NK Cell Patterns in Idiopathic Inflammatory Myopathies with Pulmonary Affection
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Cohort
2.2. Sampling and Flow Cytometric Analysis of Peripheral Blood Mononuclear Cells
2.3. Gating Strategy
2.4. Statistical Analysis
2.5. Data Availability
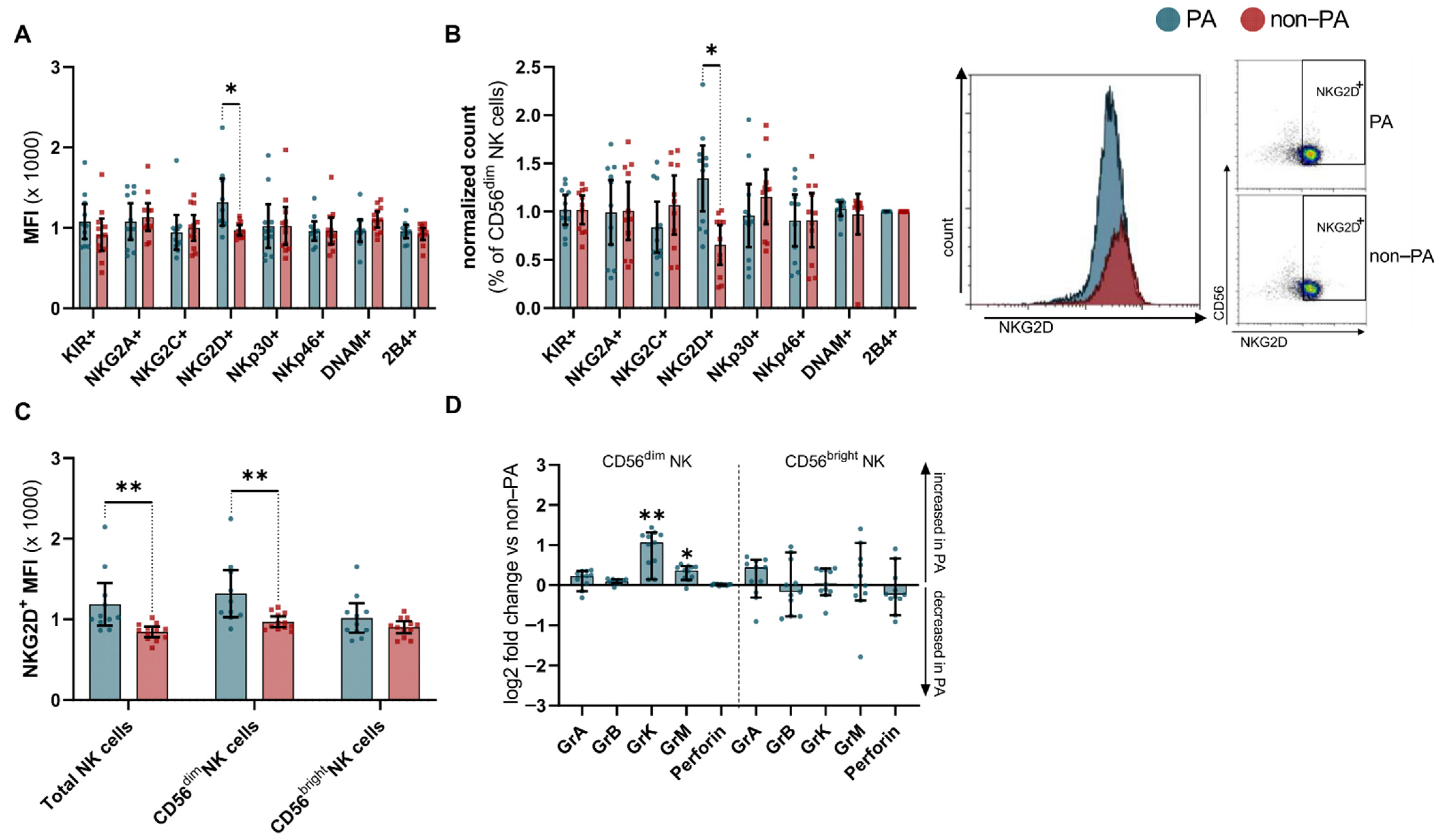
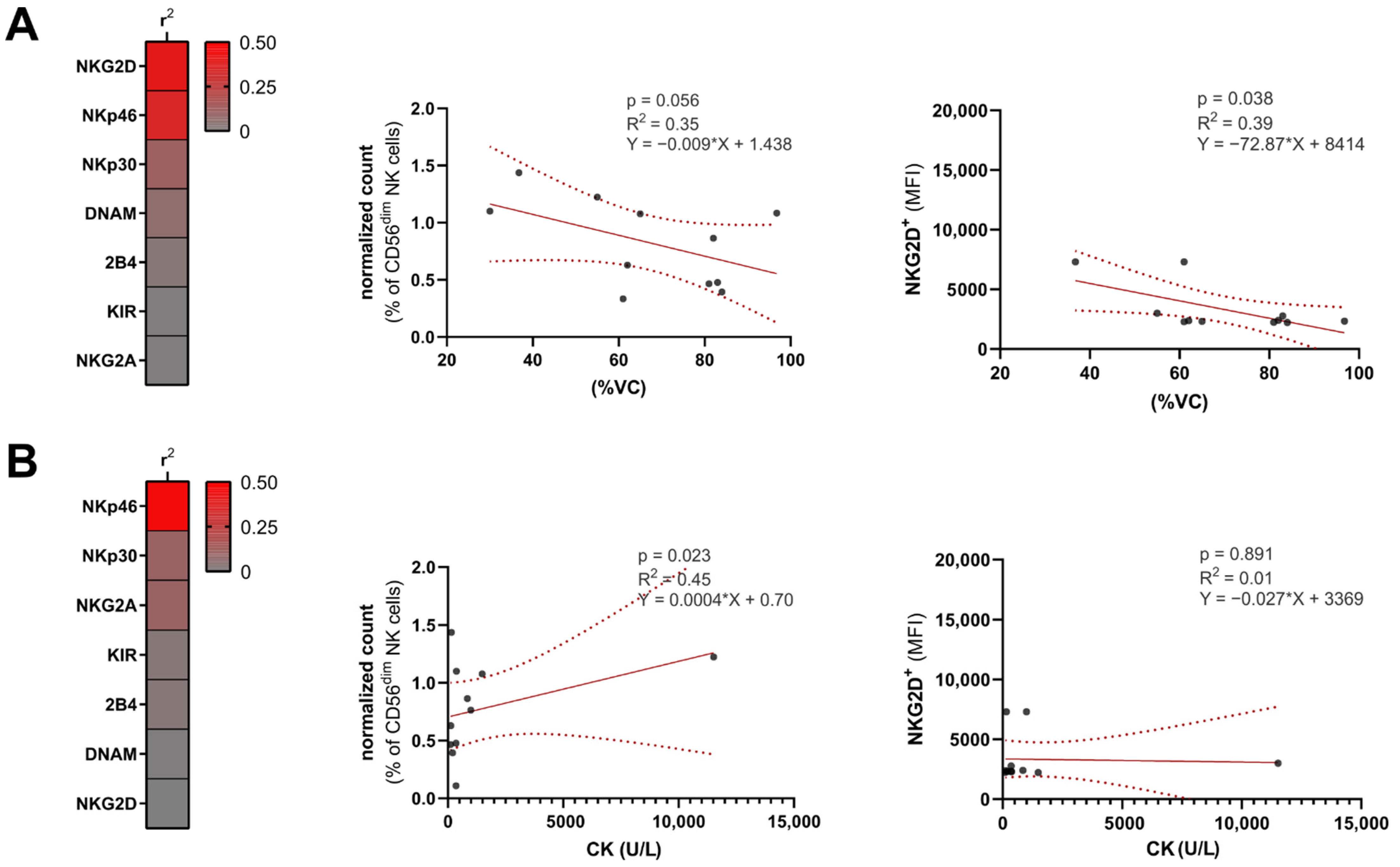
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mecoli, C.A.; Christopher-Stine, L. Management of Interstitial Lung Disease in Patients with Myositis Specific Autoantibodies. Curr. Rheumatol. Rep. 2018, 20, 27. [Google Scholar] [CrossRef]
- Hallowell, R.W.; Danoff, S.K. Interstitial lung disease associated with the idiopathic inflammatory myopathies and the antisynthetase syndrome. Curr. Opin. Rheumatol. 2014, 26, 684–689. [Google Scholar] [CrossRef]
- Hervier, B.; Uzunhan, Y. Inflammatory Myopathy-Related Interstitial Lung Disease: From Pathophysiology to Treatment. Front. Med. 2020, 6. [Google Scholar] [CrossRef]
- Shappley, C.L.; Paik, J.J.; Saketkoo, L.A. Myositis-Related Interstitial Lung Diseases: Diagnostic Features, Treatment, and Complications. Curr. Treat. Options Rheumatol. 2019, 5, 56–83. [Google Scholar] [CrossRef]
- Selva-O’Callaghan, A.; Pinal-Fernandez, I.; Trallero-Araguás, E.; Milisenda, J.C.; Grau-Junyent, J.M.; Mammen, A.L. Classification and management of adult inflammatory myopathies. Lancet Neurol. 2018, 17, 816–828. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Tjärnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; De Visser, M.; Alfredsson, L.; A. Amato, A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 2017, 76, 1955–1964. [Google Scholar] [CrossRef]
- Labrador-Horrillo, M.; Martinez, M.A.; Selva-O’Callaghan, A.; Trallero-Araguas, E.; Balada, E.; Vilardell-Tarres, M.; Juarez, C. Anti-MDA5 Antibodies in a Large Mediterranean Population of Adults with Dermatomyositis. J. Immunol. Res. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Gono, T.; Kaneko, H.; Kawaguchi, Y.; Hanaoka, M.; Kataoka, S.; Kuwana, M.; Takagi, K.; Ichida, H.; Katsumata, Y.; Ota, Y.; et al. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology 2014, 53, 2196–2203. [Google Scholar] [CrossRef] [Green Version]
- Fasano, S.; Gordon, P.; Hajji, R.; Loyo, E.; Isenberg, D.A. Rituximab in the treatment of inflammatory myopathies: A review. Rheumatology 2017, 56, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Andersson, H.; Sem, M.; Lund, M.B.; Aaløkken, T.M.; Günther, A.; Walle-Hansen, R.; Garen, T.; Molberg, Ø. Long-term experience with rituximab in anti-synthetase syndrome-related interstitial lung disease. Rheumatology 2015, 54, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Biron, C.A.; Nguyen, K.B.; Pien, G.C.; Cousens, L.P.; Salazar-Mather, T.P. NATURAL KILLER CELLS IN ANTIVIRAL DEFENSE: Function and Regulation by Innate Cytokines. Annu. Rev. Immunol. 1999, 17, 189–220. [Google Scholar] [CrossRef]
- Lünemann, A.; Lünemann, J.D.; Münz, C. Regulatory NK-Cell Functions in Inflammation and Autoimmunity. Mol. Med. 2009, 15, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.-D.; Zhou, Q. Natural killer cells as indispensable players and therapeutic targets in autoimmunity. Autoimmunity 2010, 44, 3–10. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Howard, O.Z.; Dong, H.F.; Yang, D.; Raben, N.; Nagaraju, K.; Rosen, A.; Casciola-Rosen, L.; Härtlein, M.; Kron, M.; Yang, D.; et al. Histidyl–tRNA Synthetase and Asparaginyl–tRNA Synthetase, Autoantigens in Myositis, Activate Chemokine Receptors on T Lymphocytes and Immature Dendritic Cells. J. Exp. Med. 2002, 196, 781–791. [Google Scholar] [CrossRef]
- Levine, S.M.; Raben, N.; Xie, D.; Askin, F.B.; Tuder, R.M.; Mullins, M.; Rosen, A.; Casciola-Rosen, L.A. Novel conformation of histidyl–transfer RNA synthetase in the lung. Arthritis Rheum. 2007, 56, 2729–2739. [Google Scholar] [CrossRef]
- Zhou, J.J.; Wang, F.; Xu, Z.; Lo, W.-S.; Lau, C.-F.; Chiang, K.P.; Nangle, L.A.; Ashlock, M.A.; Mendlein, J.D.; Yang, X.-L.; et al. Secreted Histidyl-tRNA Synthetase Splice Variants Elaborate Major Epitopes for Autoantibodies in Inflammatory Myositis. J. Biol. Chem. 2014, 289, 19269–19275. [Google Scholar] [CrossRef] [Green Version]
- Hervier, B.; Perez, M.; Allenbach, Y.; Devilliers, H.; Cohen, F.; Uzunhan, Y.; Ouakrim, H.; Dorgham, K.; Méritet, J.-F.; Longchampt, E.; et al. Involvement of NK Cells and NKp30 Pathway in Antisynthetase Syndrome. J. Immunol. 2016, 197, 1621–1630. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Day, J.; Guimaraes, F.S.; Wicks, I.P.; Louis, C. Natural killer cells in inflammatory autoimmune diseases. Clin. Transl. Immunol. 2021, 10, e1250. [Google Scholar] [CrossRef]
- Gonzalez-Amaro, R.; Alcocer-Varela, J.; Alarcon-Segovia, D. Natural killer cell activity in dermatomyo-sitis-polymyositis. J. Rheumatol. 1987, 14, 307–310. [Google Scholar] [PubMed]
- Ernste, F.C.; Crowson, C.S.; De Padilla, C.L.; Hein, M.S.; Reed, A.M. Longitudinal Peripheral Blood Lymphocyte Subsets Correlate with Decreased Disease Activity in Juvenile Dermatomyositis. J. Rheumatol. 2013, 40, 1200–1211. [Google Scholar] [CrossRef]
- Mammen, A.L.; Allenbach, Y.; Stenzel, W.; Benveniste, O.; De Bleecker, J.; Boyer, O.; Casciola-Rosen, L.; Christopher-Stine, L.; Damoiseaux, J.; Gitiaux, C.; et al. 239th ENMC International Workshop: Classification of dermatomyositis, Amsterdam, the Netherlands, 14–16 December 2018. Neuromuscul. Disord. 2020, 30, 70–92. [Google Scholar] [CrossRef]
- Posevitz-Fejfár, A.; Posevitz, V.; Gross, C.; Bhatia, U.; Kurth, F.; Schütte, V.; Bar-Or, A.; Meuth, S.G.; Wiendl, H. Effects of Blood Transportation on Human Peripheral Mononuclear Cell Yield, Phenotype and Function: Implications for Immune Cell Biobanking. PLoS ONE 2014, 9, e115920. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.H.; Lee, E.B.; Shin, K.C.; Im, C.H.; Chung, D.H.; Han, S.K.; Song, Y.W. Interstitial lung disease in patients with polymyositis, dermatomyositis and amyopathic dermatomyositis. Rheumatology 2005, 44, 1282–1286. [Google Scholar] [CrossRef] [Green Version]
- Gianchecchi, E.; Delfino, D.V.; Fierabracci, A. NK cells in autoimmune diseases: Linking innate and adaptive immune responses. Autoimmun. Rev. 2018, 17, 142–154. [Google Scholar] [CrossRef]
- Chen, I.-J.; Wu, Y.-J.J.; Lin, C.-W.; Fan, K.-W.; Luo, S.-F.; Ho, H.-H.; Liou, L.-B.; Tsai, W.-P.; Chen, J.-Y.; Yang, C.H.; et al. Interstitial lung disease in polymyositis and dermatomyositis. Clin. Rheumatol. 2009, 28, 639–646. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, G.; Gao, D.; Liu, G.; Pan, L.; Ni, L.; Li, Z.; Wang, Q. Factors Associated with Interstitial Lung Disease in Patients with Polymyositis and Dermatomyositis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0155381. [Google Scholar] [CrossRef] [Green Version]
- Moretta, A.; Marcenaro, E.; Parolini, S.; Ferlazzo, G.; Moretta, L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 2007, 15, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Schwane, V.; Huynh-Tran, V.H.; Vollmers, S.; Yakup, V.M.; Sauter, J.; Schmidt, A.H.; Peine, S.; Altfeld, M.; Richert, L.; Körner, C. Distinct Signatures in the Receptor Repertoire Discriminate CD56bright and CD56dim Natural Killer Cells. Front. Immunol. 2020, 11, 568927. [Google Scholar] [CrossRef]
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.; Caligiuri, M.A. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef] [Green Version]
- Hervier, B.; Béziat, V.; Haroche, J.; Mathian, A.; Lebon, P.; Ghillani-Dalbin, P.; Musset, L.; Debre, P.; Amoura, Z.; Vieillard, V. Phenotype and function of natural killer cells in systemic lupus erythematosus: Excess interferon-γ production in patients with active disease. Arthritis Rheum. 2011, 63, 1698–1706. [Google Scholar] [CrossRef]
- Gross, C.C.; Schulte-Mecklenbeck, A.; Rünzi, A.; Kuhlmann, T.; Posevitz-Fejfár, A.; Schwab, N.; Schneider-Hohendorf, T.; Herich, S.; Held, K.; Konjević, M.; et al. Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation. Proc. Natl. Acad. Sci. USA 2016, 113, E2973–E2982. [Google Scholar] [CrossRef] [Green Version]
- Gross, C.; Schulte-Mecklenbeck, A.; Wiendl, H.; Marcenaro, E.; De Rosbo, N.K.; Uccelli, A.; Laroni, A. Regulatory Functions of Natural Killer Cells in Multiple Sclerosis. Front. Immunol. 2016, 7, 606. [Google Scholar] [CrossRef] [Green Version]
- Schleinitz, N.; Vely, F.; Harlé, J.-R.; Vivier, E. Natural killer cells in human autoimmune diseases. Immunology 2010, 131, 451–458. [Google Scholar] [CrossRef]
- Johansson, S.; Berg, L.; Hall, H.; Höglund, P. NK cells: Elusive players in autoimmunity. Trends Immunol. 2005, 26, 613–618. [Google Scholar] [CrossRef]
- Ruck, T.; Bittner, S.; Afzali, A.M.; Göbel, K.; Glumm, S.; Kraft, P.; Sommer, C.; Kleinschnitz, C.; Preuße, C.; Stenzel, W.; et al. The NKG2D-IL-15 signaling pathway contributes to T-cell mediated pathology in inflammatory myopathies. Oncotarget 2015, 6, 43230–43243. [Google Scholar] [CrossRef]
- Raulet, D.H.; Gasser, S.; Gowen, B.; Deng, W.; Jung, H. Regulation of Ligands for the NKG2D Activating Receptor. Annu. Rev. Immunol. 2013, 31, 413–441. [Google Scholar] [CrossRef] [Green Version]
- Wensveen, F.; Jelenčić, V.; Polić, B. NKG2D: A Master Regulator of Immune Cell Responsiveness. Front. Immunol. 2018, 9, 441. [Google Scholar] [CrossRef]
- Prinz, D.; Klein, K.; List, J.; Knab, V.M.; Menzl, I.; Leidenfrost, N.; Heller, G.; Polić, B.; Putz, E.M.; Witalisz-Siepracka, A.; et al. Loss of NKG2D in murine NK cells leads to increased perforin production upon long-term stimulation with IL-2. Eur. J. Immunol. 2020, 50, 880–890. [Google Scholar] [CrossRef] [Green Version]
- Ruck, T.; Bittner, S.; Gross, C.; Breuer, J.; Albrecht, S.; Korr, S.; Göbel, K.; Pankratz, S.; Henschel, C.M.; Schwab, N.; et al. CD4+NKG2D+ T Cells Exhibit Enhanced Migratory and Encephalitogenic Properties in Neuroinflammation. PLoS ONE 2013, 8, e81455. [Google Scholar] [CrossRef]
- Molfetta, R.; Quatrini, L.; Santoni, A.; Paolini, R. Regulation of NKG2D-Dependent NK Cell Functions: The Yin and the Yang of Receptor Endocytosis. Int. J. Mol. Sci. 2017, 18, 1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelenčić, V.; Šestan, M.; Kavazovic, I.; Lenartić, M.; Marinović, S.; Holmes, T.D.; Prchal-Murphy, M.; Lisnić, B.; Sexl, V.; Bryceson, Y.T.; et al. NK cell receptor NKG2D sets activation threshold for the NCR1 receptor early in NK cell development. Nat. Immunol. 2018, 19, 1083–1092. [Google Scholar] [CrossRef]
- Borchers, M.T.; Harris, N.L.; Wesselkamper, S.C.; Vitucci, M.; Cosman, D. NKG2D ligands are expressed on stressed human airway epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2006, 291, L222–L231. [Google Scholar] [CrossRef] [PubMed]
- Wensink, A.C.; Wiewel, M.A.; Jongeneel, L.H.; Boes, M.; van der Poll, T.; Hack, C.E.; Bovenschen, N. Granzyme M and K release in human experimental endotoxemia. Immunobiol. 2016, 221, 773–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afzali, A.M.; Ruck, T.; Wiendl, H.; Meuth, S.G. Animal models in idiopathic inflammatory myopathies: How to overcome a translational roadblock? Autoimmun. Rev. 2017, 16, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Markasz, L.; Stuber, G.; Vanherberghen, B.; Flaberg, E.; Olah, E.; Carbone, E.; Eksborg, S.; Klein, E.; Skribek, H.; Szekely, L. Effect of frequently used chemotherapeutic drugs on the cytotoxic activity of human natural killer cells. Mol. Cancer Ther. 2007, 6, 644–654. [Google Scholar] [CrossRef] [Green Version]


| Patient | Age | Sex | Diagnosis | Antibody | Known Malignancy | CK Level (U/L) | Characteristics of PA | Treatments |
|---|---|---|---|---|---|---|---|---|
| 1 | 54 | M | ASyS | JO1 | - | 1046 | none | MMF, steroids |
| 2 | 43 | M | ASyS | JO1 | - | 249 | IVIG, steroids | |
| 3 | 49 | M | DM | MI-2 | - | 2422 | none | |
| 4 | 53 | M | PM | negative | - | 1588 | IVIG | |
| 5 | 43 | M | DM | NXP2 | oropharyngeal | 4576 | steroids | |
| 6 | 66 | F | DM | negative | - | 11,980 | none | |
| 7 | 68 | M | DM | negative | colorectal | 99 | MTX, steroids, IVIG | |
| 8 | 51 | F | DM | TIF-1 | - | 61 | AZA | |
| 9 | 79 | M | ASyS | PL7 | - | 243 | none | |
| 10 | 48 | M | ASyS | negative | - | 580 | steroids | |
| 11 | 68 | M | DM | TIF-1 | - | 399 | none | |
| 12 | 60 | M | ASyS | PL12 | - | 115 | none | |
| 13 | 41 | M | ASyS | PL7 | - | 355 | pulmonary fibrosis with diffusion restriction | CYP, steroids |
| 14 | 37 | M | ASyS | JO1 | - | 354 | alveolitis, pulmonary fibrosis | CYP, steroids |
| 15 | 56 | F | ASyS | EJ | - | 845 | usual interstitial pneumonia | CYP, steroids |
| 16 | 63 | F | DM | TIF-1 | - | 204 | emphysema, pulmonary fibrosis with diffusion restriction | CyS, steroids |
| 17 | 60 | F | ASyS | JO1 | - | 165 | pulmonary fibrosis with diffusion restriction | IVIG |
| 18 | 76 | F | DM | MI2 | - | 1489 | pulmonary fibrosis with diffusion restriction | AZA |
| 19 | 46 | M | DM | MDA5 | - | 110 | pulmonary fibrosis with diffusion restriction | CyS |
| 20 | 47 | F | ASyS | JO1 | - | 10,000 | pulmonary fibrosis with diffusion restriction | MTX |
| 21 | 46 | M | ASyS | PL7 | - | 11,520 | pulmonary fibrosis with diffusion restriction | Steroids |
| 22 | 35 | F | DM | SSA | - | 373 | pulmonary fibrosis with diffusion restriction | steroids, IVIG |
| 23 | 57 | M | ASyS | PL7 | - | 150 | pulmonary fibrosis with diffusion restriction | AZA, steroids |
| 24 | 66 | F | ASyS | PL7 | - | 373 | interstitial pneumonia | MTX |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlitzki, M.; Nelke, C.; Rolfes, L.; Hasseli, R.; Tomaras, S.; Feist, E.; Schänzer, A.; Räuber, S.; Regner, L.; Preuße, C.; et al. NK Cell Patterns in Idiopathic Inflammatory Myopathies with Pulmonary Affection. Cells 2021, 10, 2551. https://doi.org/10.3390/cells10102551
Pawlitzki M, Nelke C, Rolfes L, Hasseli R, Tomaras S, Feist E, Schänzer A, Räuber S, Regner L, Preuße C, et al. NK Cell Patterns in Idiopathic Inflammatory Myopathies with Pulmonary Affection. Cells. 2021; 10(10):2551. https://doi.org/10.3390/cells10102551
Chicago/Turabian StylePawlitzki, Marc, Christopher Nelke, Leoni Rolfes, Rebecca Hasseli, Stylianos Tomaras, Eugen Feist, Anne Schänzer, Saskia Räuber, Liesa Regner, Corinna Preuße, and et al. 2021. "NK Cell Patterns in Idiopathic Inflammatory Myopathies with Pulmonary Affection" Cells 10, no. 10: 2551. https://doi.org/10.3390/cells10102551
APA StylePawlitzki, M., Nelke, C., Rolfes, L., Hasseli, R., Tomaras, S., Feist, E., Schänzer, A., Räuber, S., Regner, L., Preuße, C., Allenbach, Y., Benveniste, O., Wiendl, H., Stenzel, W., Meuth, S. G., & Ruck, T. (2021). NK Cell Patterns in Idiopathic Inflammatory Myopathies with Pulmonary Affection. Cells, 10(10), 2551. https://doi.org/10.3390/cells10102551








