Altered Brain Cholinergic and Synaptic Markers in Obese Zucker Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Animal Handling
2.2. Animals
2.3. Biochemical Analysis
2.4. Tissue Handling
2.5. Western Blot (WB) and Quantification
2.6. Immunohistochemistry (IHC) and Image Analysis
2.7. Immunofluorescence
2.8. Statistical Analysis
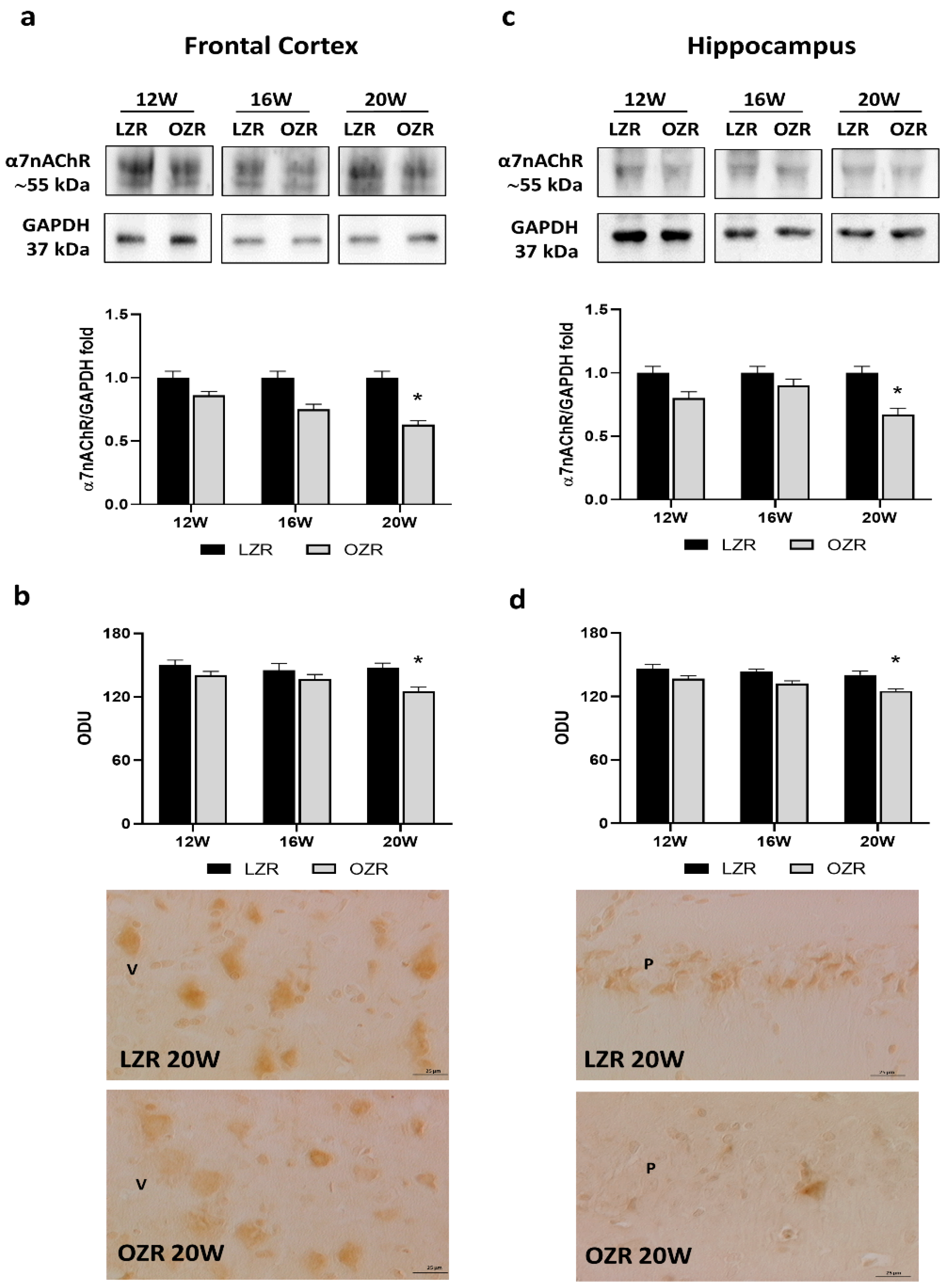
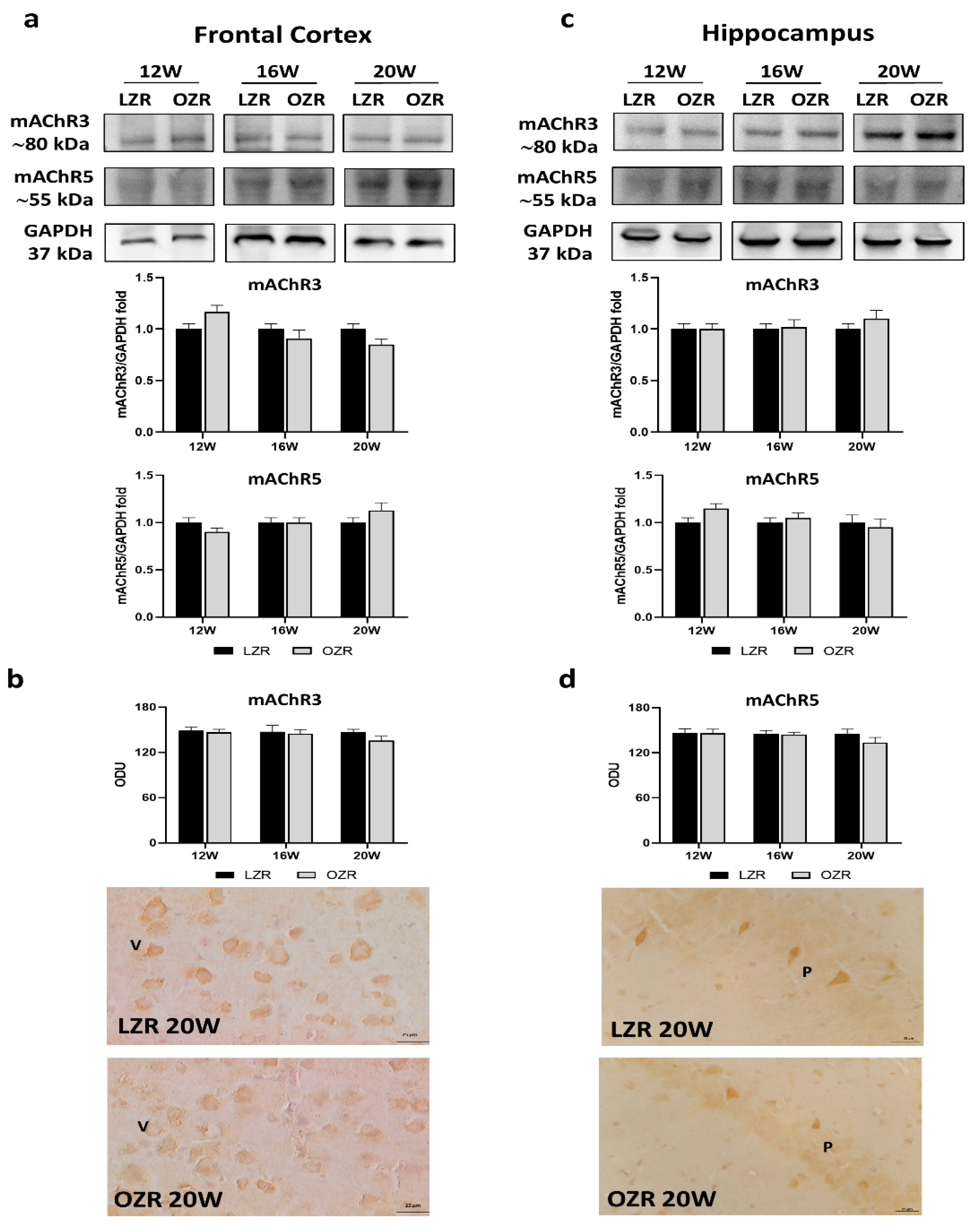
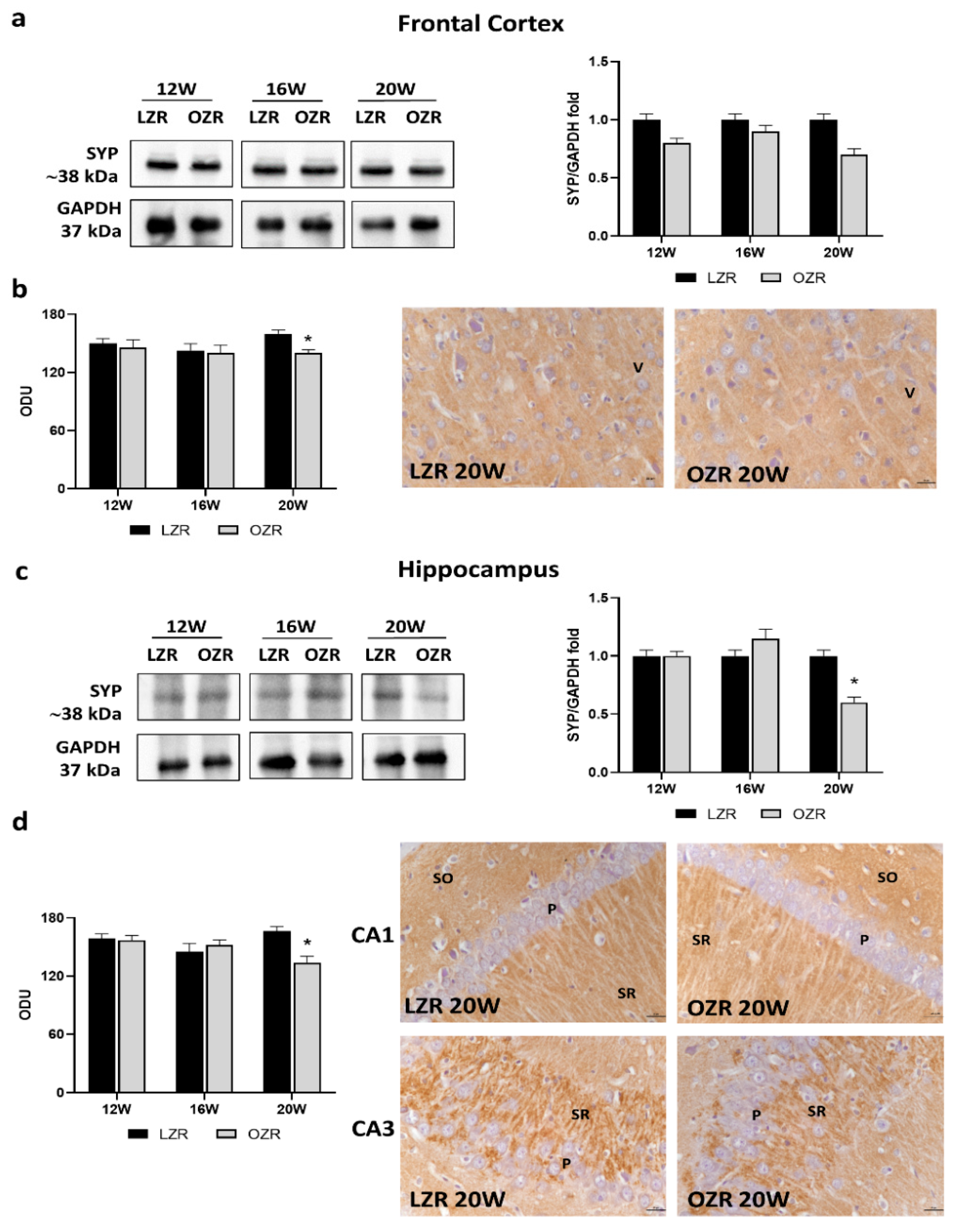
3. Results
3.1. General and Blood Analysis
3.2. Cholinergic Marker: Vesicular Acetylcholine Transporter
3.3. Synaptic Markers
3.3.1. Cholinergic Receptors
3.3.2. Synaptic Vesicle Glycoproteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grundy, S.M. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef] [Green Version]
- Van Dieren, S.; Beulens, J.W.; van der Schouw, Y.T.; Grobbee, D.E.; Neal, B. The global burden of diabetes and its complications: An emerging pandemic. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, S3–S8. [Google Scholar] [CrossRef]
- Blüher, M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 163–177. [Google Scholar] [CrossRef]
- Pillon, N.J.; Loos, R.J.F.; Marshall, S.M.; Zierath, J.R. Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care. Cell 2021, 184, 1530–1544. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Norman, E.D.; Lee, K.; Cutler, R.G.; Telljohann, R.S.; Egan, J.M.; Mattson, M.P. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 2008, 18, 1085–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, C.D.; Pistell, P.J.; Ingram, D.K.; Johnson, W.D.; Liu, Y.; Fernandez-Kim, S.O.; White, C.L.; Purpera, M.N.; Uranga, R.M.; Bruce-Keller, A.J.; et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: Implications for decreased nrf2 signaling. J. Neurochem. 2010, 114, 1581–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeilly, A.D.; Williamson, R.; Balfour, D.J.; Stewart, C.A.; Sutherland, C. A high-fat-diet-induced cognitive deficit in rats that is not prevented by improving insulin sensitivity with metformin. Diabetologia 2012, 55, 3061–3070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillemot-Legris, O.; Muccioli, G.G. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. 2017, 40, 237–253. [Google Scholar] [CrossRef]
- Van Dyken, P.; Lacoste, B. Impact of Metabolic Syndrome on Neuroinflammation and the Blood-Brain Barrier. Front. Neurosci. 2018, 12, 930. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [Green Version]
- Könner, A.C.; Brüning, J.C. Toll-like receptors: Linking inflammation to metabolism. Trends Endocrinol. Metab. 2011, 22, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Micioni Di Bonaventura, M.V.; Martinelli, I.; Moruzzi, M.; Micioni Di Bonaventura, E.; Giusepponi, M.E.; Polidori, C.; Lupidi, G.; Tayebati, S.K.; Amenta, F.; Cifani, C.; et al. Brain alterations in high fat diet induced obesity: Effects of tart cherry seeds and juice. Nutrients 2020, 12, 623. [Google Scholar] [CrossRef] [Green Version]
- Tomassoni, D.; Nwankwo, I.E.; Gabrielli, M.G.; Bhatt, S.; Muhammad, A.B.; Lokhandwala, M.F.; Tayebati, S.K.; Amenta, F. Astrogliosis in the brain of obese Zucker rat: A model of metabolic syndrome. Neurosci. Lett. 2013, 543, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Tomassoni, D.; Martinelli, I.; Moruzzi, M.; Micioni Di Bonaventura, M.V.; Cifani, C.; Amenta, F.; Tayebati, S.K. Obesity and Age-Related Changes in the Brain of the Zucker Lepr fa/fa Rats. Nutrients. 2020, 12, 1356. [Google Scholar] [CrossRef]
- Sagae, S.C.; Grassiolli, S.; Raineki, C.; Balbo, S.L.; Marques da Silva, A.C. Sex differences in brain cholinergic activity in MSG-obese rats submitted to exercise. Can. J. Physiol. Pharmacol. 2011, 89, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Long, J.; Su, Z.; Xu, B.; Lin, M.; Chen, Y.; Long, D. Restored presynaptic synaptophysin and cholinergic inputs contribute to the protective effects of physical running on spatial memory in aged mice. Neurobiol. Dis. 2019, 132, 104586. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Z.; Xue, B.; Shi, H. Activation of the Cholinergic Antiinflammatory Pathway Ameliorates Obesity-Induced Inflammation and Insulin Resistance. Endocrinology 2011, 152, 836–846. [Google Scholar] [CrossRef] [Green Version]
- Chavan, S.S.; Tracey, K.J. Essential neuroscience in immunology. J. Immunol. 2017, 198, 3389–3397. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. Neural regulation of immunity: Molecular mechanisms and clinical translation. Nat. Neurosci. 2017, 20, 156–166. [Google Scholar] [CrossRef]
- Martinelli, I.; Tomassoni, D.; Moruzzi, M.; Traini, E.; Amenta, F.; Tayebati, S.K. Obesity and Metabolic Syndrome Affect the Cholinergic Transmission and Cognitive Functions. CNS Neurol. Disord. Drug Targets 2017, 16, 664–676. [Google Scholar] [CrossRef]
- Chang, E.H.; Chavan, S.S.; Pavlov, V.A. Cholinergic Control of Inflammation, Metabolic Dysfunction, and Cognitive Impairment in Obesity-Associated Disorders: Mechanisms and Novel Therapeutic Opportunities. Front. Neurosci. 2019, 13, 263. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, V.A.; Tracey, K.J. The vagus nerve and the inflammatory reflex–linking immunity and metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Su, L.; Ren, H.; Wang, C.; Chen, J.; Fu, X. Donepezil Attenuates Obesity-Associated Oxidative Stress and Central Inflammation and Improves Memory Deficit in Mice Fed a High-Fat Diet. Dement. Geriatr. Cogn. Disord. 2019, 48, 154–163. [Google Scholar] [CrossRef]
- Consolim-Colombo, F.M.; Sangaleti, C.T.; Costa, F.O.; Morais, T.L.; Lopes, H.F.; Motta, J.M.; Irigoyen, M.C.; Bortoloto, L.A.; Rochitte, C.E.; Harris, Y.T.; et al. Galantamine alleviates inflammation and insulin resistance in patients with metabolic syndrome in a randomized trial. JCI Insight 2017, 2, e93340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.F.P.; Alves, P.L.; Alponti, R.F.; Silveira, P.F.; Abdalla, F.M.F. Effects of obesity induced by high-calorie diet and its treatment with exenatide on muscarinic acetylcholine receptors in rat hippocampus. Biochem. Pharmacol. 2019, 169, 113630. [Google Scholar] [CrossRef]
- Bocarsly, M.E.; Fasolino, M.; Kane, G.A.; LaMarca, E.A.; Kirschen, G.W.; Karatsoreos, I.N.; McEwen, B.S.; Gould, E. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc. Natl. Acad. Sci. USA 2015, 112, 15731–15736. [Google Scholar] [CrossRef] [Green Version]
- Marcello, E.; Epis, R.; Saraceno, C.; Di Luca, M. Synaptic dysfunction in Alzheimer’s disease. Adv. Exp. Med. Biol. 2012, 970, 573–601. [Google Scholar] [CrossRef]
- Wang, D.B.; Kinoshita, Y.; Kinoshita, C.; Uo, T.; Sopher, B.L.; Cudaback, E.; Keene, C.D.; Bilousova, T.; Gylys, K.; Case, A.; et al. Loss of endophilin-B1 exacerbates Alzheimer’s disease pathology. Brain 2015, 138, 2005–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivanesan, S.; Tan, A.; Rajadas, J. Pathogenesis of Abeta oligomers in synaptic failure. Curr. Alzheimer Res. 2013, 10, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Martinelli, I.; Tomassoni, D.; Moruzzi, M.; Roy, P.; Cifani, C.; Amenta, F.; Tayebati, S.K. Cardiovascular Changes Related to Metabolic Syndrome: Evidence in Obese Zucker Rats. Int. J. Mol. Sci. 2020, 21, 2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amenta, F.; Peleg, E.; Tomassoni, D.; Sabbatini, M.; Rosenthal, T. Effect of treatment with lercanidipine on heart of Cohen-Rosenthal diabetic hypertensive rats. Hypertension 2003, 41, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Hattori, T.; Ohtake, M.; Abe, M.; Amakusa, Y.; Takatsu, M.; Murohara, T.; Nagata, K. Cardiac remodeling and diastolic dysfunction in DahlS.Z-Lepr(fa)/Lepr(fa) rats: A new animal model of metabolic syndrome. Hypertens. Res. 2012, 35, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Riojas-Hernández, A.; Bernal-Ramírez, J.; Rodríguez-Mier, D.; Morales-Marroquín, F.E.; Domínguez-Barragán, E.M.; Borja-Villa, C.; Rivera-Álvarez, I.; García-Rivas, G.; Altamirano, J.; García, N. Enhanced oxidative stress sensitizes the mitochondrial permeability transition pore to opening in heart from Zucker Fa/fa rats with type 2 diabetes. Life Sci. 2015, 141, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Tayebati, S.K.; Di Tullio, M.A.; Amenta, F. Vesicular acetylcholine transporter (VAChT) in the brain of spontaneously hypertensive rats (SHR): Effect of treatment with an acetylcholinesterase inhibitor. Clin. Exp. Hypertens. 2008, 30, 732–743. [Google Scholar] [CrossRef]
- Tayebati, S.K.; Di Tullio, M.A.; Tomassoni, D.; Amenta, F. Localization of the m5 muscarinic cholinergic receptor in rat circle of Willis and pial arteries. Neuroscience 2003, 122, 205–211. [Google Scholar] [CrossRef]
- Jones, I.W.; Wonnacott, S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J. Neurosci. 2004, 24, 11244–11252. [Google Scholar] [CrossRef]
- Fabian-Fine, R.; Skehel, P.; Errington, M.L.; Davies, H.A.; Sher, E.; Stewart, M.G.; Fine, A. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J. Neurosci. 2001, 21, 7993–8003. [Google Scholar] [CrossRef]
- Caulfield, M.P. Muscarinic receptors--characterization, coupling and function. Pharmacol. Ther. 1993, 58, 319–379. [Google Scholar] [CrossRef]
- Tayebati, S.K.; Amenta, F.; El-Assouad, D.; Zaccheo, D. Muscarinic cholinergic receptor subtypes in the hippocampus of aged rats. Mech. Ageing Dev. 2002, 123, 521–528. [Google Scholar] [CrossRef]
- Lebois, E.P.; Thorn, C.; Edgerton, J.R.; Popiolek, M.; Xi, S. Muscarinic receptor subtype distribution in the central nervous system and relevance to aging and Alzheimer’s disease. Neuropharmacology 2018, 13, 362–373. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farr, S.A.; Yamada, K.A.; Butterfield, D.A.; Abdul, H.M.; Xu, L.; Miller, N.E.; Banks, W.A.; Morley, J.E. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology 2008, 149, 2628–2636. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Hayes, M.R.; Greenwald, H.S.; Fortin, S.M.; Gianessi, C.A.; Gilbert, J.R.; Grill, H.J. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology 2011, 36, 1859–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeilly, A.D.; Williamson, R.; Sutherland, C.; Balfour, D.J.; Stewart, C.A. High fat feeding promotes simultaneous decline in insulin sensitivity and cognitive performance in a delayed matching and nonmatching to position task. Behav. Brain Res. 2011, 217, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, M.S.; Thuret, S. Nutrition, adult hippocampal neurogenesis and mental health. Br. Med. Bull. 2012, 103, 89–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurung, S.; Agbaga, M.P.; Myers, D.A. Cognitive differences between Sprague-Dawley rats selectively bred for sensitivity or resistance to diet induced obesity. Behav. Brain Res. 2016, 311, 122–130. [Google Scholar] [CrossRef]
- Sánchez-Sarasúa, S.; Moustafa, S.; García-Avilés, Á.; López-Climent, M.F.; Gómez-Cadenas, A.; Olucha-Bordonau, F.E.; Sánchez-Pérez, A.M. The effect of abscisic acid chronic treatment on neuroinflammatory markers and memory in a rat model of high-fat diet induced neuroinflammation. Nutr. Metab. 2016, 13, 73. [Google Scholar] [CrossRef] [Green Version]
- Martins, I.V.; Rivers-Auty, J.; Allan, S.M.; Lawrence, C.B. Mitochondrial Abnormalities and Synaptic Loss Underlie Memory Deficits Seen in Mouse Models of Obesity and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 915–932. [Google Scholar] [CrossRef] [Green Version]
- Grossman, S.P. Direct adrenergic and cholinergic stimulation of hypothalamic mechanisms. Am. J. Physiol. 1962, 202, 872–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chance, W.T.; Lints, C.E. Eating following cholinergic stimulation of the hypothalamus. Physiol. Psychol. 1977, 5, 440–444. [Google Scholar] [CrossRef] [Green Version]
- Ohkuma, S.; Ma, F.H.; Kishi, M.; Kuriyama, K. Alteration of acetylcholine metabolism in the brain of zucker fatty rat. Neurochem. Int. 1990, 16, 99–103. [Google Scholar] [CrossRef]
- Goodman, C.B.; Soliman, K.F. Altered brain cholinergic enzymes activity in the genetically obese rat. Experientia 1991, 47, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Baskin, D.G.; Stein, L.J.; Ikeda, H.; Woods, S.C.; Figlewicz, D.P.; Porte, D.; Greenwood, M.R.; Dorsa, D.M. Genetically obese Zucker rats have abnormally low brain insulin content. Life Sci. 1985, 36, 627–633. [Google Scholar] [CrossRef]
- Prado, V.F.; Martins-Silva, C.; de Castro, B.M.; Lima, R.F.; Barros, D.M.; Amaral, E.; Ramsey, A.J.; Sotnikova, T.D.; Ramirez, M.R.; Kim, H.G.; et al. Mice deficient for the vesicular acetylcholine transporter are myasthenic and have deficits in object and social recognition. Neuron 2006, 51, 601–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Castro, B.M.; Pereira, G.S.; Magalhães, V.; Rossato, J.I.; De Jaeger, X.; Martins-Silva, C.; Leles, B.; Lima, P.; Gomez, M.V.; Gainetdinov, R.R.; et al. Reduced expression of the vesicular acetylcholine transporter causes learning deficits in mice. Genes Brain Behav. 2009, 8, 23–35. [Google Scholar] [CrossRef]
- Nordberg, A. Nicotinic receptor abnormalities of Alzheimer’s disease: Therapeutic implications. Biol. Psychiatry 2001, 49, 200–210. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Zhang, W.J.; Su, D.F.; Zhang, G.Q.; Miao, C.Y. Cellular responses and functions of α7 nicotinic acetylcholine receptor activation in the brain: A narrative review. Ann. Transl. Med. 2021, 9, 509. [Google Scholar] [CrossRef]
- Suzuki, T.; Hide, I.; Matsubara, A.; Hama, C.; Harada, K.; Miyano, K.; Andrä, M.; Matsubayashi, H.; Sakai, N.; Kohsaka, S.; et al. Microglial alpha7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J. Neurosci. Res. 2006, 83, 1461–1470. [Google Scholar] [CrossRef]
- Papouin, T.; Dunphy, J.M.; Tolman, M.; Dineley, K.T.; Haydon, P.G. Septal Cholinergic Neuromodulation Tunes the Astrocyte-Dependent Gating of Hippocampal NMDA Receptors to Wakefulness. Neuron 2017, 94, 840–854. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef]
- Echeverria, V.; Yarkov, A.; Aliev, G. Positive modulators of the α7 nicotinic receptor against neuroinflammation and cognitive impairment in Alzheimer’s disease. Prog. Neurobiol. 2016, 144, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Liang, D.; Bao, M.; Xie, Y.; Xu, W.; Wang, L.; Wang, Z.; Qiao, Z. Gx-50 Inhibits Neuroinflammation via α7 nAChR Activation of the JAK2/STAT3 and PI3K/AKT Pathways. J. Alzheimers Dis. 2016, 50, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, Y.; Bian, H.; Guo, L.; Zhu, H. Activation of the α7 nicotinic receptor promotes lipopolysaccharide-induced conversion of M1 microglia to M2. Am. J. Transl. Res. 2017, 9, 971–985. [Google Scholar]
- Tomassoni, D.; Tayebati, S.K.; Ciriaci, M.; Cognigni, F.; Amenta, F. Obesity-related blood brain barrier changes in obese Zucker rats. Ital. J. Anat. Embryol. 2015, 120, 192. [Google Scholar]
- Hwang, I.K.; Choi, J.H.; Nam, S.M.; Park, O.K.; Yoo, D.Y.; Kim, W.; Yi, S.S.; Won, M.H.; Seong, J.K.; Yoon, Y.S. Activation of microglia and induction of pro-inflammatory cytokines in the hippocampus of type 2 diabetic rats. Neurol. Res. 2014, 36, 824–832. [Google Scholar] [CrossRef]
- Wess, J.; Eglen, R.M.; Gautam, D. Muscarinic acetylcholine receptors: Mutant mice provide new insights for drug development. Nat. Rev. Drug Discov. 2007, 6, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Langmead, C.J.; Watson, J.; Reavill, C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 2008, 117, 232–243. [Google Scholar] [CrossRef]
- Conn, P.J.; Jones, C.K.; Lindsley, C.W. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol. Sci. 2009, 30, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Morin, J.P.; Rodríguez-Durán, L.F.; Guzmán-Ramos, K.; Perez-Cruz, C.; Ferreira, G.; Diaz-Cintra, S.; Pacheco-López, G. Palatable Hyper-Caloric Foods Impact on Neuronal Plasticity. Front. Behav. Neurosci. 2017, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Edlow, A.G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagn. 2017, 37, 95–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sestan-Pesa, M.; Horvath, T.L. Metabolism and Mental Illness. Trends Mol. Med. 2016, 22, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Dey, A.; Yu, X.; Stranahan, A.M. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav. Immun. 2016, 51, 230–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesby, J.P.; Kim, J.J.; Scadeng, M.; Woods, G.; Kado, D.M.; Olefsky, J.M.; Jeste, D.V.; Achim, C.L.; Semenova, S. Spatial cognition in adult and aged mice exposed to high-fat diet. PLoS ONE 2015, 10, e0140034. [Google Scholar] [CrossRef] [Green Version]
- Baran, S.E.; Campbell, A.M.; Kleen, J.K.; Foltz, C.H.; Wright, R.L.; Diamond, D.M.; Conrad, C.D. Combination of high fat diet and chronic stress retracts hippocampal dendrites. Neuroreport 2005, 16, 39–43. [Google Scholar] [CrossRef] [Green Version]



| Antibodies | Company and Cat. No | Dilution WB | Dilution IHC |
|---|---|---|---|
| Vesicular acetylcholine transporter (VAChT) | Santa Cruz Biotechnology Cat. sc7717 | 1:500 | 1:100 |
| Alpha7 nicotinic acetylcholine receptor (α7nAChR) | Santa Cruz Biotechnology Cat. sc5544 | 1:500 | 1:50 |
| Muscarinic acetylcholine receptor subtype 1 (mAChR1) | Santa Cruz Biotechnology Cat. sc9106 | 1:500 | 1:50 |
| Muscarinic acetylcholine receptor subtype 3 (mAChR3) | Santa Cruz Biotechnology Cat. sc7474 | 1:500 | 1:50 |
| Muscarinic acetylcholine receptor subtype 5 (mAChR5) | Santa Cruz Biotechnology Cat. sc7479 | 1:500 | 1:50 |
| Synaptophysin (SYP) | EMD Millipore Cat. MAB368 | 1:500 | 1:200 |
| Synaptic vesicle glycoprotein 2A (SV2A) | Santa Cruz Biotechnology Cat. sc11939 | 1:200 | 1:50 |
| Synaptic vesicle glycoprotein 2B (SV2B) | Santa Cruz Biotechnology Cat. sc11943 | 1:200 | 1:50 |
| Synaptic vesicle glycoprotein 2C (SV2C) | Santa Cruz Biotechnology Cat. sc11946 | 1:200 | 1:50 |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | Sigma Aldrich Cat. G9295 | 1:5000 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, I.; Tomassoni, D.; Roy, P.; Amenta, F.; Tayebati, S.K. Altered Brain Cholinergic and Synaptic Markers in Obese Zucker Rats. Cells 2021, 10, 2528. https://doi.org/10.3390/cells10102528
Martinelli I, Tomassoni D, Roy P, Amenta F, Tayebati SK. Altered Brain Cholinergic and Synaptic Markers in Obese Zucker Rats. Cells. 2021; 10(10):2528. https://doi.org/10.3390/cells10102528
Chicago/Turabian StyleMartinelli, Ilenia, Daniele Tomassoni, Proshanta Roy, Francesco Amenta, and Seyed Khosrow Tayebati. 2021. "Altered Brain Cholinergic and Synaptic Markers in Obese Zucker Rats" Cells 10, no. 10: 2528. https://doi.org/10.3390/cells10102528
APA StyleMartinelli, I., Tomassoni, D., Roy, P., Amenta, F., & Tayebati, S. K. (2021). Altered Brain Cholinergic and Synaptic Markers in Obese Zucker Rats. Cells, 10(10), 2528. https://doi.org/10.3390/cells10102528








