurol. 2011, 519, 775–789. [Google Scholar] [CrossRef] [Green Version]
- Martí-Mengual, U.; Varea, E.; Crespo, C.; Blasco-Ibáñez, J.M.; Nacher, J. Cells expressing markers of immature neurons in the amygdala of adult humans. Eur. J. Neurosci. 2013, 37, 10–22. [Google Scholar] [CrossRef]
- La Rosa, C.; Bonfanti, L. Searching for alternatives to brain regeneration. Neur. Reg. Res. 2021, 16, 2198–2200. [Google Scholar]
- Fung, S.J.; Joshi, D.; Allen, K.M.; Sivagnanasundaram, S.; Rothmond, D.A.; Saunders, R.; Noble, P.L.; Webster, M.J.; Weickert, C.S. Developmental patterns of doublecortin expression and white matter neuron density in the postnatal primate prefrontal cortex and schizophrenia. PLoS ONE 2011, 6, e25194. [Google Scholar] [CrossRef]
- Marlatt, M.W.; Philippens, I.; Manders, E.; Czéh, B.; Joels, M.; Krugers, H.; Lucassen, P.J. Distinct structural plasticity in the hippocampus and amygdala of the middle-aged common marmoset (Callithrix jacchus). Exp. Neurol. 2011, 230, 291–301. [Google Scholar] [CrossRef]
- Bonfanti, L.; Peretto, P. Adult neurogenesis in mammals: A theme with many variations. Eur. J. Neurosci. 2011, 34, 930–950. [Google Scholar] [CrossRef]
- Feliciano, D.M.; Bordey, A.; Bonfanti, L. Noncanonical sites of adult neurogenesis in the mammalian brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a018846. [Google Scholar] [CrossRef] [Green Version]
- Francis, F.; Koulakoff, A.; Boucher, D.; Chafey, P.; Schaar, B.; Vinet, M.C.; Friocourt, G.; McDonnell, N.; Reiner, O.; Kahn, A.; et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 1999, 23, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, J.G.; Lin, P.T.; Flanagan, L.A.; Walsh, C.A. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 1999, 23, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Nacher, J.; Crespo, C.; McEwen, B.S. Doublecortin expression in the adult rat telencephalon. Eur. J. Neurosci. 2001, 14, 629–644. [Google Scholar] [CrossRef]
- Nacher, J.; Guirado, R.; Castillo-Gómez, E. Structural plasticity of interneurons in the adult brain: Role of PSA-NCAM and implications for psychiatric disorders. Neurochem. Res. 2013, 38, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Guirado, R.; Perez-Rando, M.; Sanchez-Matarredona, D.; Castillo-Gómez, E.; Liberia, T.; Rovira-Esteban, L.; Varea, E.; Crespo, C.; Blasco-Ibáñez, J.M.; Nacher, J. The dendritic spines of interneurons are dynamic structures influenced by PSA-NCAM expression. Cereb. Cortex 2014, 24, 3014–3024. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 2018, 22, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Jimenéz, E.P.; Flor-Garcia, M.; Terreros-Roncal, J.; Rabano, A.; Cafini, F.; Pallas-Bazarra, N.; Avila, J.; Llorens-Martin, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Paredes, M.F.; Zhang, Z.; Kang, G.; Pastor-Alonso, O.; Biagiotti, S.; Page, C.E.; Sandoval, K.; Knox, A.; Connolly, A.; et al. Positive controls in adults and children support that very few, if any, new neurons are born in the adult human hippocampus. J. Neurosci. 2021, 41, 2554–2565. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Hori, T.; Miyata, H.; Maehara, M.; Namba, T. Analysis of proliferating neuronal progenitors and immature neurons in the human hippocampus surgically removed from control and epileptic patients. Sci. Rep. 2019, 9, 18194. [Google Scholar] [CrossRef] [Green Version]
- Duque, A.; Spector, R. A balanced evaluation of the evidence for adult neurogenesis in humans: Implication for neuropsychiatric disorders. Brain Struct. Funct. 2019, 224, 2281–2295. [Google Scholar] [CrossRef]
- Mikkonen, M.; Soininen, B.M.; Kalviainen, R.; Tapiola, T.; Ylinen, A.; Vapalahti, M.; Paljarvi, L.; Pitkanen, A. Remodeling of neuronal circuitries in human temporal lobe epilepsy: Increased expression of highly polysialylated neural cell adhesion molecule in the hippocampus and the entorhinal cortex. Ann. Neurol. 1998, 44, 923–934. [Google Scholar] [CrossRef]
- Mikkonen, M.; Soininen, H.; Tapiola, T.; Alafuzoff, I.; Miettinen, R. Hippocampal plasticity in Alzheimer’s disease: Changes in highly polysialylated NCAM immunoreactivity in the hippocampal formation. Eur. J. Neurosci. 1999, 11, 1754–1764. [Google Scholar] [CrossRef]
- Ni Dhuill, C.M.; Fox, G.B.; Pittock, S.J.; O’Connell, A.W.; Murphy, K.J.; Regan, C.M. Polysialylated neural cell adhesion molecule expression in the dentate gyrus of the human hippocampal formation from infancy to old age. J. Neurosci. Res. 1999, 55, 99–106. [Google Scholar] [CrossRef]
- Knoth, R.; Singec, I.; Ditter, M.; Pantazis, G.; Capetian, P.; Meyer, R.P.; Horvat, V.; Volk, B.; Kempermann, G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 2010, 5, e8809. [Google Scholar] [CrossRef]
- Dennis, C.V.; Suh, L.S.; Rodriguez, M.L.; Kril, J.J.; Sutherland, G.T. Human adult neurogenesis across the ages: An immunohistochemical study. Neuropathol. Appl. Neurobiol. 2016, 42, 621–638. [Google Scholar] [CrossRef] [Green Version]
- Sanai, N.; Tramontin, A.D.; Quinones-Hinojosa, A.; Barbaro, N.M.; Gupta, N.; Kunwar, S.; Lawton, M.T.; McDermott, M.W.; Parsa, A.T.; Garcìa-Verdugo, J.M.; et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 2004, 427, 740–744. [Google Scholar] [CrossRef]
- Sanai, N.; Nguyen, T.; Ihrie, R.A.; Mirzadeh, Z.; Tsai, H.-H.; Wong, M.; Gupta, N.; Berger, M.S.; Huang, E.; Garcia-Verdugo, J.M.; et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011, 478, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Liebl, J.; Bernard, S.; Alkass, K.; Yeung, M.S.Y.; Steier, P.; Kutschera, W.; Johnson, L.; Landén, M.; Druid, H.; et al. The age of olfactory bulb neurons in humans. Neuron 2012, 74, 634–639. [Google Scholar] [CrossRef] [Green Version]
- Curtis, M.A.; Kam, M.; Nannmark, U.; Anderson, M.F.; Axell, M.Z.; Wikkelso, C.; Holtas, S.; van Roon-Mom, W.M.; Bjork-Eriksson, T.; Nordborg, C.; et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 2007, 315, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Seki, T. Understanding the real state of human adult hippocampal neurogenesis from studies of rodents and non-human primates. Front. Neurosci. 2020, 14, 839. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Namba, T.; Liu, J.; Suzuki, R.; Shioda, S.; Seki, T. Glial fibrillary acidic protein-expressing neural progenitors give rise to immature neurons via early intermediate progenitors expressing both glial fibrillary acidic protein and neuronal markers in the adult hippocampus. Neuroscience 2010, 166, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Ngwenya, L.B.; Heyworth, N.C.; Shwe, Y.; Moore, T.L.; Rosene, D.L. Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Front. Syst. Neurosci. 2015, 9, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngwenya, L.B.; Peters, A.; Rosene, D.L. Maturational sequence of newly generated neurons in the dentate gyrus of the young adult rhesus monkey. J. Comp. Neurol. 2006, 498, 204–216. [Google Scholar] [CrossRef]
- Ngwenya, L.B.; Rosene, D.L.; Peters, A. An ultrastructural characterization of the newly generated cells in the adult monkey dentate gyrus. Hippocampus 2008, 18, 210–220. [Google Scholar] [CrossRef]
- Kohler, S.J.; Williams, N.I.; Stanton, G.B.; Cameron, J.L.; Greenough, W.T. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc. Natl. Acad. Sci. USA 2011, 108, 10326–10331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Climent, M.A.; Guirado, R.; Varea, E.; Nacher, J.; Nacher, J. “Arrested development”. Immature, but not recently generated, neurons in the adult brain. Arch. Ital. Biol. 2010, 148, 159–172. [Google Scholar] [PubMed]
- La Rosa, C.; Ghibaudi, M.; Bonfanti, L. Newly generated and non-newly generated “immature” neurons in the mammalian brain: A possible reservoir of young cells to prevent brain ageing and disease? J. Clin. Med. 2019, 8, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coviello, S.; Benedetti, B.; Jakubecova, D.; Belles, M.; Klimczak, P.; Gramuntell, Y.; Couillard-Despres, S.; Nacher, J. PSA depletion induces the differentiation of immature neurons in the piriform cortex of adult mice. Int. J. Mol. Sci. 2021, 22, 5733. [Google Scholar] [CrossRef]
- Ohira, K.; Hagihara, H.; Miwa, M.; Nakamura, K.; Miyakawa, T. Fluoxetine-induced dematuration of hippocampal neurons and adult cortical neurogenesis in the common marmoset. Mol. Brain 2019, 12, 69. [Google Scholar] [CrossRef] [Green Version]
- Hagihara, H.; Murano, T.; Ohira, K.; Miwa, M.; Nakamura, K.; Miyakawa, T. Expression of progenitor cell/immature neuron markers does not present definitive evidence for adult neurogenesis. Mol. Brain 2019, 12, 108. [Google Scholar] [CrossRef]
- Saini, V.; Kaur, T.; Kalotra, S.; Kaur, G. The neuroplasticity marker PSA-NCAM: Insights into new therapeutic avenues for promoting neuroregeneration. Pharm. Res. 2020, 160, 105186. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonfanti, L.; Seki, T. The PSA-NCAM-Positive “Immature” Neurons: An Old Discovery Providing New Vistas on Brain Structural Plasticity. Cells 2021, 10, 2542. https://doi.org/10.3390/cells10102542
Bonfanti L, Seki T. The PSA-NCAM-Positive “Immature” Neurons: An Old Discovery Providing New Vistas on Brain Structural Plasticity. Cells. 2021; 10(10):2542. https://doi.org/10.3390/cells10102542
Chicago/Turabian StyleBonfanti, Luca, and Tatsunori Seki. 2021. "The PSA-NCAM-Positive “Immature” Neurons: An Old Discovery Providing New Vistas on Brain Structural Plasticity" Cells 10, no. 10: 2542. https://doi.org/10.3390/cells10102542
APA StyleBonfanti, L., & Seki, T. (2021). The PSA-NCAM-Positive “Immature” Neurons: An Old Discovery Providing New Vistas on Brain Structural Plasticity. Cells, 10(10), 2542. https://doi.org/10.3390/cells10102542






