Dabigatran Etexilate Induces Cytotoxicity in Rat Gastric Epithelial Cell Line via Mitochondrial Reactive Oxygen Species Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. Intracellular mitROS Measurement
2.4. Evaluation of Lipid Peroxidation
2.5. Measurement of Cell Membrane Viscosity using Atomic Force Microscopy
2.6. Measurement of The Mitochondrial Membrane Potential using JC-1
2.7. Statistical Analysis
3. Results
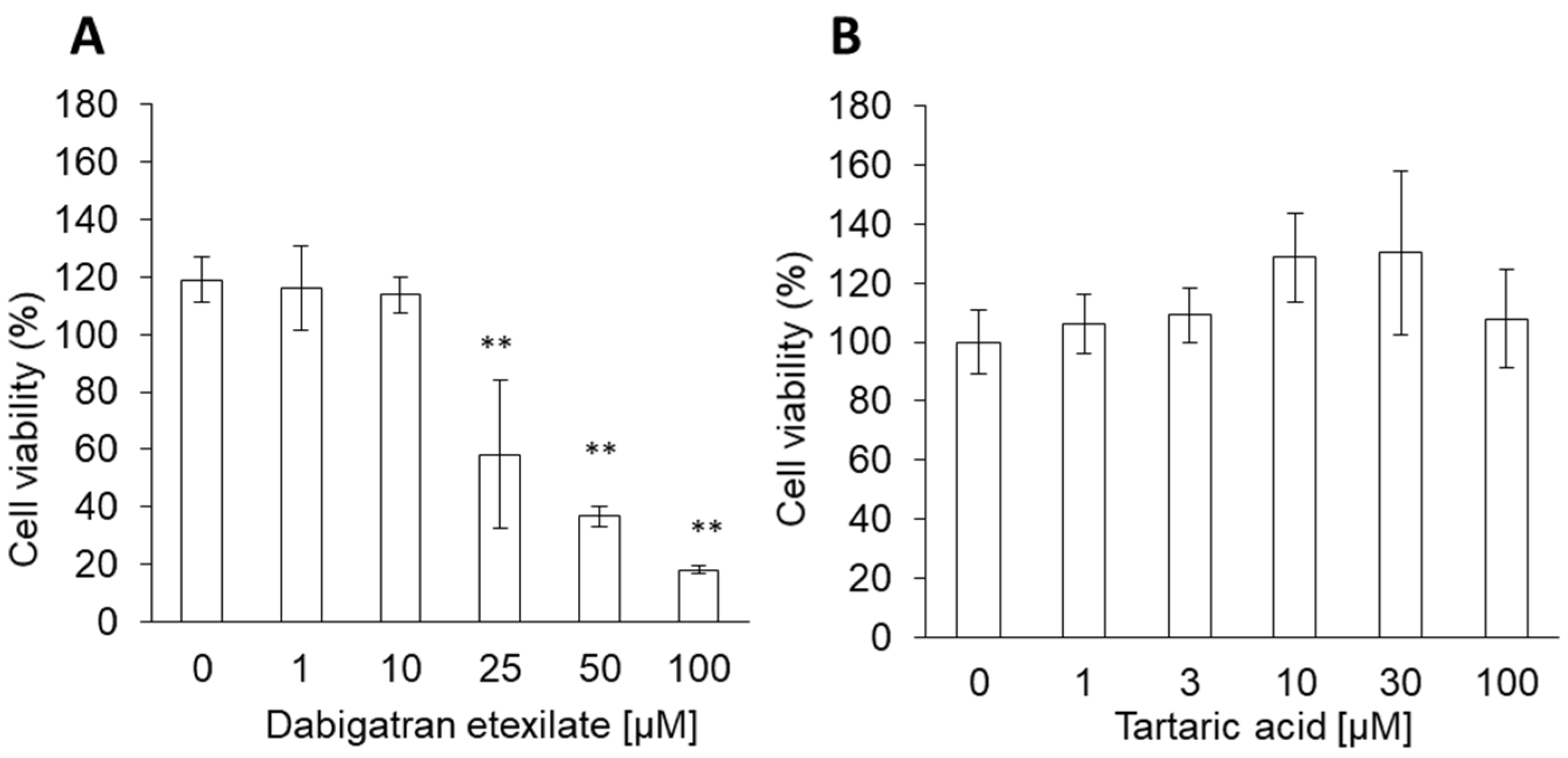
3.1. Dabigatran Etexilate induces Cytotoxicity in Normal Gastric Cells
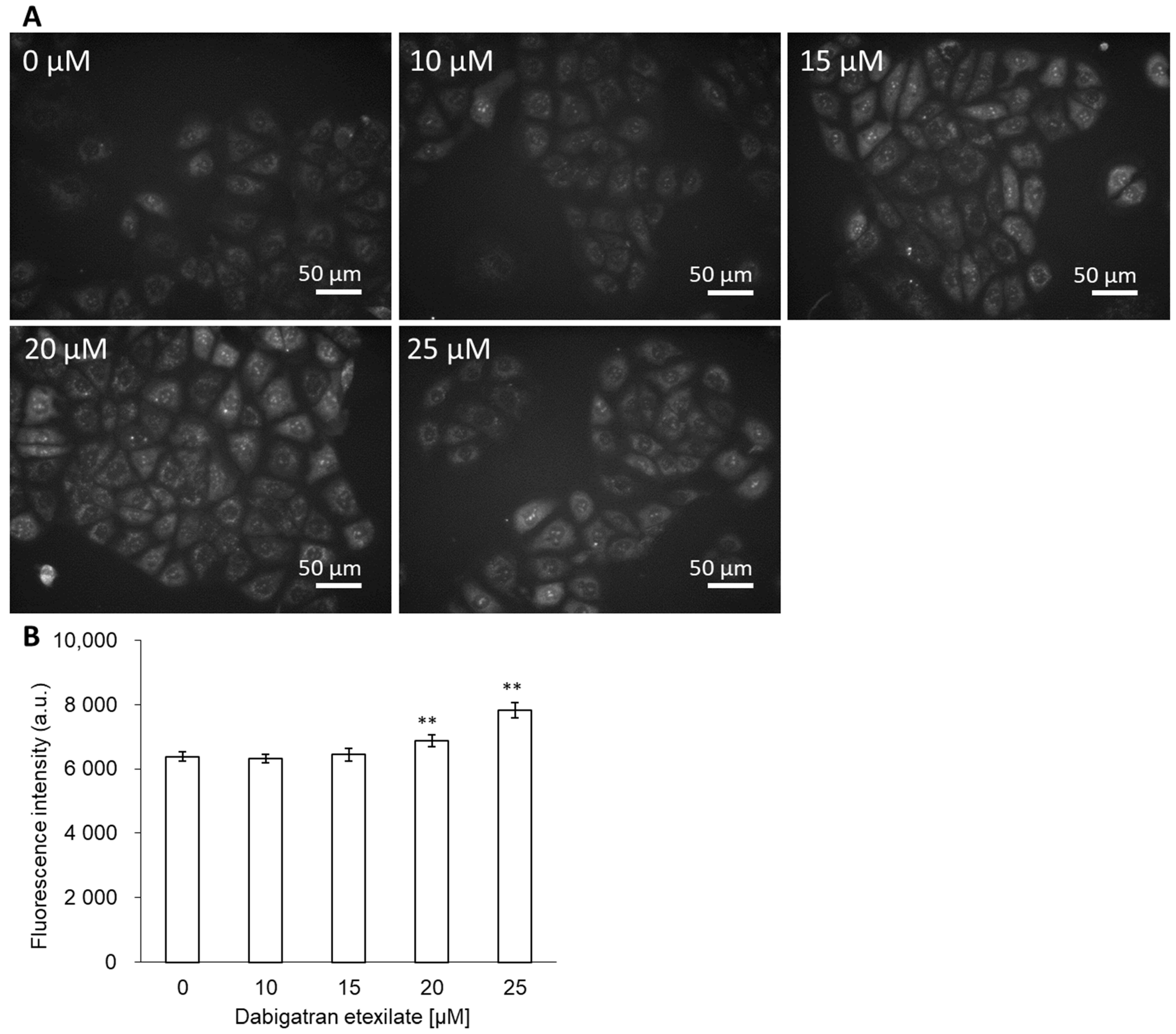
3.2. Dabigatran Increases mitROS Production
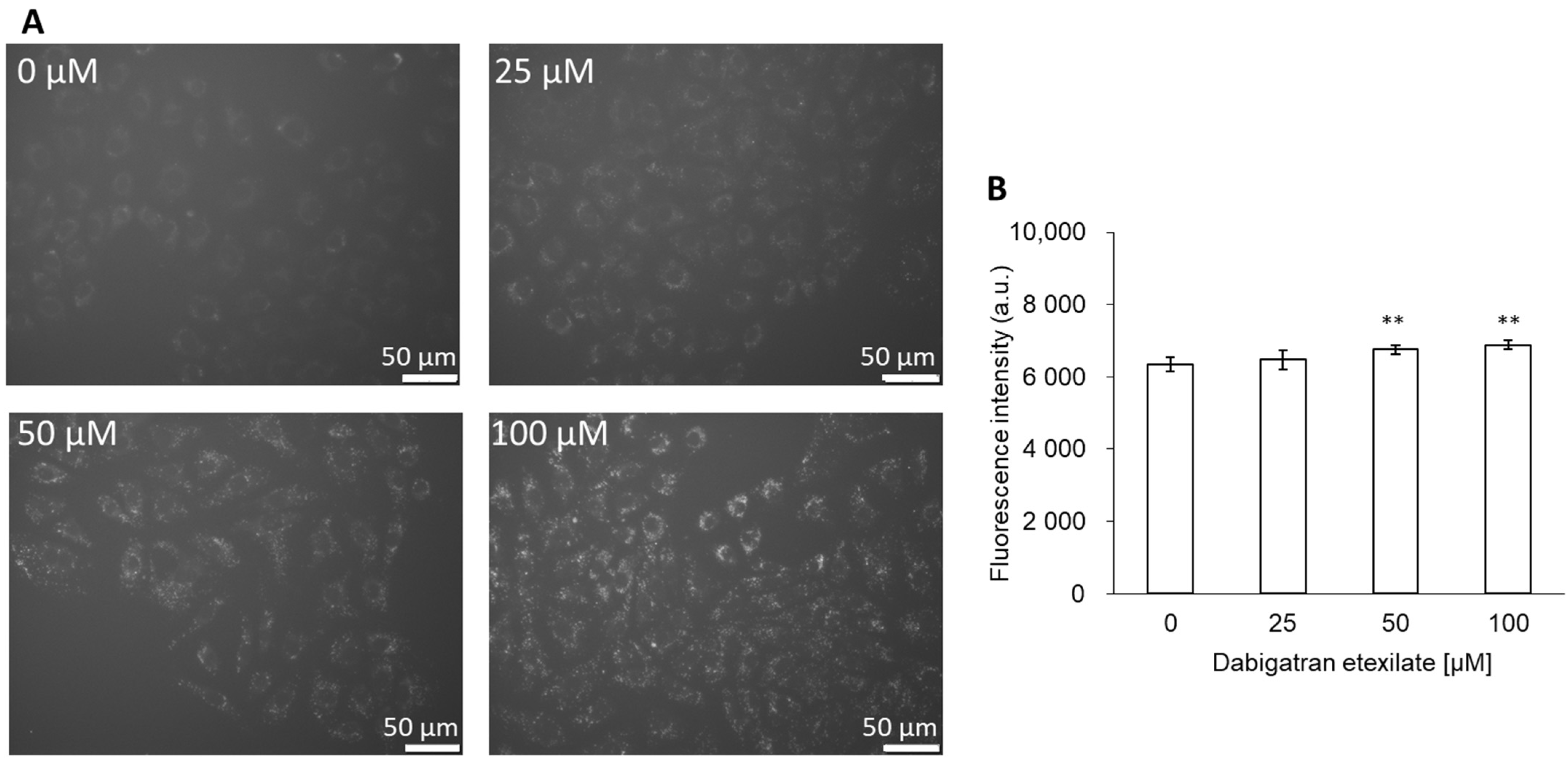
3.3. Dabigatran Etexilate induces Lipid Peroxidation
3.4. Dabigatran Etexilate Alters Cell Membrane Viscosity
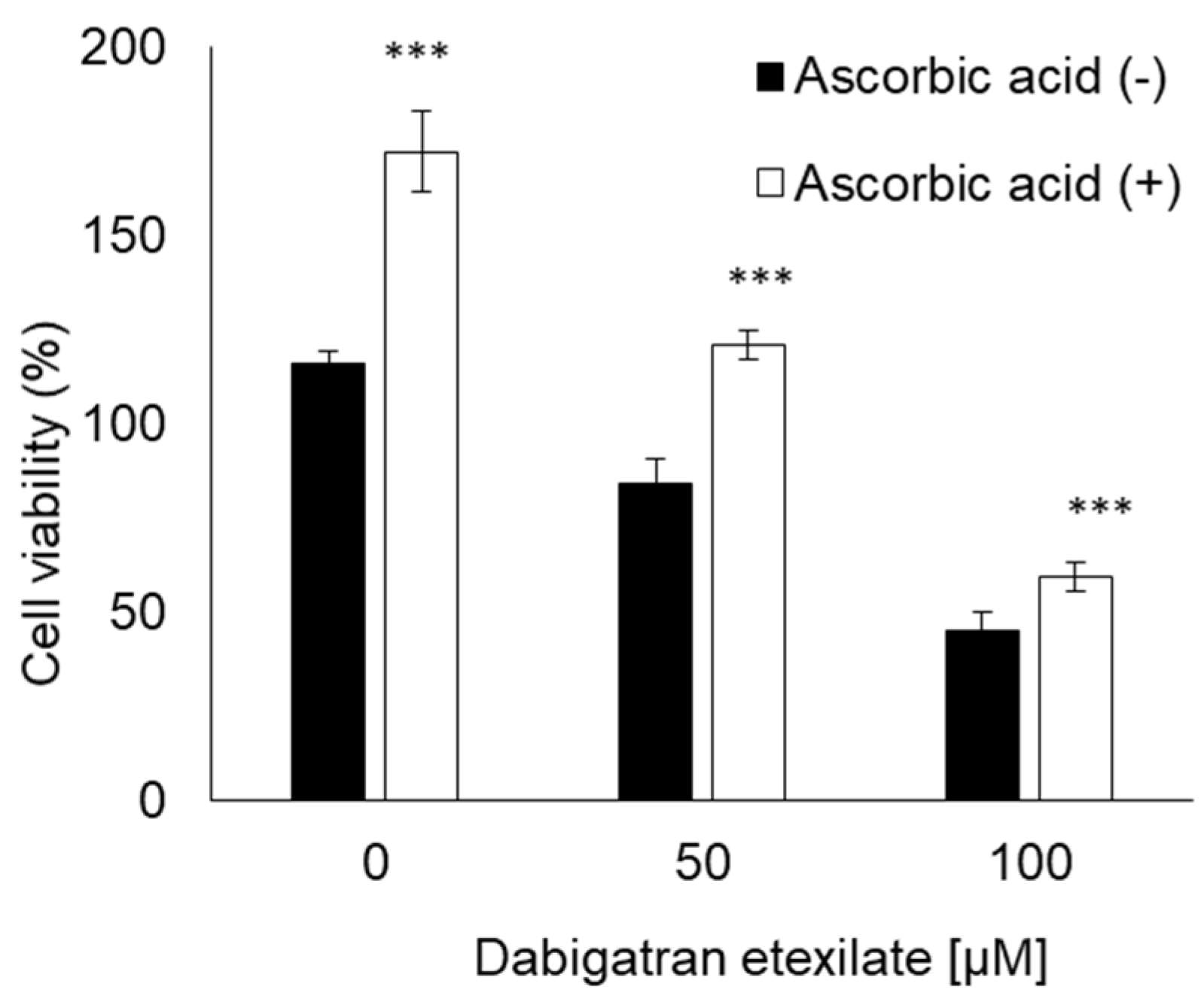
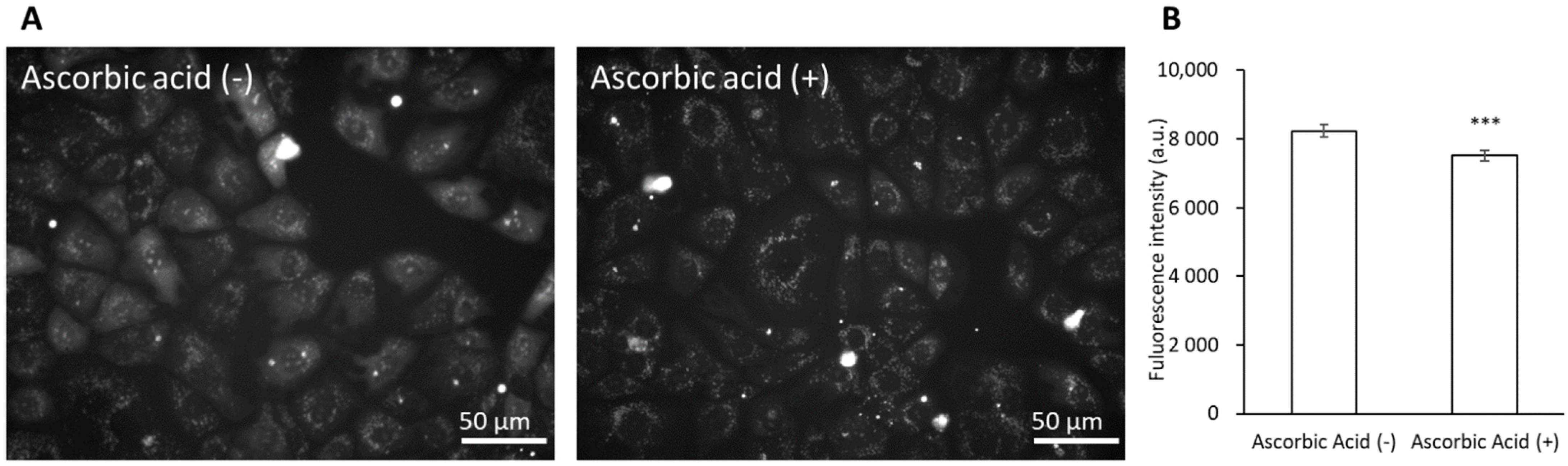
3.5. Ascorbic Acid Suppresses the Cytotoxicity of Dabigatran Etexilate via the Inhibition of mitROS Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.B.; Liu, J.; Fu, G.H.; Fang, R.Y.; Gao, F.; Chu, H.M. Comparison of dabigatran and warfarin used in patients with non-valvular atrial fibrillation: Meta-analysis of random control trial. Medicine 2018, 97, e12841. [Google Scholar] [CrossRef]
- Ageno, W.; Gallus, A.S.; Wittkowsky, A.; Crowther, M.; Hylek, E.M.; Palareti, G. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e44S–e88S. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Y.; Zhang, L.; Guan, W. Dabigatran must be used carefully: Literature review and recommendations for management of adverse events. Drug. Des. Dev. Ther. 2019, 13, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Blommel, M.L.; Blommel, A.L. Dabigatran etexilate: A novel oral direct thrombin inhibitor. AJHP Off. J. Am. Soc. Health-Syst. Pharm. 2011, 68, 1506–1519. [Google Scholar] [CrossRef]
- Gomez-Outes, A.; Terleira-Fernandez, A.I.; Calvo-Rojas, G.; Suarez-Gea, M.L.; Vargas-Castrillon, E. Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups. Thrombosis 2013, 2013, 640723. [Google Scholar] [CrossRef]
- Van Ryn, J.; Hauel, N.; Wienen, W.; Clemens, A. Dabigatran Etexilate: Pharmacology of the New, Oral Direct Thrombin Inhibitor. J. Jpn. Soc. Thromb. Hemost. 2011, 22, 143–150. [Google Scholar] [CrossRef][Green Version]
- Ellis, C.R.; Kaiser, D.W. The clinical efficacy of dabigatran etexilate for preventing stroke in atrial fibrillation patients. Vasc. Health. Risk. Manag. 2013, 9, 341–352. [Google Scholar] [CrossRef][Green Version]
- Charlton, B.; Redberg, R. The trouble with dabigatran. BMJ 2014, 349, g4681. [Google Scholar] [CrossRef] [PubMed]
- Bloom, B.J.; Filion, K.B.; Atallah, R.; Eisenberg, M.J. Meta-analysis of randomized controlled trials on the risk of bleeding with dabigatran. Am. J. Cardiol. 2014, 113, 1066–1074. [Google Scholar] [CrossRef]
- Ansell, J. Warfarin versus new agents: Interpreting the data. Hematol. Am. Soc. Hematol. Educ. Progr. 2010, 2010, 221–228. [Google Scholar] [CrossRef]
- Bytzer, P.; Connolly, S.J.; Yang, S.; Ezekowitz, M.; Formella, S.; Reilly, P.A.; Aisenberg, J. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2013, 11, 246–252. [Google Scholar] [CrossRef]
- Nagano, Y.; Matsui, H.; Tamura, M.; Shimokawa, O.; Nakamura, Y.; Kaneko, T.; Hyodo, I. NSAIDs and acidic environment induce gastric mucosal cellular mitochondrial dysfunction. Digestion 2012, 85, 131–135. [Google Scholar] [CrossRef]
- Nagano, Y.; Matsui, H.; Shimokawa, O.; Hirayama, A.; Nakamura, Y.; Tamura, M.; Rai, K.; Kaneko, T.; Hyodo, I. Bisphosphonate-induced gastrointestinal mucosal injury is mediated by mitochondrial superoxide production and lipid peroxidation. J. Clin. Biochem. Nutr. 2012, 51, 196–203. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yasuda, G.; Ito, H.; Kurokawa, H.; Terasaki, M.; Suzuki, H.; Mizokami, Y.; Matsui, H. The preventive effect of Qing Dai on bisphosphonate-induced gastric cellular injuries. J. Clin. Biochem. Nutr. 2019, 64, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Tamura, M.; Matsui, H.; Nagano, Y.; Suzuki, H.; Kaneko, T.; Mizokami, Y.; Hyodo, I. Qing Dai attenuates nonsteroidal anti-inflammatory drug-induced mitochondrial reactive oxygen species in gastrointestinal epithelial cells. J. Clin. Biochem. Nutr. 2015, 56, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Fleury, C.; Mignotte, B.; Vayssière, J.L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ahn, Y.; Hong, M.H.; Joo, S.Y.; Kim, K.H.; Sohn, I.S.; Park, H.W.; Hong, Y.J.; Kim, J.H.; Kim, W.; et al. Curcumin attenuates inflammatory responses of TNF-alpha-stimulated human endothelial cells. J. Cardiovasc. Pharmacol. 2007, 50, 41–49. [Google Scholar] [CrossRef]
- Proksch, R.; Kocun, M.; Hurley, D.; Viani, M.; Labuda, A.; Meinhold, W.; Bemis, J. Practical loss tangent imaging with amplitude-modulated atomic force microscopy. J. Appl. Phys. 2016, 119, 134901. [Google Scholar] [CrossRef]
- Proksch, R.; Yablon, D.G. Loss tangent imaging: Theory and simulations of repulsive-mode tapping atomic force microscopy. Appl. Phys. Lett. 2012, 100, 073106. [Google Scholar] [CrossRef]
- Kumar, B.; Koul, S.; Khandrika, L.; Meacham, R.B.; Koul, H.K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008, 68, 1777–1785. [Google Scholar] [CrossRef]
- Van Ryn, J.; Stangier, J.; Haertter, S.; Liesenfeld, K.H.; Wienen, W.; Feuring, M.; Clemens, A. Dabigatran etexilate--a novel, reversible, oral direct thrombin inhibitor: Interpretation of coagulation assays and reversal of anticoagulant activity. Thromb. Haemost. 2010, 103, 1116–1127. [Google Scholar] [CrossRef]
- Schiele, F.; van Ryn, J.; Canada, K.; Newsome, C.; Sepulveda, E.; Park, J.; Nar, H.; Litzenburger, T. A specific antidote for dabigatran: Functional and structural characterization. Blood 2013, 121, 3554–3562. [Google Scholar] [CrossRef]
- Yeh, C.H.; Gross, P.L.; Weitz, J.I. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood 2014, 124, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Reichman, M.E.; Wernecke, M.; Zhang, R.; Southworth, M.R.; Levenson, M.; Sheu, T.C.; Mott, K.; Goulding, M.R.; Houstoun, M.; et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015, 131, 157–164. [Google Scholar] [CrossRef]
- Sarah, S. The pharmacology and therapeutic use of dabigatran etexilate. J. Clin. Pharmacol. 2013, 53, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Comuzzo, P.; Battistutta, F. Chapter 2—Acidification and pH Control in Red Wines. In Red Wine Technology; Morata, A., Ed.; Academic Press: London, UK, 2019; pp. 17–34. [Google Scholar]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric mucosal defense and cytoprotection: Bench to bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef]
- Somasundaram, S.; Rafi, S.; Hayllar, J.; Sigthorsson, G.; Jacob, M.; Price, A.B.; Macpherson, A.; Mahmod, T.; Scott, D.; Wrigglesworth, J.M.; et al. Mitochondrial damage: A possible mechanism of the “topical” phase of NSAID induced injury to the rat intestine. Gut 1997, 41, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Nagano, Y.; Matsui, H.; Muramatsu, M.; Shimokawa, O.; Shibahara, T.; Yanaka, A.; Nakahara, A.; Matsuzaki, Y.; Tanaka, N.; Nakamura, Y. Rebamipide significantly inhibits indomethacin-induced mitochondrial damage, lipid peroxidation, and apoptosis in gastric epithelial RGM-1 cells. Dig. Dis. Sci. 2005, 50 (Suppl. 1), S76–S83. [Google Scholar] [CrossRef]
- Jancic, S.A.; Stosic, B.Z. Chapter Fourteen—Cadmium Effects on the Thyroid Gland. In Vitamins & Hormones; Litwack, G., Ed.; Academic Press: London, UK, 2014; Volume 94, pp. 391–425. [Google Scholar]
- Fonseca, F.; Pénicaud, C.; Tymczyszyn, E.E.; Gómez-Zavaglia, A.; Passot, S. Factors influencing the membrane fluidity and the impact on production of lactic acid bacteria starters. Appl. Microbiol. Biotechnol. 2019, 103, 6867–6883. [Google Scholar] [CrossRef]
- Kaneko, T.; Matsui, H.; Shimokawa, O.; Nakahara, A.; Hyodo, I. Cellular membrane fluidity measurement by fluorescence polarization in indomethacin-induced gastric cellular injury in vitro. J. Gastroenterol. 2007, 42, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. The Fluid-Mosaic Model of Membrane Structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta 2014, 1838, 1451–1466. [Google Scholar] [CrossRef]
- Cartagena, A.; Raman, A. Local viscoelastic properties of live cells investigated using dynamic and quasi-static atomic force microscopy methods. Biophys. J. 2014, 106, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Raman, A.; Trigueros, S.; Cartagena, A.; Stevenson, A.P.; Susilo, M.; Nauman, E.; Contera, S.A. Mapping nanomechanical properties of live cells using multi-harmonic atomic force microscopy. Nat. Nanotechnol. 2011, 6, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Al-Rekabi, Z.; Contera, S. Multifrequency AFM reveals lipid membrane mechanical properties and the effect of cholesterol in modulating viscoelasticity. Proc. Natl. Acad. Sci. USA 2018, 115, 2658–2663. [Google Scholar] [CrossRef]
- Cartagena-Rivera, A.X.; Wang, W.H.; Geahlen, R.L.; Raman, A. Fast, multi-frequency, and quantitative nanomechanical mapping of live cells using the atomic force microscope. Sci. Rep. 2015, 5, 11692. [Google Scholar] [CrossRef]
- Schiele, N.R.; von Flotow, F.; Tochka, Z.L.; Hockaday, L.A.; Marturano, J.E.; Thibodeau, J.J.; Kuo, C.K. Actin cytoskeleton contributes to the elastic modulus of embryonic tendon during early development. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2015, 33, 874–881. [Google Scholar] [CrossRef]
- Sevanian, A.; Muakkassah-Kelly, S.F.; Montestruque, S. The influence of phospholipase A2 and glutathione peroxidase on the elimination of membrane lipid peroxides. Arch. Biochem. Biophys. 1983, 223, 441–452. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio-Protocol 2019, 9. [Google Scholar] [CrossRef]
- Han, B.J.; Li, W.; Jiang, G.B.; Lai, S.H.; Zhang, C.; Zeng, C.C.; Liu, Y.J. Effects of daidzein in regards to cytotoxicity in vitro, apoptosis, reactive oxygen species level, cell cycle arrest and the expression of caspase and Bcl-2 family proteins. Oncol. Rep. 2015, 34, 1115–1120. [Google Scholar] [CrossRef]
- Liu, J.X.; Zhang, J.H.; Li, H.H.; Lai, F.J.; Chen, K.J.; Chen, H.; Luo, J.; Guo, H.C.; Wang, Z.H.; Lin, S.Z. Emodin induces Panc-1 cell apoptosis via declining the mitochondrial membrane potential. Oncol. Rep. 2012, 28, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Cao, K.; Xu, C.; Sun, A.; Lu, W.; Zheng, Y.; Li, H.; Hong, G.; Wu, B.; Qiu, Q.; et al. Crosstalk between Mitochondrial Fission and Oxidative Stress in Paraquat-Induced Apoptosis in Mouse Alveolar Type II Cells. Int. J. Biol. Sci. 2017, 13, 888–900. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurokawa, H.; Taninaka, A.; Shigekawa, H.; Matsui, H. Dabigatran Etexilate Induces Cytotoxicity in Rat Gastric Epithelial Cell Line via Mitochondrial Reactive Oxygen Species Production. Cells 2021, 10, 2508. https://doi.org/10.3390/cells10102508
Kurokawa H, Taninaka A, Shigekawa H, Matsui H. Dabigatran Etexilate Induces Cytotoxicity in Rat Gastric Epithelial Cell Line via Mitochondrial Reactive Oxygen Species Production. Cells. 2021; 10(10):2508. https://doi.org/10.3390/cells10102508
Chicago/Turabian StyleKurokawa, Hiromi, Atsushi Taninaka, Hidemi Shigekawa, and Hirofumi Matsui. 2021. "Dabigatran Etexilate Induces Cytotoxicity in Rat Gastric Epithelial Cell Line via Mitochondrial Reactive Oxygen Species Production" Cells 10, no. 10: 2508. https://doi.org/10.3390/cells10102508
APA StyleKurokawa, H., Taninaka, A., Shigekawa, H., & Matsui, H. (2021). Dabigatran Etexilate Induces Cytotoxicity in Rat Gastric Epithelial Cell Line via Mitochondrial Reactive Oxygen Species Production. Cells, 10(10), 2508. https://doi.org/10.3390/cells10102508





