Effects of Sex and 17 β-Estradiol on Cardiac Fibroblast Morphology and Signaling Activities In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Culture
2.2. Collagen Coated Culture Plates
2.3. Estrogen Treatment
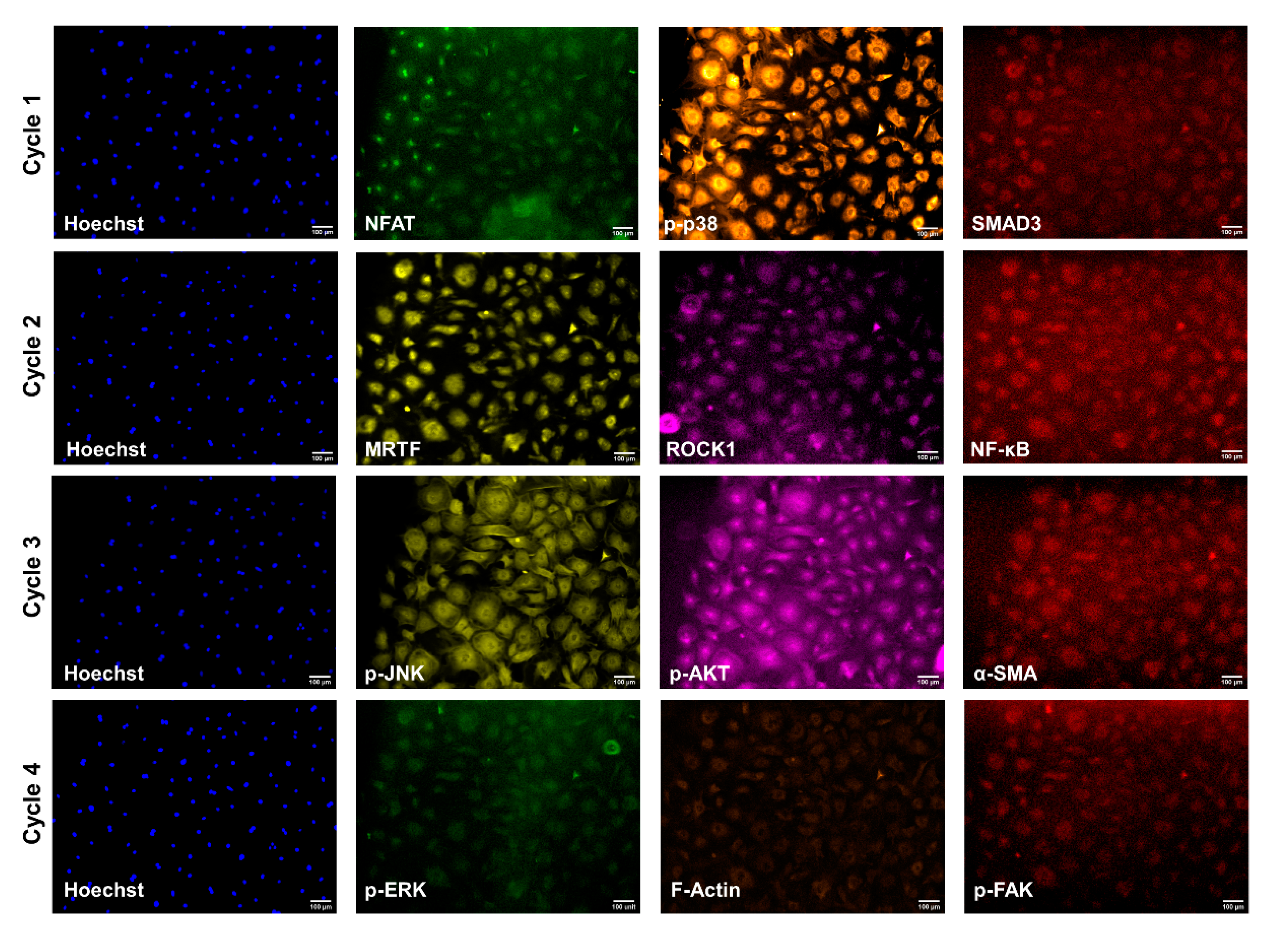
2.4. Cyclic Immunofluorescence
2.5. Post-Image Processing
2.6. Statistical Analysis
3. Results
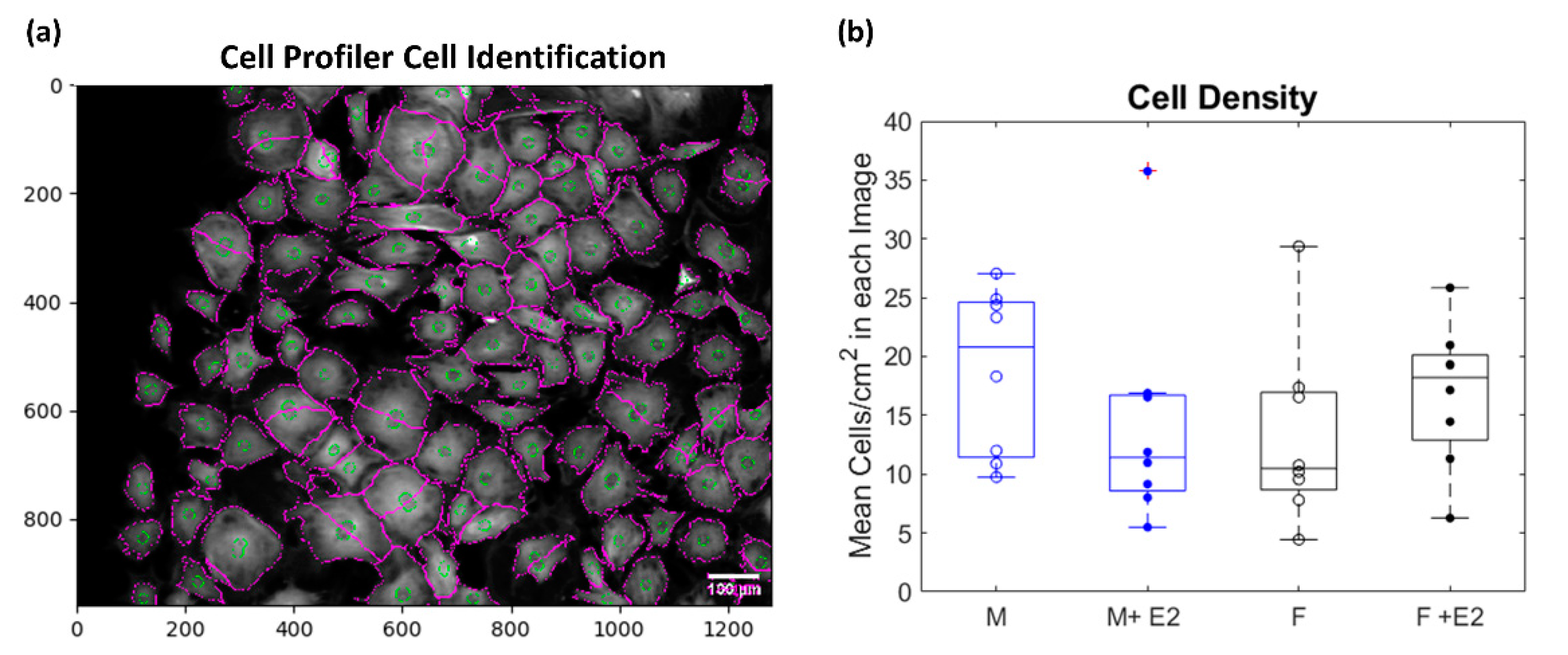
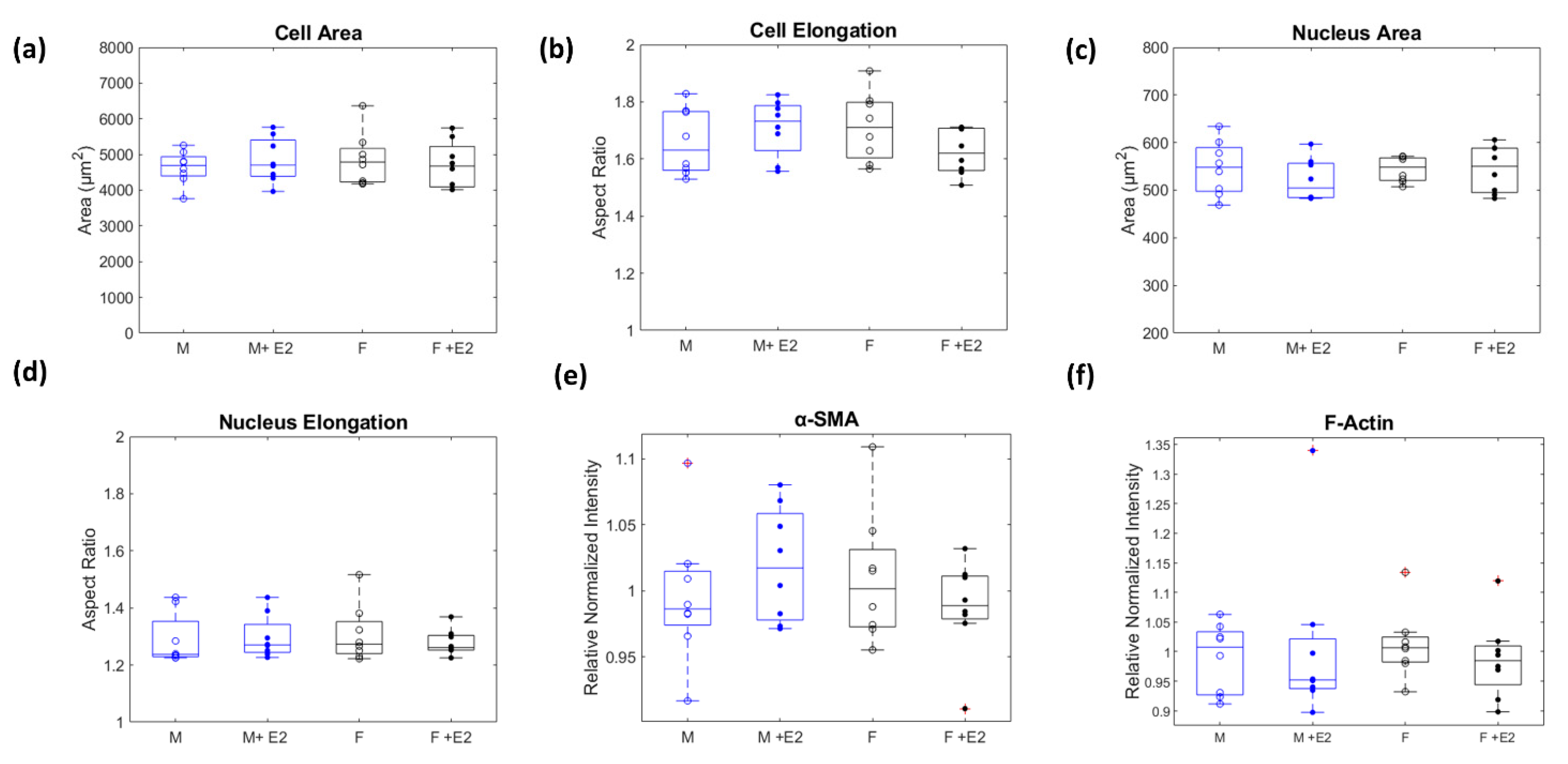
3.1. Sex-Dissagregated Analysis of CF Morphology
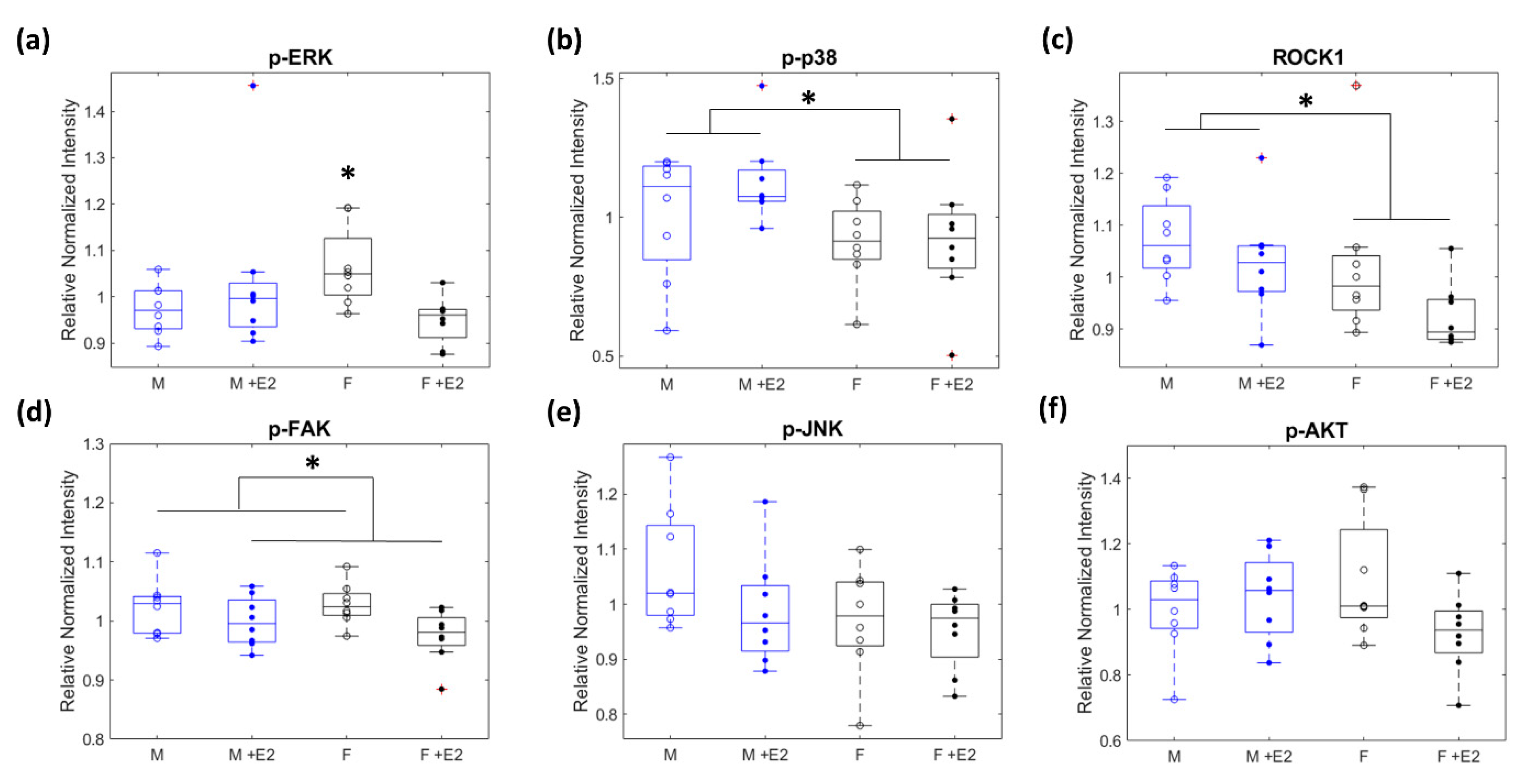
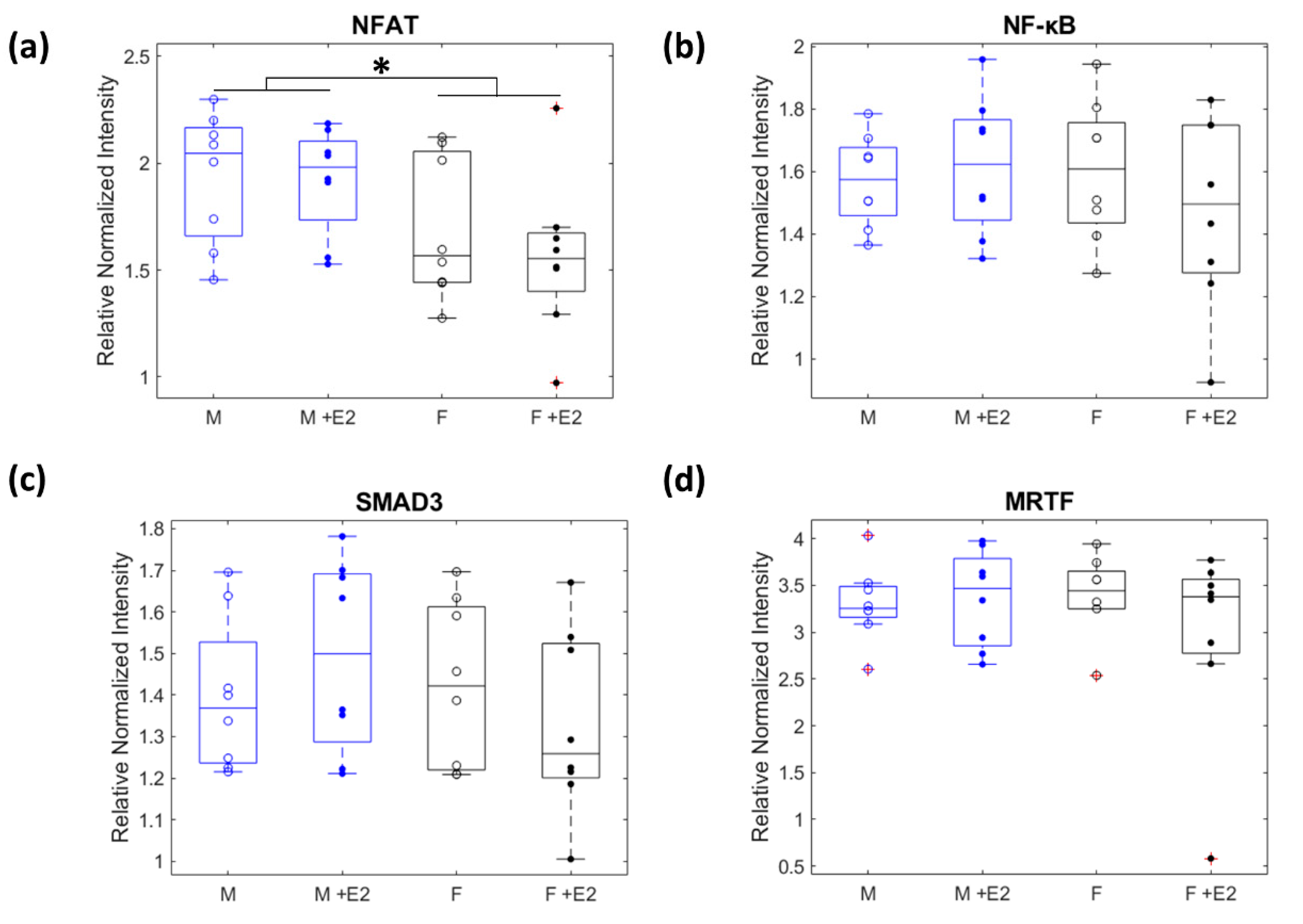
3.2. Relative Levels of Fibrotic Related Signaling Proteins
3.3. Nuclear Localization of Mechanosensitive Proteins
3.4. Correlation Analysis of Protein-Protein Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Mehta, P.A.; Cowie, M.R. Gender and Heart Failure: A Population Perspective. Heart 2006, 92, iii14–iii18. [Google Scholar] [CrossRef] [Green Version]
- Patrizio, M.; Marano, G. Gender Differences in Cardiac Hypertrophic Remodeling. Ann. Dell’istituto Super. Di Sanità 2016, 52, 223–229. [Google Scholar] [CrossRef]
- Eisenberg, E.; Di Palo, K.E.; Piña, I.L. Sex Differences in Heart Failure. Clin. Cardiol. 2018, 41, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Raparelli, V.; Proietti, M.; Lenzi, A.; Basili, S. Sex and Gender Differences in Ischemic Heart Disease: Endocrine Vascular Disease Approach (EVA) Study Design. J. Cardiovasc. Transl. Res. 2020, 13, 14–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, N.S. Understanding Hormones, Menopause, and Heart Failure: Still a Work in Progress. J. Am. Coll. Cardiol. 2017, 69, 2527–2529. [Google Scholar] [CrossRef] [PubMed]
- Medzikovic, L.; Aryan, L.; Eghbali, M. Connecting Sex Differences, Estrogen Signaling, and MicroRNAs in Cardiac Fibrosis. J. Mol. Med. 2019, 97, 1385–1398. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A. Hormone-Replacement Therapy: Current Thinking. Nat. Rev. Endocrinol. 2017, 13, 220–231. [Google Scholar] [CrossRef]
- Zhao, D.; Guallar, E.; Ouyang, P.; Subramanya, V.; Vaidya, D.; Ndumele, C.E.; Lima, J.A.; Allison, M.A.; Shah, S.J.; Bertoni, A.G.; et al. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. J. Am. Coll. Cardiol. 2018, 71, 2555–2566. [Google Scholar] [CrossRef]
- Mahmoodzadeh, S.; Dworatzek, E.; Fritschka, S.; Pham, T.H.; Regitz-Zagrosek, V. 17β-Estradiol Inhibits Matrix Metalloproteinase-2 Transcription via MAP Kinase in Fibroblasts. Cardiovasc. Res. 2010, 85, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Shao, Y.; Huang, Y.; Yao, T.; Lu, L.-M. 17β-Estradiol Inhibits Angiotensin II-Induced Collagen Synthesis of Cultured Rat Cardiac Fibroblasts via Modulating Angiotensin II Receptors. Eur. J. Pharmacol. 2007, 567, 186–192. [Google Scholar] [CrossRef]
- Wu, C.-H.; Liu, J.-Y.; Wu, J.-P.; Hsieh, Y.-H.; Liu, C.-J.; Hwang, J.-M.; Lee, S.-D.; Chen, L.-M.; Chang, M.-H.; Kuo, W.-W.; et al. 17β-Estradiol Reduces Cardiac Hypertrophy Mediated through the up-Regulation of PI3K/Akt and the Suppression of Calcineurin/NF-AT3 Signaling Pathways in Rats. Life Sci. 2005, 78, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Moore-Morris, T.; Guimarães-Camboa, N.; Yutzey, K.E.; Pucéat, M.; Evans, S.M. Cardiac Fibroblasts: From Development to Heart Failure. J. Mol. Med. 2015, 93, 823–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dworatzek, E.; Mahmoodzadeh, S.; Schriever, C.; Kusumoto, K.; Kramer, L.; Santos, G.; Fliegner, D.; Leung, Y.-K.; Ho, S.-M.; Zimmermann, W.-H.; et al. Sex-Specific Regulation of Collagen I and III Expression by 17β-Estradiol in Cardiac Fibroblasts: Role of Estrogen Receptors. Cardiovasc. Res. 2019, 115, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.-H.; Chen, J.-J.; Chen, C.-H.; Lin, H.; Cheng, C.-F.; Lian, W.-S.; Chen, Y.-L.; Juan, S.-H.; Liu, J.-C.; Liou, J.-Y.; et al. Inhibition of Angiotensin II Induced Endothelin-1 Gene Expression by 17-β-Oestradiol in Rat Cardiac Fibroblasts. Heart 2005, 91, 664–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iorga, A.; Umar, S.; Ruffenach, G.; Aryan, L.; Li, J.; Sharma, S.; Motayagheni, N.; Nadadur, R.D.; Bopassa, J.C.; Eghbali, M. Estrogen Rescues Heart Failure through Estrogen Receptor Beta Activation. Biol. Sex Differ. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Janmey, P.A.; Fletcher, D.A.; Reinhart-King, C.A. Stiffness Sensing by Cells. Physiol. Rev. 2020, 100, 695–724. [Google Scholar] [CrossRef]
- MacLean, J.; Pasumarthi, K.B.S. Signaling Mechanisms Regulating Fibroblast Activation, Phenoconversion and Fibrosis in the Heart. Indian J. Biochem. Biophys. 2014, 51, 476–482. [Google Scholar]
- Herum, K.M.; Lunde, I.G.; McCulloch, A.D.; Christensen, G. The Soft- and Hard-Heartedness of Cardiac Fibroblasts: Mechanotransduction Signaling Pathways in Fibrosis of the Heart. J. Clin. Med. 2017, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- McLellan, M.A.; Skelly, D.A.; Dona, M.S.I.; Squiers, G.T.; Farrugia, G.E.; Gaynor, T.L.; Cohen, C.D.; Pandey, R.; Diep, H.; Vinh, A.; et al. High-Resolution Transcriptomic Profiling of the Heart During Chronic Stress Reveals Cellular Drivers of Cardiac Fibrosis and Hypertrophy. Circulation 2020, 142, 1448–1463. [Google Scholar] [CrossRef]
- Berry, M.F.; Engler, A.J.; Woo, Y.J.; Pirolli, T.J.; Bish, L.T.; Jayasankar, V.; Morine, K.J.; Gardner, T.J.; Discher, D.E.; Sweeney, H.L. Mesenchymal Stem Cell Injection after Myocardial Infarction Improves Myocardial Compliance. Am. J. Physiol. -Heart Circ. Physiol. 2006, 290, H2196–H2203. [Google Scholar] [CrossRef]
- Engler, A.J.; Carag-Krieger, C.; Johnson, C.P.; Raab, M.; Tang, H.-Y.; Speicher, D.W.; Sanger, J.W.; Sanger, J.M.; Discher, D.E. Embryonic Cardiomyocytes Beat Best on a Matrix with Heart-like Elasticity: Scar-like Rigidity Inhibits Beating. J. Cell Sci. 2008, 121, 3794–3802. [Google Scholar] [CrossRef] [Green Version]
- Janmey, P.A.; Miller, R.T. Mechanisms of Mechanical Signaling in Development and Disease. J. Cell Sci. 2011, 124, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Herum, K.M.; Choppe, J.; Kumar, A.; Engler, A.J.; McCulloch, A.D. Mechanical Regulation of Cardiac Fibroblast Profibrotic Phenotypes. Mol. Biol. Cell 2017, 28, 1871–1882. [Google Scholar] [CrossRef]
- Rogers, J.D.; Holmes, J.W.; Saucerman, J.J.; Richardson, W.J. Mechano-Chemo Signaling Interactions Modulate Matrix Production by Cardiac Fibroblasts. Matrix Biol. Plus 2021, 10, 100055. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, V.; Clark, J.; Fix, C.; Law, B.A.; Morales, M.O.; Qiao, X.; Ako-Asare, K.; Goldsmith, J.G.; Carver, W.; Murray, D.B.; et al. Type II Diabetes Promotes a Myofibroblast Phenotype in Cardiac Fibroblasts. Life Sci. 2013, 92, 669–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Fallahi-Sichani, M.; Chen, J.; Sorger, P.K. Cyclic Immunofluorescence (CycIF), A Highly Multiplexed Method for Single-cell Imaging. Curr. Protoc. Chem. Biol. 2016, 8, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Fluorescence SpectraViewer. Available online: https://www.thermofisher.com/order/fluorescence-spectraviewer (accessed on 2 July 2021).
- McQuin, C.; Goodman, A.; Chernyshev, V.; Kamentsky, L.; Cimini, B.A.; Karhohs, K.W.; Doan, M.; Ding, L.; Rafelski, S.M.; Thirstrup, D.; et al. CellProfiler 3.0: Next-Generation Image Processing for Biology. PLoS Biol. 2018, 16, e2005970. [Google Scholar] [CrossRef] [Green Version]
- de Simone, G.; Devereux, R.B.; Daniels, S.R.; Meyer, R.A. Gender Differences in Left Ventricular Growth. Hypertension 1995, 26, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Schaible, T.F.; Scheuer, J. Comparison of Heart Function in Male and Female Rats. Basic Res. Cardiol. 1984, 79, 402–412. [Google Scholar] [CrossRef]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-Smooth Muscle Actin Expression Upregulates Fibroblast Contractile Activity. Mol. Biol. Cell 2001, 12, 2730–2741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash, R.; Schmidt, A.G.; Pathak, A.; Gerst, M.J.; Biniakiewicz, D.; Kadambi, V.J.; Hoit, B.D.; Abraham, W.T.; Kranias, E.G. Differential Regulation of P38 Mitogen-Activated Protein Kinase Mediates Gender-Dependent Catecholamine-Induced Hypertrophy. Cardiovasc. Res. 2003, 57, 704–714. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Fan, G.; Zhao, H.; Wang, Z.; Li, F.; Zhang, P.; Zhang, J.; Wang, X.; Wang, W. Targeted Inhibition of Focal Adhesion Kinase Attenuates Cardiac Fibrosis and Preserves Heart Function in Adverse Cardiac Remodeling. Sci. Rep. 2017, 7, 43146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, G.-P.; Wang, W.; Zhao, H.; Cai, L.; Zhang, P.-D.; Yang, Z.-H.; Zhang, J.; Wang, X. Pharmacological Inhibition of Focal Adhesion Kinase Attenuates Cardiac Fibrosis in Mice Cardiac Fibroblast and Post-Myocardial-Infarction Models. Cell. Physiol. Biochem. 2015, 37, 515–526. [Google Scholar] [CrossRef]
- Rigiracciolo, D.C.; Santolla, M.F.; Lappano, R.; Vivacqua, A.; Cirillo, F.; Galli, G.R.; Talia, M.; Muglia, L.; Pellegrino, M.; Nohata, N.; et al. Focal Adhesion Kinase (FAK) Activation by Estrogens Involves GPER in Triple-Negative Breast Cancer Cells. J. Exp. Clin. Cancer Res. 2019, 38, 58. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-L.; Wu, H.-M.; Lin, C.-Y.; Lin, Y.-J.; Chao, A.; Wang, T.-H.; Hsueh, S.; Lai, C.-H.; Wang, H.-S. Estradiol and Tamoxifen Induce Cell Migration through GPR30 and Activation of Focal Adhesion Kinase (FAK) in Endometrial Cancers with Low or without Nuclear Estrogen Receptor α (ERα). PLoS ONE 2013, 8, e72999. [Google Scholar] [CrossRef] [Green Version]
- Tanner, M.A.; Thomas, T.P.; Maitz, C.A.; Grisanti, L.A. B2-Adrenergic Receptors Increase Cardiac Fibroblast Proliferation Through the Gαs/ERK1/2-Dependent Secretion of Interleukin-6. Int. J. Mol. Sci. 2020, 21, 8507. [Google Scholar] [CrossRef]
- Matarrese, P.; Maccari, S.; Vona, R.; Gambardella, L.; Stati, T.; Marano, G. Role of β-Adrenergic Receptors and Estrogen in Cardiac Repair after Myocardial Infarction: An Overview. Int. J. Mol. Sci. 2021, 22, 8957. [Google Scholar] [CrossRef]
- Peter, A.K.; Walker, C.J.; Ceccato, T.; Trexler, C.L.; Ozeroff, C.D.; Lugo, K.R.; Perry, A.R.; Anseth, K.S.; Leinwand, L.A. Cardiac Fibroblasts Mediate a Sexually Dimorphic Fibrotic Response to Β-Adrenergic Stimulation. J. Am. Heart Assoc. 2021, 10, e018876. [Google Scholar] [CrossRef]
- Hsu, H.-J.; Lee, C.-F.; Kaunas, R. A Dynamic Stochastic Model of Frequency-Dependent Stress Fiber Alignment Induced by Cyclic Stretch. PLoS ONE 2009, 4, e4853. [Google Scholar] [CrossRef] [Green Version]
- Moretti, M.; Prina-Mello, A.; Reid, A.J.; Barron, V.; Prendergast, P.J. Endothelial Cell Alignment on Cyclically-Stretched Silicone Surfaces. J. Mater. Sci.: Mater. Med. 2004, 15, 1159–1164. [Google Scholar] [CrossRef]
- Huang, C.; Miyazaki, K.; Akaishi, S.; Watanabe, A.; Hyakusoku, H.; Ogawa, R. Biological Effects of Cellular Stretch on Human Dermal Fibroblasts. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, e351–e361. [Google Scholar] [CrossRef]
- Chen, K.; Vigliotti, A.; Bacca, M.; McMeeking, R.M.; Deshpande, V.S.; Holmes, J.W. Role of Boundary Conditions in Determining Cell Alignment in Response to Stretch. Proc. Natl. Acad. Sci. USA 2018, 115, 986–991. [Google Scholar] [CrossRef] [Green Version]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Zuppinger, C. 3D Cardiac Cell Culture: A Critical Review of Current Technologies and Applications. Front. Cardiovasc. Med. 2019, 6, 87. [Google Scholar] [CrossRef] [Green Version]
- Li, C.X.; Talele, N.P.; Boo, S.; Koehler, A.; Knee-Walden, E.; Balestrini, J.L.; Speight, P.; Kapus, A.; Hinz, B. MicroRNA-21 Preserves the Fibrotic Mechanical Memory of Mesenchymal Stem Cells. Nat. Mater. 2017, 16, 379–389. [Google Scholar] [CrossRef]
- Turner, N.A. Inflammatory and Fibrotic Responses of Cardiac Fibroblasts to Myocardial Damage Associated Molecular Patterns (DAMPs). J. Mol. Cell. Cardiol. 2016, 94, 189–200. [Google Scholar] [CrossRef]
- Ueda, K.; Adachi, Y.; Liu, P.; Fukuma, N.; Takimoto, E. Regulatory Actions of Estrogen Receptor Signaling in the Cardiovascular System. Front. Endocrinol. 2020, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Menazza, S.; Murphy, E. The Expanding Complexity of Estrogen Receptor Signaling in the Cardiovascular System. Circ. Res. 2016, 118, 994–1007. [Google Scholar] [CrossRef]
- Zeigler, A.C.; Richardson, W.J.; Holmes, J.W.; Saucerman, J.J. Computational Modeling of Cardiac Fibroblasts and Fibrosis. J. Mol. Cell. Cardiol. 2016, 93, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Rogers, J.D.; Richardson, W.J. Fibroblast Mechanotransduction Network Predicts Targets for Mechano-Adaptive Infarct Therapies. bioRxiv 2020. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watts, K.; Richardson, W.J. Effects of Sex and 17 β-Estradiol on Cardiac Fibroblast Morphology and Signaling Activities In Vitro. Cells 2021, 10, 2564. https://doi.org/10.3390/cells10102564
Watts K, Richardson WJ. Effects of Sex and 17 β-Estradiol on Cardiac Fibroblast Morphology and Signaling Activities In Vitro. Cells. 2021; 10(10):2564. https://doi.org/10.3390/cells10102564
Chicago/Turabian StyleWatts, Kelsey, and William J. Richardson. 2021. "Effects of Sex and 17 β-Estradiol on Cardiac Fibroblast Morphology and Signaling Activities In Vitro" Cells 10, no. 10: 2564. https://doi.org/10.3390/cells10102564
APA StyleWatts, K., & Richardson, W. J. (2021). Effects of Sex and 17 β-Estradiol on Cardiac Fibroblast Morphology and Signaling Activities In Vitro. Cells, 10(10), 2564. https://doi.org/10.3390/cells10102564





