Early Effects of HTLV-1 Infection on the Activation, Exhaustion, and Differentiation of T-Cells in Humanized NSG Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Isolation of Hematopoietic Stem Cells from Human Fetal Liver Samples
2.3. Establishment of HIS-NSG Mice
2.4. HTLV-1 Infection of HIS-NSG Mice and Processing of Samples
2.5. Monoclonal Antibodies and Flow Cytometry Assays
2.6. Quantification of HTLV-1 Proviral Load (PVL)
2.7. Statistical Analysis
3. Results
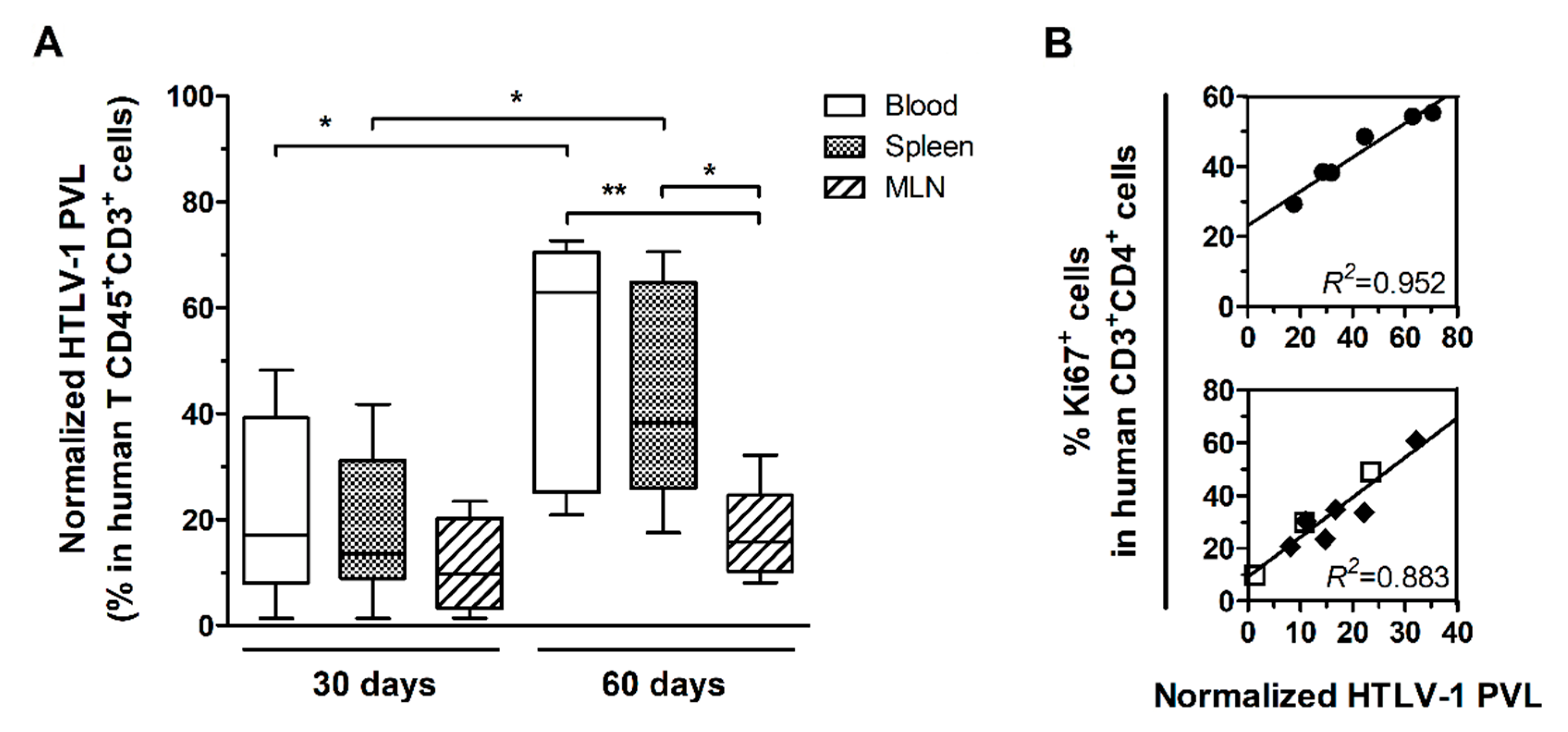
3.1. HTLV-1 PVL in the Blood, Spleen, and MLN of HTLV-1-Infected HIS-NSG Mice
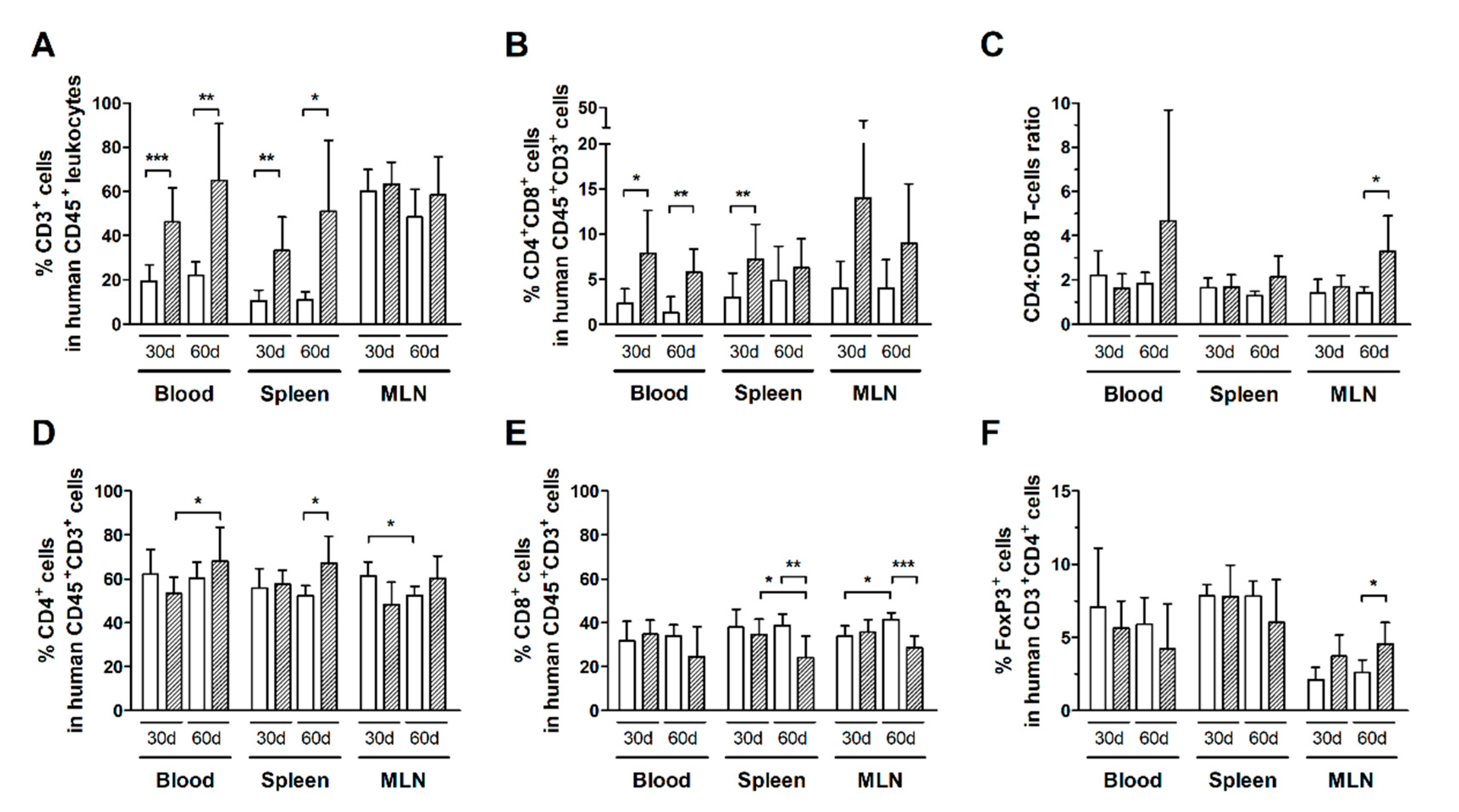
3.2. Expansion of CD4+ and CD8+ T-Cell Subsets Is Stimulated by HTLV-1 Infection
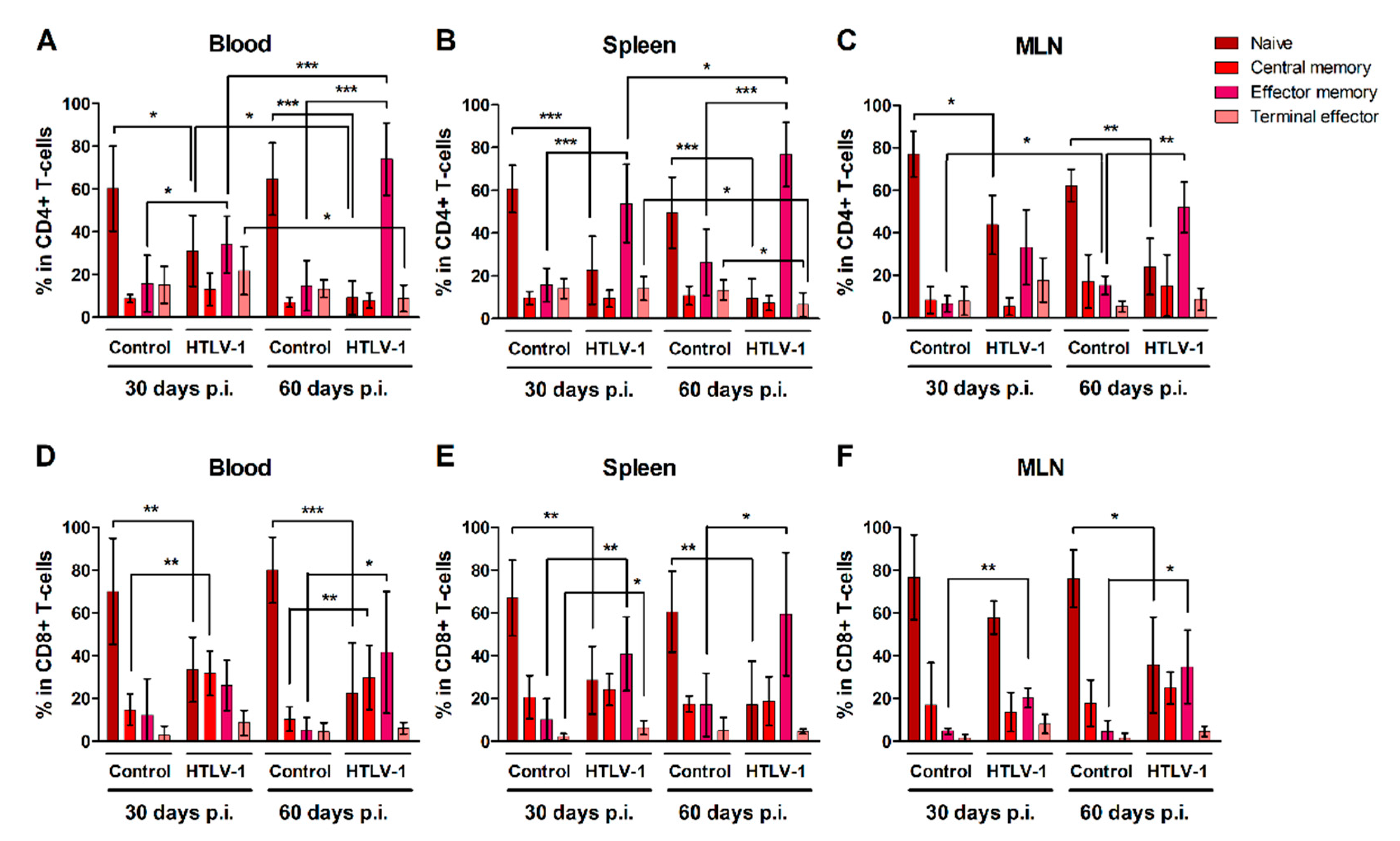
3.3. HTLV-1-Infection Induces CD4+ and CD8+ T-Cells to Mature into Effector Memory Cells
3.4. Expression of Th1-Related Chemokine Receptors in T-Cells during HTLV-1 Infection
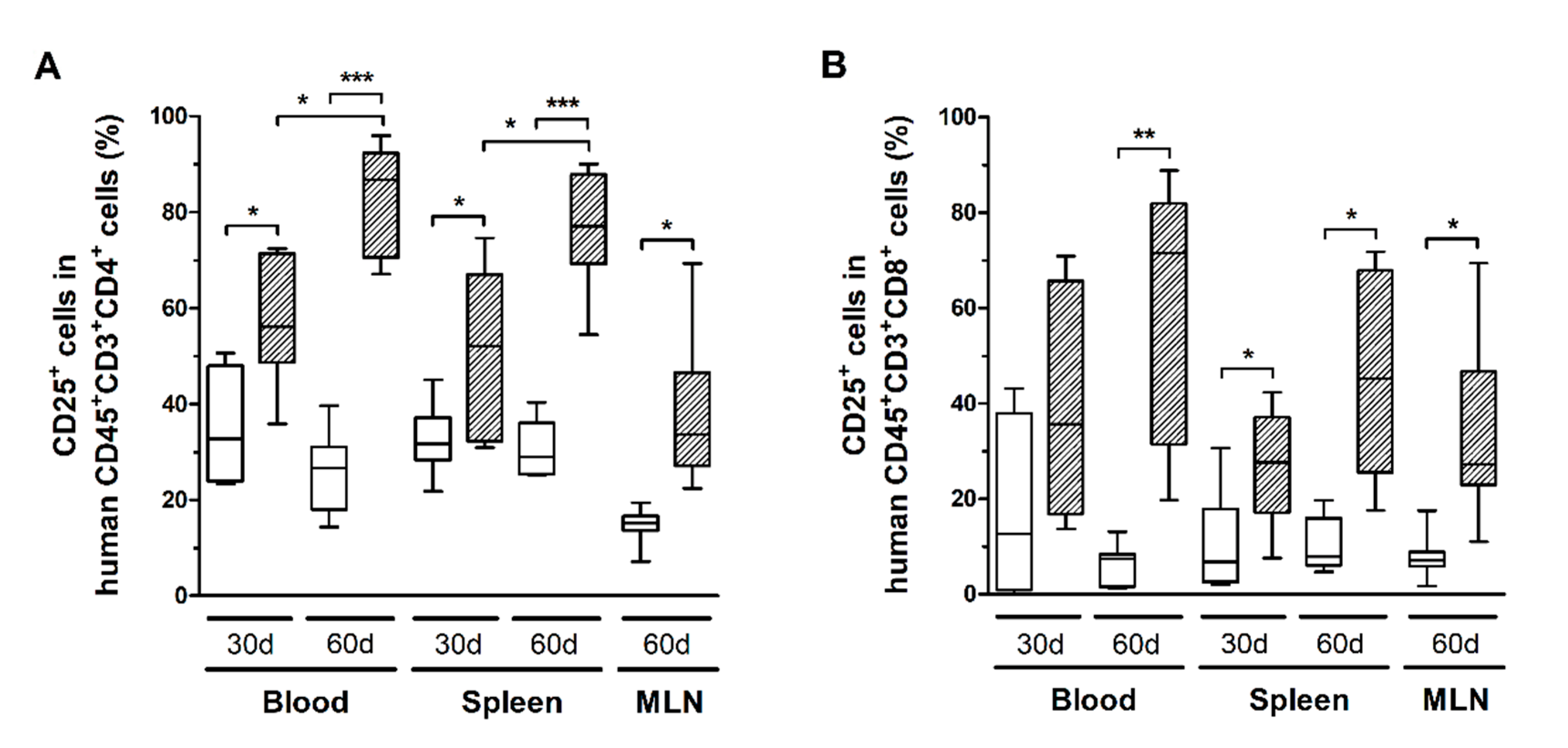
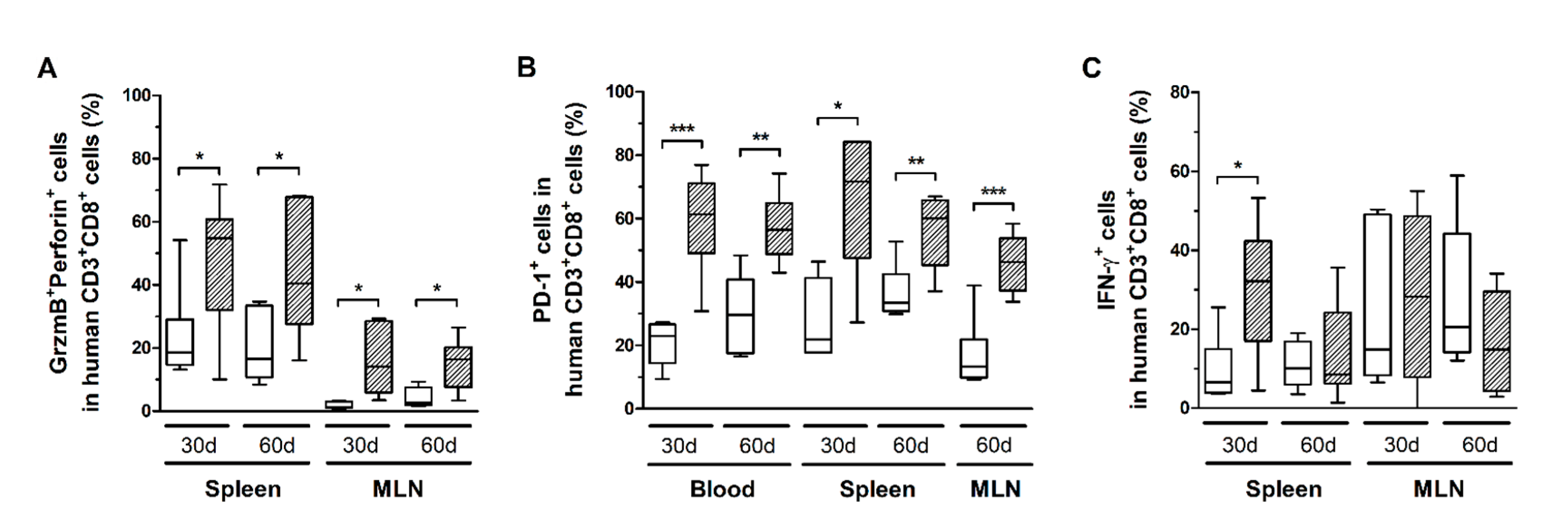
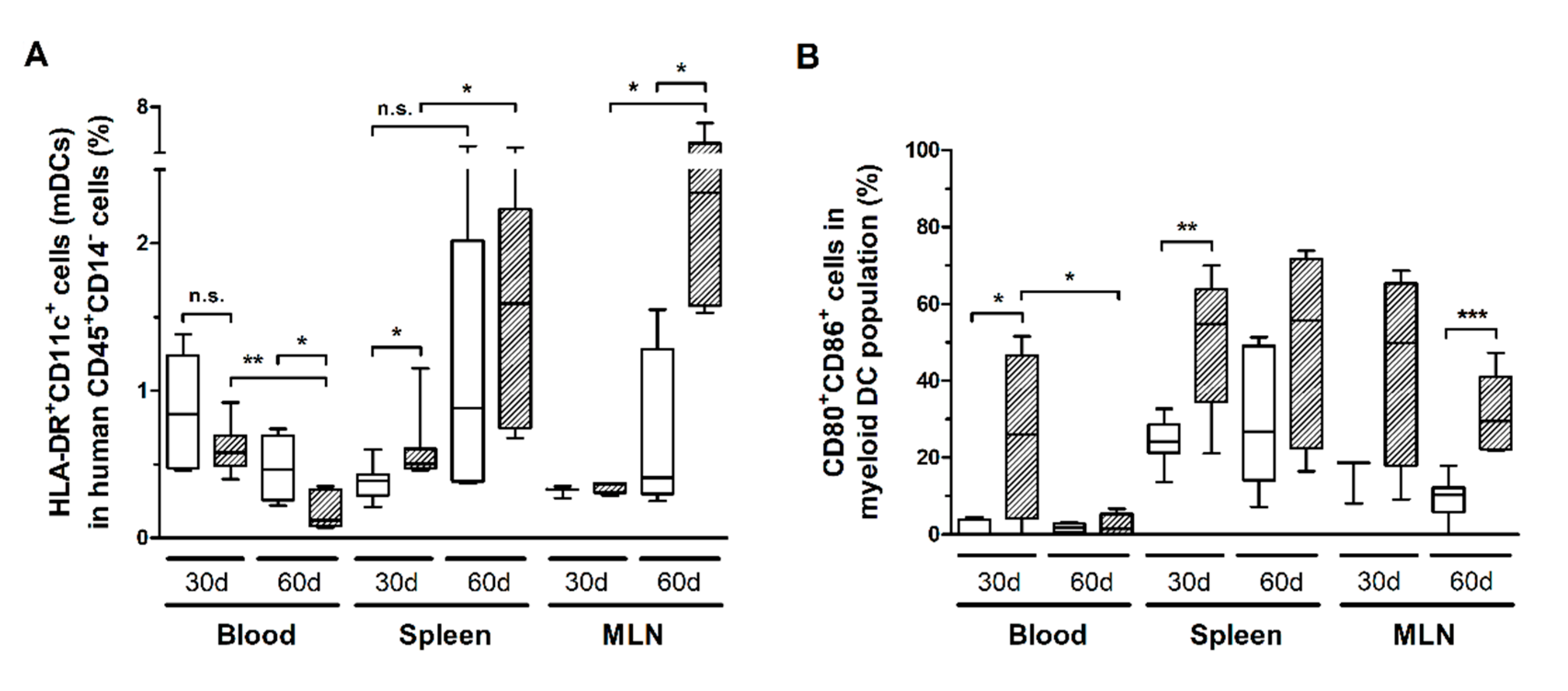
3.5. HTLV-1 Infection Induces the Activation and Exhaustion of T-Cells in HIS-NSG Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T. Adult T-Cell Leukemia: Molecular Basis for Clonal Expansion and Transformation of HTLV-1–Infected T Cells. Blood 2017, 129, 1071–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panfil, A.R.; Martinez, M.P.; Ratner, L.; Green, P.L. Human T-Cell Leukemia Virus-Associated Malignancy. Curr. Opin. Virol. 2016, 20, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, A.Q.; Silva, M.T.T. The HTLV-1 Neurological Complex. Lancet Neurol. 2006, 5, 1068–1076. [Google Scholar] [CrossRef]
- Bangham, C.R.M.; Araujo, A.; Yamano, Y.; Taylor, G.P. HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis. Nat. Rev. Dis. Primer 2015, 1, 15012. [Google Scholar] [CrossRef] [PubMed]
- Asquith, B.; Zhang, Y.; Mosley, A.J.; de Lara, C.M.; Wallace, D.L.; Worth, A.; Kaftantzi, L.; Meekings, K.; Griffin, G.E.; Tanaka, Y.; et al. In Vivo T Lymphocyte Dynamics in Humans and the Impact of Human T-Lymphotropic Virus 1 Infection. Proc. Natl. Acad. Sci. USA 2007, 104, 8035–8040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito-Melo, G.E.A.; Martins-Filho, O.A.; Carneiro-Proietti, A.B.F.; Catalan-Soares, B.; Ribas, J.G.; Thorum, G.W.; Barbosa-Stancioli, E.F.; Grupo Interdisciplinar de Pesquisas Em HTLV. Phenotypic Study of Peripheral Blood Leucocytes in HTLV-I-Infected Individuals from Minas Gerais, Brazil. Scand. J. Immunol. 2002, 55, 621–628. [Google Scholar] [CrossRef]
- Kress, A.K.; Grassmann, R.; Fleckenstein, B. Cell Surface Markers in HTLV-1 Pathogenesis. Viruses 2011, 3, 1439–1459. [Google Scholar] [CrossRef]
- Coutinho, R.; Grassi, M.F.R.; Korngold, A.B.; Olavarria, V.N.; Galvão-Castro, B.; Mascarenhas, R.E. Human T Lymphotropic Virus Type 1 (HTLV-1) Proviral Load Induces Activation of T-Lymphocytes in Asymptomatic Carriers. BMC Infect. Dis. 2014, 14, 453. [Google Scholar] [CrossRef] [Green Version]
- Tattermusch, S.; Skinner, J.A.; Chaussabel, D.; Banchereau, J.; Berry, M.P.; McNab, F.W.; O’Garra, A.; Taylor, G.P.; Bangham, C.R.M. Systems Biology Approaches Reveal a Specific Interferon-Inducible Signature in HTLV-1 Associated Myelopathy. PLoS Pathog. 2012, 8, e1002480. [Google Scholar] [CrossRef] [Green Version]
- Espíndola, O.M.; Oliveira, L.C.; Ferreira, P.M.S.; Leite, A.C.C.B.; Lima, M.A.S.D.; Andrada-Serpa, M.J. High IFN-Gamma/IL-10 Expression Ratio and Increased Frequency of Persistent Human T-Cell Lymphotropic Virus Type 1-Infected Clones Are Associated with Human T-Cell Lymphotropic Virus Type 1-Associated Myelopathy/Tropical Spastic Paraparesis Development. Intervirology 2015, 58, 106–114. [Google Scholar] [CrossRef]
- Kattan, T.; MacNamara, A.; Rowan, A.G.; Nose, H.; Mosley, A.J.; Tanaka, Y.; Taylor, G.P.; Asquith, B.; Bangham, C.R.M. The Avidity and Lytic Efficiency of the CTL Response to HTLV-1. J. Immunol. 2009, 182, 5723–5729. [Google Scholar] [CrossRef] [Green Version]
- Hilburn, S.; Rowan, A.; Demontis, M.-A.; MacNamara, A.; Asquith, B.; Bangham, C.R.M.; Taylor, G.P. In Vivo Expression of Human T-Lymphotropic Virus Type 1 Basic Leucine-Zipper Protein Generates Specific CD8+ and CD4+ T-Lymphocyte Responses That Correlate with Clinical Outcome. J. Infect. Dis. 2011, 203, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdonck, K.; González, E.; Van Dooren, S.; Vandamme, A.-M.; Vanham, G.; Gotuzzo, E. Human T-Lymphotropic Virus 1: Recent Knowledge about an Ancient Infection. Lancet Infect. Dis. 2007, 7, 266–281. [Google Scholar] [CrossRef]
- Boxus, M.; Twizere, J.-C.; Legros, S.; Dewulf, J.-F.; Kettmann, R.; Willems, L. The HTLV-1 Tax Interactome. Retrovirology 2008, 5, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Z.; Xiao, G. Human T-Cell Lymphotropic Virus: A Model of NF-ΚB-Associated Tumorigenesis. Viruses 2011, 3, 714–749. [Google Scholar] [CrossRef]
- Zhao, T. The Role of HBZ in HTLV-1-Induced Oncogenesis. Viruses 2016, 8, 34. [Google Scholar] [CrossRef]
- Fochi, S.; Mutascio, S.; Bertazzoni, U.; Zipeto, D.; Romanelli, M.G. HTLV Deregulation of the NF-ΚB Pathway: An Update on Tax and Antisense Proteins Role. Front. Microbiol. 2018, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Barbeau, B.; Peloponese, J.-M.; Mesnard, J.-M. Functional Comparison of Antisense Proteins of HTLV-1 and HTLV-2 in Viral Pathogenesis. Front. Microbiol. 2013, 4, 226. [Google Scholar] [CrossRef] [Green Version]
- Satou, Y.; Yasunaga, J.; Yoshida, M.; Matsuoka, M. HTLV-I Basic Leucine Zipper Factor Gene MRNA Supports Proliferation of Adult T Cell Leukemia Cells. Proc. Natl. Acad. Sci. USA 2006, 103, 720–725. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, M.; Jeang, K.-T. Human T-Cell Leukaemia Virus Type 1 (HTLV-1) Infectivity and Cellular Transformation. Nat. Rev. Cancer 2007, 7, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Sawa, H.; Lewis, M.J.; Orba, Y.; Sheehy, N.; Yamamoto, Y.; Ichinohe, T.; Tsunetsugu-Yokota, Y.; Katano, H.; Takahashi, H.; et al. Thymus-Derived Leukemia-Lymphoma in Mice Transgenic for the Tax Gene of Human T-Lymphotropic Virus Type I. Nat. Med. 2006, 12, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Ohsugi, T.; Kumasaka, T.; Okada, S.; Urano, T. The Tax Protein of HTLV-1 Promotes Oncogenesis in Not Only Immature T Cells but Also Mature T Cells. Nat. Med. 2007, 13, 527–528. [Google Scholar] [CrossRef]
- Satou, Y.; Yasunaga, J.; Zhao, T.; Yoshida, M.; Miyazato, P.; Takai, K.; Shimizu, K.; Ohshima, K.; Green, P.L.; Ohkura, N.; et al. HTLV-1 BZIP Factor Induces T-Cell Lymphoma and Systemic Inflammation In Vivo. PLoS Pathog. 2011, 7, e1001274. [Google Scholar] [CrossRef] [PubMed]
- Dodon, M.D.; Villaudy, J.; Gazzolo, L.; Haines, R.; Lairmore, M. What We Are Learning on HTLV-1 Pathogenesis from Animal Models. Front. Microbiol. 2012, 3, 320. [Google Scholar] [CrossRef] [Green Version]
- Villaudy, J.; Wencker, M.; Gadot, N.; Gillet, N.A.; Scoazec, J.-Y.; Gazzolo, L.; Manz, M.G.; Bangham, C.R.M.; Dodon, M.D. HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2−/− Gamma c−/− Mice. PLoS Pathog. 2011, 7, e1002231. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, K.; Xun, R.; Tei, M.; Ueno, T.; Tanaka, M.; Takenouchi, N.; Fujisawa, J.-I. An Animal Model of Adult T-Cell Leukemia: Humanized Mice with HTLV-1-Specific Immunity. Blood 2014, 123, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Percher, F.; Curis, C.; Pérès, E.; Artesi, M.; Rosewick, N.; Jeannin, P.; Gessain, A.; Gout, O.; Mahieux, R.; Ceccaldi, P.-E.; et al. HTLV-1-Induced Leukotriene B4 Secretion by T Cells Promotes T Cell Recruitment and Virus Propagation. Nat. Commun. 2017, 8, 15890. [Google Scholar] [CrossRef]
- Pérès, E.; Blin, J.; Ricci, E.P.; Artesi, M.; Hahaut, V.; Van den Broeke, A.; Corbin, A.; Gazzolo, L.; Ratner, L.; Jalinot, P.; et al. PDZ Domain-Binding Motif of Tax Sustains T-Cell Proliferation in HTLV-1-Infected Humanized Mice. PLoS Pathog. 2018, 14, e1006933. [Google Scholar] [CrossRef]
- Vicario, M.; Mattiolo, A.; Montini, B.; Piano, M.A.; Cavallari, I.; Amadori, A.; Chieco-Bianchi, L.; Calabrò, M.L. A Preclinical Model for the ATLL Lymphoma Subtype with Insights into the Role of Microenvironment in HTLV-1-Mediated Lymphomagenesis. Front. Microbiol. 2018, 9, 1215. [Google Scholar] [CrossRef]
- Legrand, N.; Weijer, K.; Spits, H. Experimental Model for the Study of the Human Immune System. In Innate Immunity; Ewbank, J., Vivier, E., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 65–82. ISBN 978-1-58829-746-4. [Google Scholar]
- Legrand, N.; Weijer, K.; Spits, H. Experimental Model for the Study of the Human Immune System: Production and Monitoring of “Human Immune System” Rag2−/−Gamma c−/− Mice. Methods Mol. Biol. Clifton N. J. 2008, 415, 65–82. [Google Scholar] [CrossRef]
- Silva, M.T.T.; Harab, R.C.; Leite, A.C.; Schor, D.; Araújo, A.; Andrada-Serpa, M.J. Human T Lymphotropic Virus Type 1 (HTLV-1) Proviral Load in Asymptomatic Carriers, HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis, and Other Neurological Abnormalities Associated with HTLV-1 Infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007, 44, 689–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, Y.B.; Landay, A.L.; Zack, J.A.; Kitchen, S.G.; Al-Harthi, L. Upregulation of CD4 on CD8+ T Cells: CD4dimCD8bright T Cells Constitute an Activated Phenotype of CD8+ T Cells. Immunology 2001, 103, 270–280. [Google Scholar] [CrossRef]
- Pérès, E.; Bagdassarian, E.; This, S.; Villaudy, J.; Rigal, D.; Gazzolo, L.; Duc Dodon, M. From Immunodeficiency to Humanization: The Contribution of Mouse Models to Explore HTLV-1 Leukemogenesis. Viruses 2015, 7, 6371–6386. [Google Scholar] [CrossRef]
- Iwanaga, M.; Watanabe, T.; Yamaguchi, K. Adult T-Cell Leukemia: A Review of Epidemiological Evidence. Front. Microbiol. 2012, 3, 322. [Google Scholar] [CrossRef] [Green Version]
- Hermine, O.; Ramos, J.C.; Tobinai, K. A Review of New Findings in Adult T-Cell Leukemia–Lymphoma: A Focus on Current and Emerging Treatment Strategies. Adv. Ther. 2018, 35, 135–152. [Google Scholar] [CrossRef] [Green Version]
- Shimoyama, M.; Minato, K.; Tobinai, K.; Nagai, M.; Setoya, T.; Takenaka, T.; Ishihara, K.; Watanabe, S.; Hoshino, H.; Miwa, M.; et al. Atypical Adult T-Cell Leukemia-Lymphoma: Diverse Clinical Manifestations of Adult T-Cell Leukemia-Lymphoma. Jpn. J. Clin. Oncol. 1983, 13 (Suppl. 2), 165–187. [Google Scholar]
- Okayama, A.; Tachibana, N.; Ishihara, S.; Nagatomo, Y.; Murai, K.; Okamoto, M.; Shima, T.; Sagawa, K.; Tsubouchi, H.; Stuver, S.; et al. Increased Expression of Interleukin-2 Receptor Alpha on Peripheral Blood Mononuclear Cells in HTLV-I Tax/Rex MRNA-Positive Asymptomatic Carriers. J. Acquir. Immune Defic. Syndr. Hum. Retrovirology Off. Publ. Int. Retrovirology Assoc. 1997, 15, 70–75. [Google Scholar] [CrossRef]
- Gille, C.; Orlikowsky, T.W.; Spring, B.; Hartwig, U.F.; Wilhelm, A.; Wirth, A.; Goecke, B.; Handgretinger, R.; Poets, C.F.; André, M.C. Monocytes Derived from Humanized Neonatal NOD/SCID/IL2Rγ(Null) Mice Are Phenotypically Immature and Exhibit Functional Impairments. Hum. Immunol. 2012, 73, 346–354. [Google Scholar] [CrossRef]
- Brehm, M.A.; Wiles, M.V.; Greiner, D.L.; Shultz, L.D. Generation of Improved Humanized Mouse Models for Human Infectious Diseases. J. Immunol. Methods 2014, 410, 3–17. [Google Scholar] [CrossRef] [PubMed]
- de Castro-Amarante, M.F.; Pise-Masison, C.A.; McKinnon, K.; Washington Parks, R.; Galli, V.; Omsland, M.; Andresen, V.; Massoud, R.; Brunetto, G.; Caruso, B.; et al. Human T Cell Leukemia Virus Type 1 Infection of the Three Monocyte Subsets Contributes to Viral Burden in Humans. J. Virol. 2015, 90, 2195–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waschbisch, A.; Sammet, L.; Schröder, S.; Lee, D.-H.; Barrantes-Freer, A.; Stadelmann, C.; Linker, R.A. Analysis of CD4+ CD8+ Double-Positive T Cells in Blood, Cerebrospinal Fluid and Multiple Sclerosis Lesions. Clin. Exp. Immunol. 2014, 177, 404–411. [Google Scholar] [CrossRef]
- Nascimbeni, M.; Shin, E.-C.; Chiriboga, L.; Kleiner, D.E.; Rehermann, B. Peripheral CD4+ CD8+ T Cells Are Differentiated Effector Memory Cells with Antiviral Functions. Blood 2004, 104, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Frahm, M.A.; Picking, R.A.; Kuruc, J.D.; McGee, K.S.; Gay, C.L.; Eron, J.J.; Hicks, C.B.; Tomaras, G.D.; Ferrari, G. CD4+ CD8+ T Cells Represent a Significant Portion of the Anti-HIV T Cell Response to Acute HIV Infection. J. Immunol. 2012, 188, 4289–4296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimbeni, M.; Pol, S.; Saunier, B. Distinct CD4+ CD8+ Double-Positive T Cells in the Blood and Liver of Patients during Chronic Hepatitis B and C. PLoS ONE 2011, 6, e20145. [Google Scholar] [CrossRef] [PubMed]
- Macchi, B.; Graziani, G.; Zhang, J.; Mastino, A. Emergence of Double-Positive CD4/CD8 Cells from Adult Peripheral Blood Mononuclear Cells Infected with Human T Cell Leukemia Virus Type I (HTLV-I). Cell. Immunol. 1993, 149, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Mahnke, Y.D.; Brodie, T.M.; Sallusto, F.; Roederer, M.; Lugli, E. The Who’s Who of T-Cell Differentiation: Human Memory T-Cell Subsets. Eur. J. Immunol. 2013, 43, 2797–2809. [Google Scholar] [CrossRef]
- Hazenberg, M.D.; Stuart, J.W.; Otto, S.A.; Borleffs, J.C.; Boucher, C.A.; de Boer, R.J.; Miedema, F.; Hamann, D. T-Cell Division in Human Immunodeficiency Virus (HIV)-1 Infection Is Mainly Due to Immune Activation: A Longitudinal Analysis in Patients before and during Highly Active Antiretroviral Therapy (HAART). Blood 2000, 95, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Scholzen, T.; Gerdes, J. The Ki-67 Protein: From the Known and the Unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Gattinoni, L.; Lugli, E.; Ji, Y.; Pos, Z.; Paulos, C.M.; Quigley, M.F.; Almeida, J.R.; Gostick, E.; Yu, Z.; Carpenito, C.; et al. A Human Memory T Cell Subset with Stem Cell-like Properties. Nat. Med. 2011, 17, 1290–1297. [Google Scholar] [CrossRef]
- Macallan, D.C.; Wallace, D.; Zhang, Y.; De Lara, C.; Worth, A.T.; Ghattas, H.; Griffin, G.E.; Beverley, P.C.L.; Tough, D.F. Rapid Turnover of Effector-Memory CD4+ T Cells in Healthy Humans. J. Exp. Med. 2004, 200, 255–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karube, K.; Ohshima, K.; Tsuchiya, T.; Yamaguchi, T.; Kawano, R.; Suzumiya, J.; Utsunomiya, A.; Harada, M.; Kikuchi, M. Expression of FoxP3, a Key Molecule in CD4 CD25 Regulatory T Cells, in Adult T-Cell Leukaemia/Lymphoma Cells. Br. J. Haematol. 2004, 126, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ishii, N.; Ine, S.; Ikeda, S.; Fujimura, T.; Ndhlovu, L.C.; Soroosh, P.; Tada, K.; Harigae, H.; Kameoka, J.; et al. Regulatory T Cell-like Activity of Foxp3+ Adult T Cell Leukemia Cells. Int. Immunol. 2006, 18, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [Green Version]
- Toulza, F.; Nosaka, K.; Takiguchi, M.; Pagliuca, T.; Mitsuya, H.; Tanaka, Y.; Taylor, G.P.; Bangham, C.R.M. FoxP3+ Regulatory T Cells Are Distinct from Leukemia Cells in HTLV-1-Associated Adult T-Cell Leukemia. Int. J. Cancer 2009, 125, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Toulza, F.; Heaps, A.; Tanaka, Y.; Taylor, G.P.; Bangham, C.R.M. High Frequency of CD4+ FoxP3+ Cells in HTLV-1 Infection: Inverse Correlation with HTLV-1-Specific CTL Response. Blood 2008, 111, 5047–5053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring Function in Exhausted CD8 T Cells during Chronic Viral Infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.J.; De Pierres, C.; et al. PD-1 Expression on HIV-Specific T Cells Is Associated with T-Cell Exhaustion and Disease Progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, L.; Janbazian, L.; Chomont, N.; Said, E.A.; Gimmig, S.; Bessette, B.; Boulassel, M.-R.; Delwart, E.; Sepulveda, H.; Balderas, R.S.; et al. Upregulation of PD-1 Expression on HIV-Specific CD8+ T Cells Leads to Reversible Immune Dysfunction. Nat. Med. 2006, 12, 1198–1202. [Google Scholar] [CrossRef]
- Fisicaro, P.; Valdatta, C.; Massari, M.; Loggi, E.; Biasini, E.; Sacchelli, L.; Cavallo, M.C.; Silini, E.M.; Andreone, P.; Missale, G.; et al. Antiviral Intrahepatic T-Cell Responses Can Be Restored by Blocking Programmed Death-1 Pathway in Chronic Hepatitis B. Gastroenterology 2010, 138, 682–693e4. [Google Scholar] [CrossRef]
- Sumida, K.; Shimoda, S.; Iwasaka, S.; Hisamoto, S.; Kawanaka, H.; Akahoshi, T.; Ikegami, T.; Shirabe, K.; Shimono, N.; Maehara, Y.; et al. Characteristics of Splenic CD8+ T Cell Exhaustion in Patients with Hepatitis C. Clin. Exp. Immunol. 2013, 174, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Owusu Sekyere, S.; Suneetha, P.V.; Kraft, A.R.M.; Zhang, S.; Dietz, J.; Sarrazin, C.; Manns, M.P.; Schlaphoff, V.; Cornberg, M.; Wedemeyer, H. A Heterogeneous Hierarchy of Co-Regulatory Receptors Regulates Exhaustion of HCV-Specific CD8 T Cells in Patients with Chronic Hepatitis C. J. Hepatol. 2015, 62, 31–40. [Google Scholar] [CrossRef]
- Woodland, D.L.; Kohlmeier, J.E. Migration, Maintenance and Recall of Memory T Cells in Peripheral Tissues. Nat. Rev. Immunol. 2009, 9, 153–161. [Google Scholar] [CrossRef] [PubMed]
- White, G.E.; Iqbal, A.J.; Greaves, D.R. CC Chemokine Receptors and Chronic Inflammation—Therapeutic Opportunities and Pharmacological Challenges. Pharmacol. Rev. 2013, 65, 47–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamano, Y.; Araya, N.; Sato, T.; Utsunomiya, A.; Azakami, K.; Hasegawa, D.; Izumi, T.; Fujita, H.; Aratani, S.; Yagishita, N.; et al. Abnormally High Levels of Virus-Infected IFN-Gamma+ CCR4+ CD4+ CD25+ T Cells in a Retrovirus-Associated Neuroinflammatory Disorder. PLoS ONE 2009, 4, e6517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshie, O.; Fujisawa, R.; Nakayama, T.; Harasawa, H.; Tago, H.; Izawa, D.; Hieshima, K.; Tatsumi, Y.; Matsushima, K.; Hasegawa, H.; et al. Frequent Expression of CCR4 in Adult T-Cell Leukemia and Human T-Cell Leukemia Virus Type 1-Transformed T Cells. Blood 2002, 99, 1505–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugata, K.; Yasunaga, J.-I.; Kinosada, H.; Mitobe, Y.; Furuta, R.; Mahgoub, M.; Onishi, C.; Nakashima, K.; Ohshima, K.; Matsuoka, M. HTLV-1 Viral Factor HBZ Induces CCR4 to Promote T-Cell Migration and Proliferation. Cancer Res. 2016, 76, 5068–5079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hieshima, K.; Nagakubo, D.; Nakayama, T.; Shirakawa, A.-K.; Jin, Z.; Yoshie, O. Tax-Inducible Production of CC Chemokine Ligand 22 by Human T Cell Leukemia Virus Type 1 (HTLV-1)-Infected T Cells Promotes Preferential Transmission of HTLV-1 to CCR4-Expressing CD4+ T Cells. J. Immunol. 2008, 180, 931–939. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espíndola, O.d.M.; Siteur-van Rijnstra, E.; Frankin, E.; Weijer, K.; van der Velden, Y.U.; Berkhout, B.; Blom, B.; Villaudy, J. Early Effects of HTLV-1 Infection on the Activation, Exhaustion, and Differentiation of T-Cells in Humanized NSG Mice. Cells 2021, 10, 2514. https://doi.org/10.3390/cells10102514
Espíndola OdM, Siteur-van Rijnstra E, Frankin E, Weijer K, van der Velden YU, Berkhout B, Blom B, Villaudy J. Early Effects of HTLV-1 Infection on the Activation, Exhaustion, and Differentiation of T-Cells in Humanized NSG Mice. Cells. 2021; 10(10):2514. https://doi.org/10.3390/cells10102514
Chicago/Turabian StyleEspíndola, Otávio de Melo, Esther Siteur-van Rijnstra, Esmay Frankin, Kees Weijer, Yme Ubeles van der Velden, Ben Berkhout, Bianca Blom, and Julien Villaudy. 2021. "Early Effects of HTLV-1 Infection on the Activation, Exhaustion, and Differentiation of T-Cells in Humanized NSG Mice" Cells 10, no. 10: 2514. https://doi.org/10.3390/cells10102514
APA StyleEspíndola, O. d. M., Siteur-van Rijnstra, E., Frankin, E., Weijer, K., van der Velden, Y. U., Berkhout, B., Blom, B., & Villaudy, J. (2021). Early Effects of HTLV-1 Infection on the Activation, Exhaustion, and Differentiation of T-Cells in Humanized NSG Mice. Cells, 10(10), 2514. https://doi.org/10.3390/cells10102514






