Increased Phosphatase of Regenerating Liver-1 by Placental Stem Cells Promotes Hepatic Regeneration in a Bile-Duct-Ligated Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Liver Specimens
2.2. Generation of Animal Models
2.3. Serological Analysis
2.4. Immunohistochemical Staining
2.5. Cultivation of CP-MSCs and Primary Hepatocytes
2.6. In Vitro Co-Culture Experiments
2.7. Proliferation Assay Using BrdU Staining
2.8. Western Blot Analysis
2.9. Enzyme-Linked Immunosorbant Assay (ELISA)
2.10. Invasion of CP-MSCs Using a Transwell Insert System
2.11. ICG Clearance Test
2.12. Gelatin Zymography
2.13. Statistical Analyses
3. Results
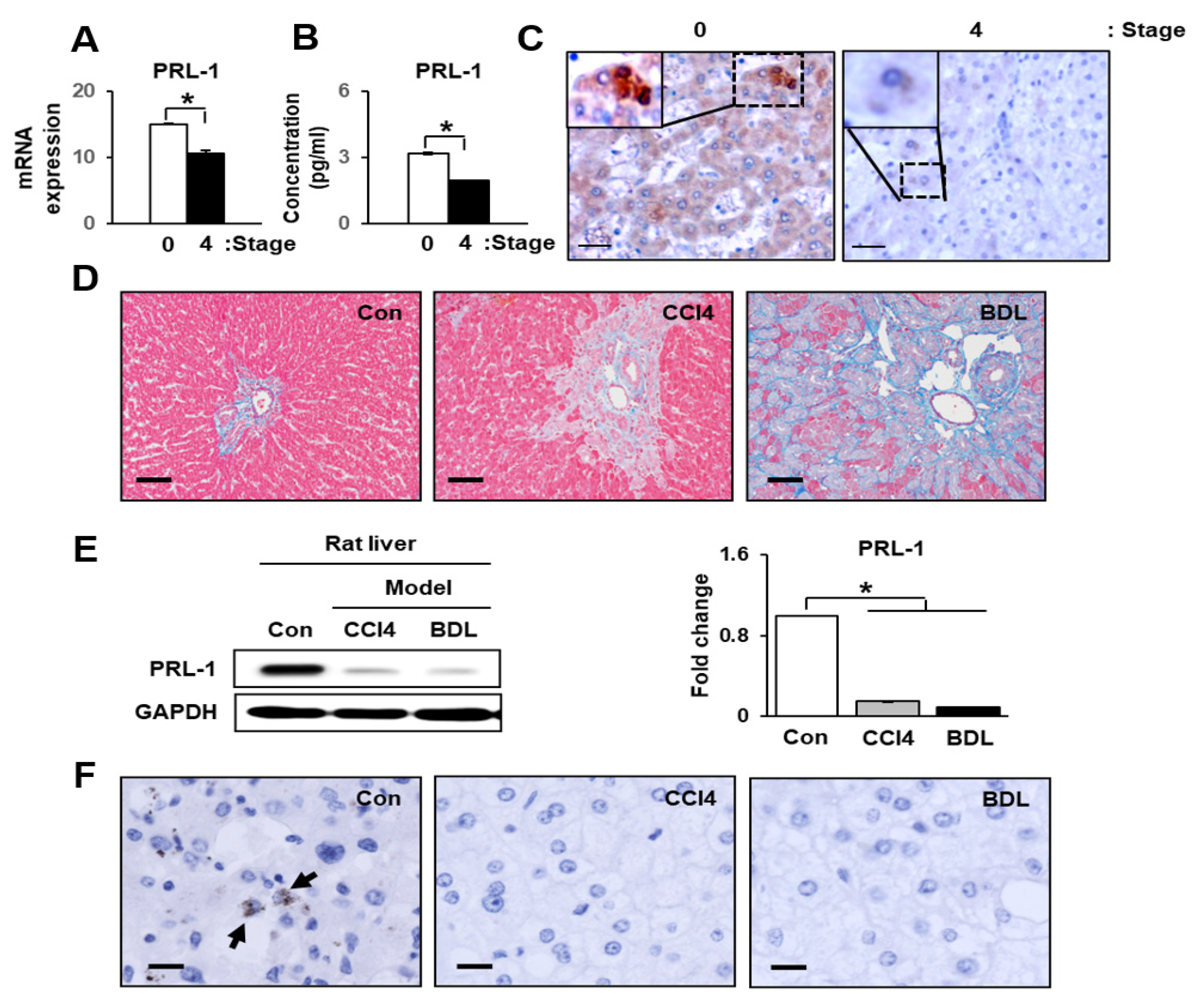
3.1. Hepatic Function and PRL-1 Expression in Damaged Human Livers
3.2. PRL-1 Expression Was Decreased in Damaged Rat Livers
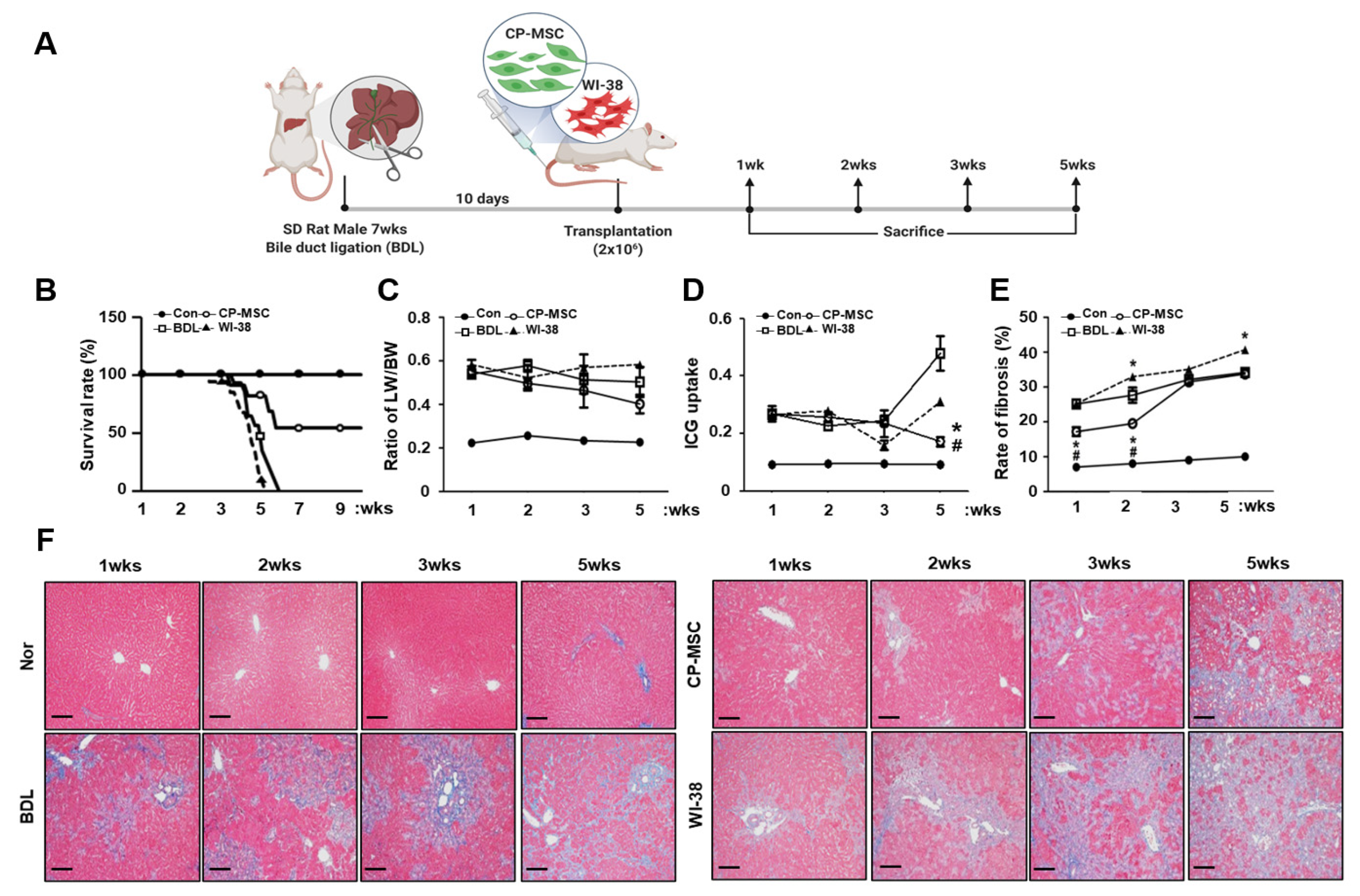
3.3. CP-MSC Transplantation Increased the Survival Rate in a BDL Rat Model via Enhanced Hepatic Regeneration
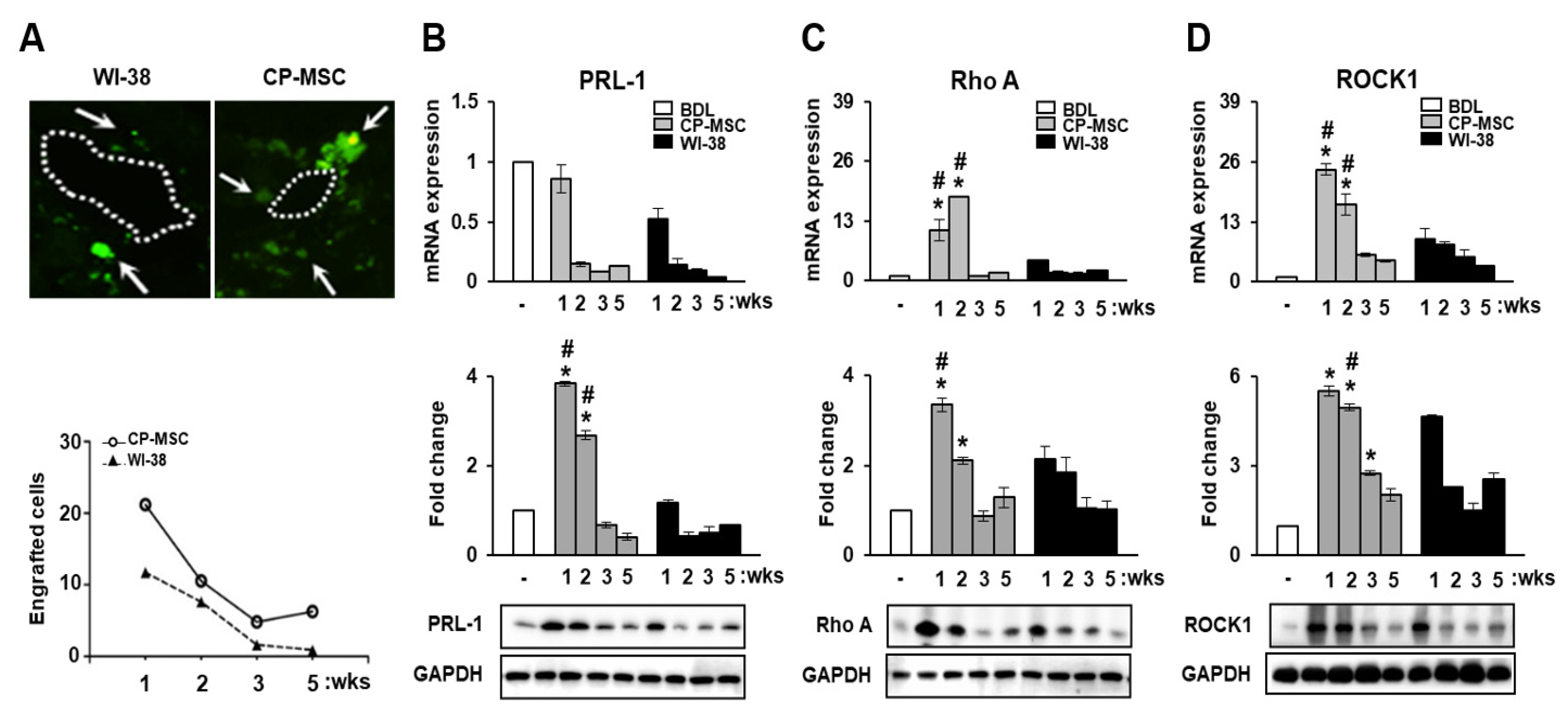
3.4. CP-MSC Transplantation Promoted PRL-1 Expression as Well as That of the Migration-Related Rho Family Proteins
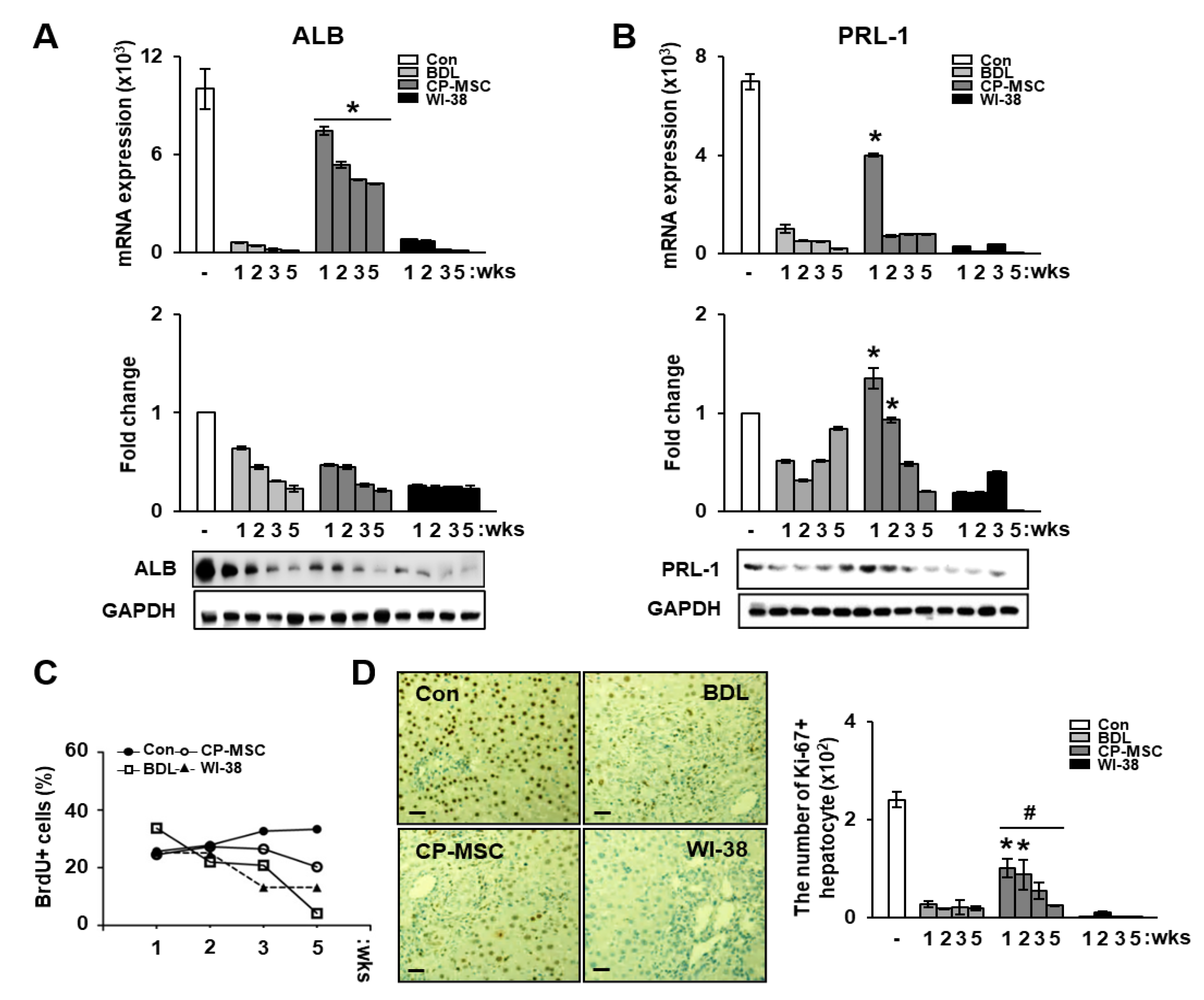
3.5. Increased PRL-1 Expression by CP-MSCs Is Involved in Regeneration and Proliferation in BDL Rat Liver
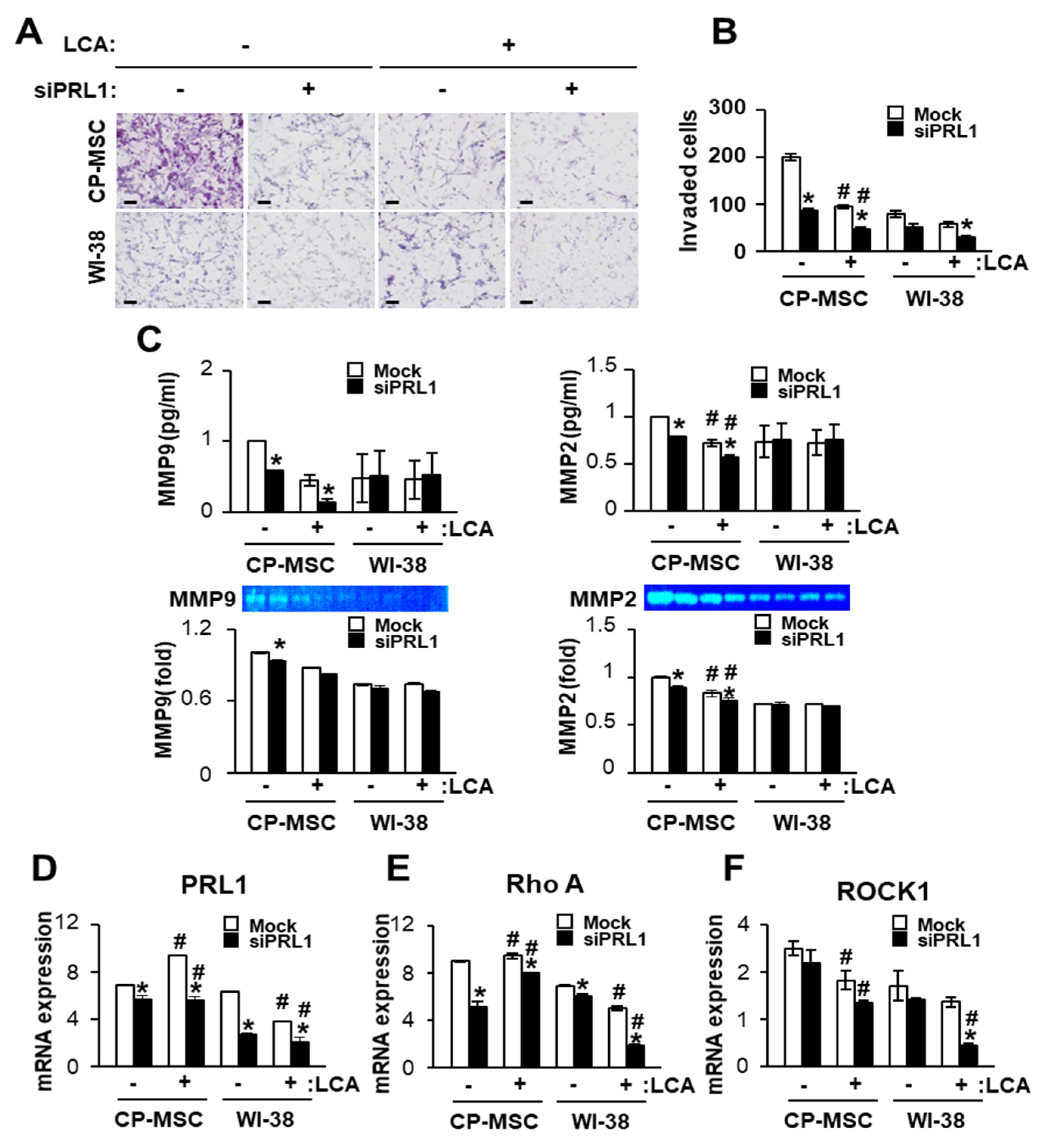
3.6. Down-Regulated PRL-1 Decreased the Migration of CP-MSCs via Migration-Related Rho Family and Matrix Metalloproteinase Protein Expression
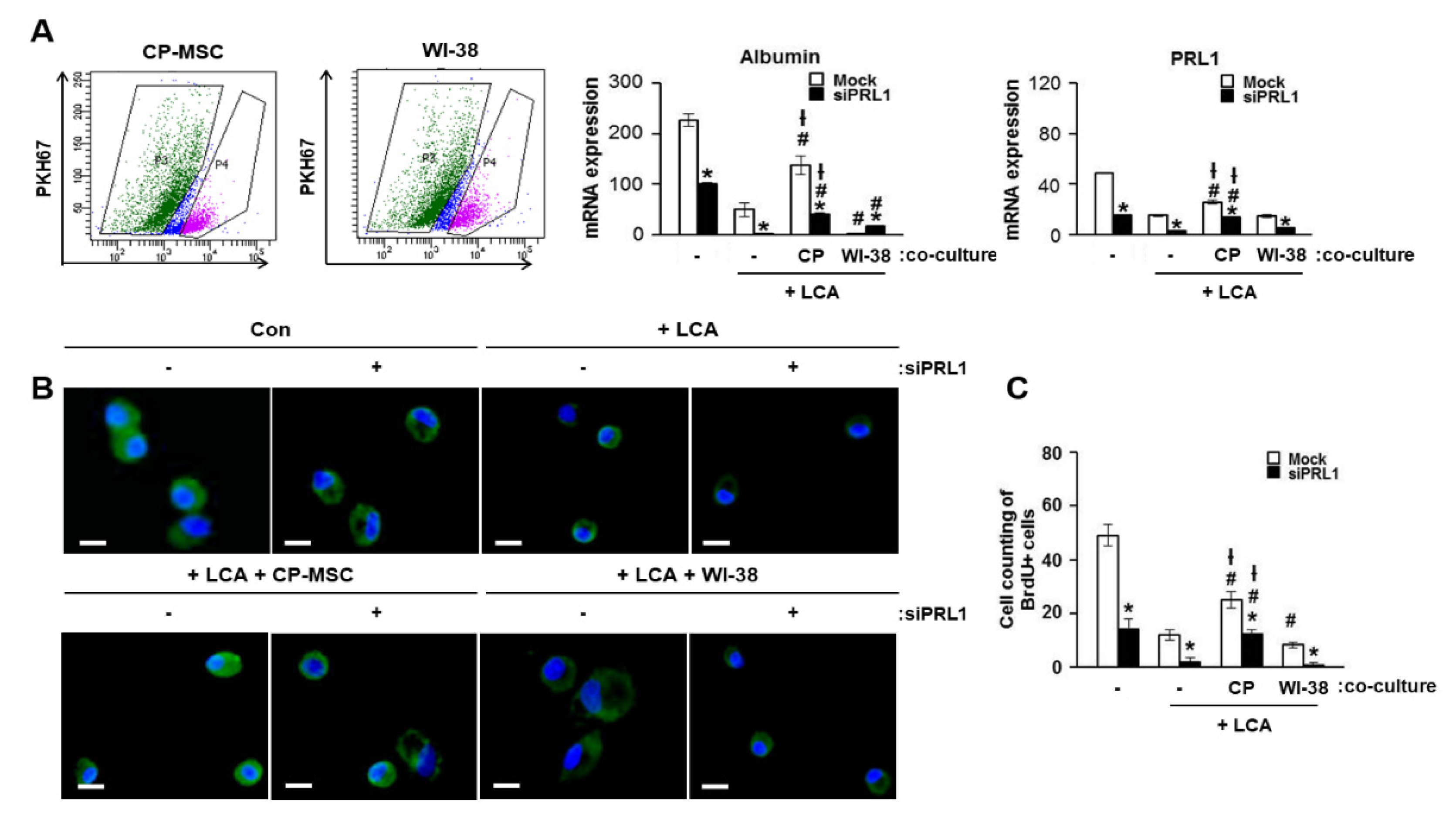
3.7. Increased PRL-1 Expression by CP-MSCs Is Involved in the Regeneration and Proliferation of Damaged Hepatocytes
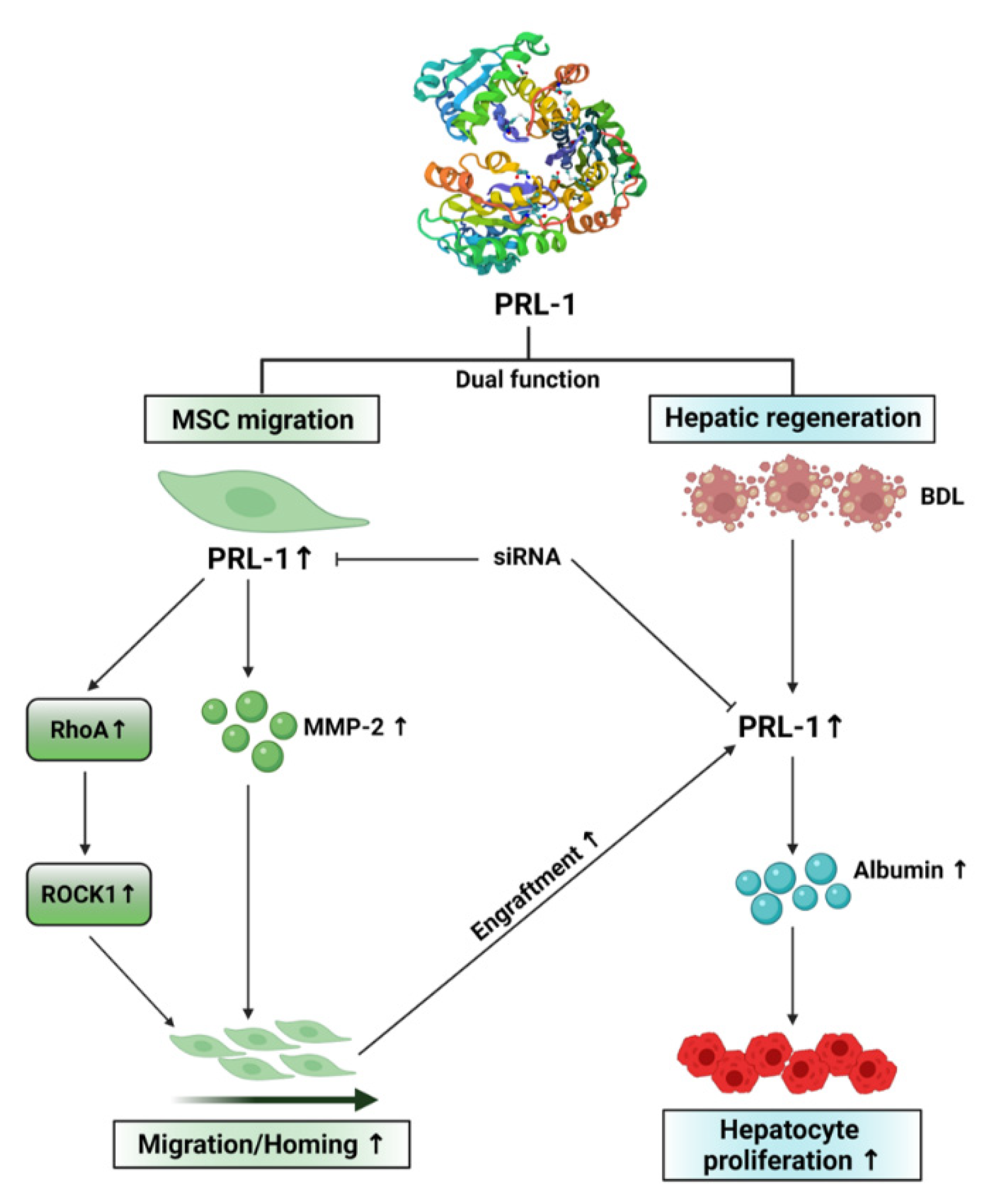
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedman, S.L.; Sheppard, D.; Duffield, J.S.; Violette, S. Therapy for fibrotic diseases: Nearing the starting line. Sci. Transl. Med. 2013, 5, 167sr1. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Gea, V.; Friedman, S.L. Pathogenesis of Liver Fibrosis. Annu. Rev. Pathol. 2011, 6, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Mechanisms of Hepatic Fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoes, P.; Kesar, V.; Ahmad, J. Spectrum of biliary complications following live donor liver transplantation. World J. Hepatol. 2015, 7, 1856–1865. [Google Scholar] [CrossRef]
- Chen, L.; He, W.; Huang, K.; Zheng, L.; Gao, L.; Liu, J.; Huang, A. Increased expression of Notch 1 in a rat liver transplantation model. Mol. Med. Rep. 2015, 12, 5293–5297. [Google Scholar] [CrossRef] [PubMed]
- Le Guen, V.; Judor, J.-P.; Boeffard, F.; Gauttier, V.; Ferry, N.; Soulillou, J.-P.; Brouard, S.; Conchon, S. Alloantigen gene transfer to hepatocytes promotes tolerance to pancreatic islet graft by inducing CD8 + regulatory T cells. J. Hepatol. 2017, 66, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.K.; Hung, S.; Chuang, C.; Chen, C.; Shih, Y.V.; Fang, S.Y.; Yang, V.W.; Lee, O.K. Stem cell therapy for liver disease: Parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology 2008, 134, 2111–2121. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Meng, Y.; Han, Z.; Ye, F.; Wei, L.; Zong, C. Mesenchymal stem cell therapy for liver disease: Full of chances and challenges. Cell Biosci. 2020, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, G.; Parungao, J.M.; Hanouneh, I.A.; Parsi, M.A. Biliary complications following liver transplantation. World J. Gastroenterol. 2013, 19, 2841–2846. [Google Scholar] [CrossRef]
- Zeng, Q.; Hong, W.; Tan, Y. Mouse PRL-2 and PRL-3, two potentially prenylated protein tyrosine phosphatases homologous to PRL-1. Biochem. Biophys. Res. Commun. 1998, 244, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Mohn, K.L.; Laz, T.M.; Hsu, J.C.; Melby, A.E.; Bravo, R.; Taub, R. The immediate-early growth response in regenerating liver and insulin-stimulated H-35 cells: Comparison with serum-stimulated 3T3 cells and identification of 41 novel immediate-early genes. Mol. Cell. Biol. 1991, 11, 381–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumaual, C.M.; Sandusky, G.E.; Soo, H.W.; Werner, S.R.; Crowell, P.L.; Randall, S. Tissue-specific alterations of PRL-1 and PRL-2 expression in cancer. Am. J. Transl. Res. 2012, 4, 83–101. [Google Scholar]
- Liu, L.-Z.; He, Y.-Z.; Dong, P.-P.; Ma, L.-J.; Wang, Z.-C.; Liu, X.-Y.; Duan, M.; Yang, L.-X.; Shi, J.-Y.; Zhou, J.; et al. Protein tyrosine phosphatase PTP4A1 promotes proliferation and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma via the PI3K/AKT pathway. Oncotarget 2016, 7, 75210–75220. [Google Scholar] [CrossRef] [Green Version]
- Shinmei, S.; Sentani, K.; Hayashi, T.; Sakamoto, N.; Goto, K.; Oo, H.Z.; Naito, Y.; Teishima, J.; Matsubara, A.; Oue, N.; et al. Identification of PRL1 as a novel diagnostic and therapeutic target for castration-resistant prostate cancer by the Escherichia coli ampicillin secretion trap (CAST) method. Urol. Oncol. 2014, 32, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Dong, J.-M.; Guo, K.; Li, J.; Tan, H.-X.; Koh, V.; Pallen, C.J.; Manser, E.; Hong, W. PRL-3 and PRL-1 promote cell migration, invasion, and metastasis. Cancer Res. 2003, 63, 2716–2722. [Google Scholar] [PubMed]
- Rios, P.; Li, X.; Köhn, M. Molecular mechanisms of the PRL phosphatases. FEBS J. 2012, 280, 505–524. [Google Scholar] [CrossRef]
- Achiwa, H.; Lazo, J.S. PRL-1 Tyrosine Phosphatase regulates c-Src levels, adherence, and invasion in human lung cancer cells. Cancer Res. 2007, 67, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, F.; Zhang, Z.-Y. PRL1 promotes cell migration and invasion by increasing MMP2 and MMP9 expression through Src and ERK1/2 pathways. Biochemistry 2009, 48, 1838–1846. [Google Scholar] [CrossRef] [Green Version]
- Kean, T.J.; Lin, P.; Caplan, A.; Dennis, J.E. MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013, 2013, 732742. [Google Scholar] [CrossRef] [Green Version]
- Ullah, M.; Liu, D.; Thakor, A.S. Mesenchymal stromal cell homing: Mechanisms and strategies for improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.; Whittall, C.; Weksler, B.; Middleton, J. Chemokines stimulate bidirectional migration of human mesenchymal stem cells across bone marrow endothelial cells. Stem Cells Dev. 2012, 21, 476–486. [Google Scholar] [CrossRef]
- Saxena, N.; Mogha, P.; Dash, S.; Majumder, A.; Jadhav, S.; Sen, S. Matrix elasticity regulates mesenchymal stem cell chemotaxis. J. Cell Sci. 2018, 131, 131. [Google Scholar] [CrossRef] [Green Version]
- Murali, A.; Rajalingam, K. Small Rho GTPases in the control of cell shape and mobility. Cell Mol. Life Sci. 2014, 71, 1703–1721. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, W.; Martin, D.; Gutkind, J.S. The Galpha13-Rho signaling axis is required for SDF-1-induced migration through CXCR4. J. Biol. Chem. 2006, 281, 39542–39549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.J.; Jung, J.; Na, K.H.; Moon, J.S.; Lee, H.J.; Kim, J.H.; Kim, G.I.; Kwon, S.W.; Hwang, S.G.; Kim, G.J. Anti-fibrotic effect of chorionic plate-derived mesenchymal stem cells isolated from human placenta in a rat model of CCl4-injured liver: Potential application to the treatment of hepatic diseases. J. Cell. Biochem. 2010, 111, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Choi, J.H.; Lee, E.; Park, J.-W.; Oh, I.-H.; Hwang, S.-G.; Kim, K.-S.; Kim, G.J. Human placenta-derived mesenchymal stem cells promote hepatic regeneration in CCl4-injured rat liver model via increased autophagic mechanism. Stem Cells 2013, 31, 1584–1596. [Google Scholar] [CrossRef]
- Jun, J.H.; Kim, J.Y.; Choi, J.H.; Lim, J.-Y.; Kim, K.; Kim, G.J. Exosomes from placenta-derived mesenchymal stem cells are involved in liver regeneration in hepatic failure induced by bile duct ligation. Stem Cells Int. 2020, 2020, 5485738. [Google Scholar] [CrossRef]
- Askenasy, N.; Farkas, D.L. Optical imaging of PKH‐labeled hematopoietic cells in recipient bone marrow in vivo. Stem Cells 2002, 20, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Ueng, S.W.; Lee, M.S.; Lin, S.; Chan, E.-C.; Liu, S.-J. Development of a biodegradable alginate carrier system for antibiotics and bone cells. J. Orthop. Res. 2006, 25, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Matsuoka, M.; French, S. Experimental models of hepatic fibrosis: A review. Semin. Liver Dis. 1990, 10, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Geier, A.; Dietrich, C.G.; Voigt, S.; Kim, S.; Gerloff, T.; Kullak-Ublick, G.A.; Lorenzen, J.; Matern, S.; Gartung, C. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology 2003, 38, 345–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, L.; Broering, D.C.; Meyer, J.; Vashist, Y.; Goettsche, J.; Wilms, C.; Rogiers, X. The induction of the immediate-early-genes Egr-1, PAI-1 and PRL-1 during liver regeneration in surgical models is related to increased portal flow. J. Hepatol. 2002, 37, 606–612. [Google Scholar] [CrossRef]
- Diamond, R.H.; Peters, C.; Jung, S.P.; Greenbaum, L.E.; Haber, B.A.; Silberg, D.G.; Traber, P.G.; Taub, R. Expression of PRL-1 nuclear PTPase is associated with proliferation in liver but with differentiation in intestine. Am. J. Physiol. 1996, 271, G121–G129. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lim, S.M.; Yoo, Y.I.; Jung, J.; Park, J.-W.; Kim, G.J. Microenvironmental interaction between hypoxia and endothelial cells controls the migration ability of placenta-derived mesenchymal stem cells via α4 integrin and rho signaling. J. Cell. Biochem. 2016, 117, 1145–1157. [Google Scholar] [CrossRef]
- Kollar, K.; Cook, M.M.; Atkinson, K.; Brooke, G. Molecular mechanisms involved in mesenchymal stem cell migration to the site of acute myocardial infarction. Int. J. Cell Biol. 2009, 2009, 904682. [Google Scholar] [CrossRef]
- Russo, F.P.; Alison, M.R.; Bigger, B.; Amofah, E.; Florou, A.; Amin, F.; Bou–Gharios, G.; Jeffery, R.; Iredale, J.P.; Forbes, S. The bone marrow functionally contributes to liver fibrosis. Gastroenterology 2006, 130, 1807–1821. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Chen, X.; Chen, J.; Gautam, S.C.; Xu, Y.; Chopp, M. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology 2002, 7, 113–117. [Google Scholar] [CrossRef]
- Ponte, A.L.; Marais, E.; Gallay, N.; Langonné, A.; Delorme, B.; Herault, O.; Charbord, P.; Domenech, J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [CrossRef]
- Barbash, I.M.; Chouraqui, P.; Baron, J.; Feinberg, M.S.; Etzion, S.; Tessone, A.; Miller, L.; Guetta, E.; Zipori, D.; Kedes, L.H.; et al. Systemic delivery of bone marrow–derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell mi-gration, and body distribution. Circulation 2003, 108, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Shahror, R.A.; Ali, A.; Wu, C.-C.; Chiang, Y.-H.; Chen, K.-Y. Enhanced homing of mesenchymal stem cells overexpressing fibroblast growth factor 21 to injury site in a mouse model of traumatic brain injury. Int. J. Mol. Sci. 2019, 20, 2624. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Zhou, F.; He, D.; Zhang, L.; Shen, J. Overexpression of CXCR7 promotes mesenchymal stem cells to repair phosgene-induced acute lung injury in rats. Biomed. Pharmacother. 2019, 109, 1233–1239. [Google Scholar] [CrossRef]
- Yao, W.; Lay, Y.-A.E.; Kot, A.; Liu, R.; Zhang, H.; Chen, H.; Lam, K.; Lane, N.E. Improved mobilization of exogenous mesenchymal stem cells to bone for fracture healing and sex difference. Stem Cells 2016, 34, 2587–2600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Jun, J.H.; Park, S.Y.; Yang, S.W.; Bae, S.H.; Kim, G.J. Dynamic regulation of miRNA expression by functionally enhanced placental mesenchymal stem cells promotes hepatic regeneration in a rat model with bile duct ligation. Int. J. Mol. Sci. 2019, 20, 5299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Park, S.; Lee, H.-J.; Lew, H.; Kim, G.J. Functionally enhanced placenta-derived mesenchymal stem cells inhibit adipogenesis in orbital fibroblasts with Graves’ ophthalmopathy. Stem Cell Res. Ther. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zeng, H.; Zhang, X.; Zhao, Y.; Sha, H.; Ge, X.; Zhang, M.; Gao, X.; Xu, Q. Phosphatase of regenerating liver-3 promotes motility and metastasis of mouse melanoma cells. Am. J. Pathol. 2004, 164, 2039–2054. [Google Scholar] [CrossRef] [Green Version]
- Parker, B.S.; Argani, P.; Cook, B.P.; Liangfeng, H.; Chartrand, S.D.; Zhang, M.; Saha, S.; Bardelli, A.; Jiang, Y.; St Martin, T.B.; et al. Alterations in vascular gene expression in invasive breast carcinoma. Cancer Res. 2004, 64, 7857–7866. [Google Scholar] [CrossRef] [Green Version]
- Östman, A.; Hellberg, C.; Böhmer, F.D. Protein-tyrosine phosphatases and cancer. Nat. Rev. Cancer 2006, 6, 307–320. [Google Scholar] [CrossRef]
- Burridge, K.; Sastry, S.K.; Sallee, J.L. Regulation of cell adhesion by protein-tyrosine phosphatases. I. Cell-matrix adhesion. J. Biol. Chem. 2006, 281, 15593–15596. [Google Scholar] [CrossRef] [Green Version]
- Shiota, M.; Tanihiro, T.; Nakagawa, Y.; Aoki, N.; Ishida, N.; Miyazaki, K.; Ullrich, A.; Miyazaki, H. Protein tyrosine phosphatase PTP20 induces actin cytoskeleton reorganization by dephosphorylating p190 RhoGAP in rat ovarian granulosa cells stimulated with follicle-stimulating hormone. Mol. Endocrinol. 2003, 17, 534–549. [Google Scholar] [CrossRef] [Green Version]
- Dumaual, C.M.; Sandusky, G.E.; Crowell, P.L.; Randall, S.K. Cellular Localization of PRL-1 and PRL-2 Gene Expression in Normal Adult Human Tissues. J. Histochem. Cytochem. 2006, 54, 1401–1412. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Genin, A.; Spinner, N.B.; Diamond, R.H.; Taub, R. The gene encoding human nuclear protein tyrosine phosphatase, PRL-1. Cloning, chromosomal localization, and identification of an intron enhancer. J. Biol. Chem. 1998, 273, 17286–17295. [Google Scholar] [CrossRef] [Green Version]
- Gnainsky, Y.; Spira, G.; Paizi, M.; Bruck, R.; Nagler, A.; Genina, O.; Taub, R.; Halevy, O.; Pines, M. Involvement of the tyrosine phosphatase early gene of liver regeneration (PRL–1) in cell cycle and in liver regeneration and fibrosis effect of halofuginone. Cell Tissue Res. 2006, 324, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Du, K.; Ramirez, S.; Diamond, R.H.; Taub, R. Mitogenic up-regulation of the PRL-1 protein-tyrosine phosphatase gene by Egr-1. Egr-1 activation is an early event in liver regeneration. J. Biol. Chem. 1999, 274, 4513–4520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Choi, J.H.; Jun, J.H.; Park, S.; Jung, J.; Bae, S.H.; Kim, G.J. Enhanced PRL-1 expression in placenta-derived mesenchymal stem cells accelerates hepatic function via mitochondrial dynamics in a cirrhotic rat model. Stem Cell Res. Ther. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- He, R.-J.; Yu, Z.-H.; Zhang, R.-Y.; Zhang, Z.-Y. Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol. Sin. 2014, 35, 1227–1246. [Google Scholar] [CrossRef] [Green Version]
| Parameter | Stage | ||||
|---|---|---|---|---|---|
| 0 (n = 5) | 1 (n = 5) | 2 (n = 5) | 3 (n = 5) | 4 (n = 5) | |
| AST | 162.8 ± 78.9 | 78.4 ± 9.6 | 97.4 ± 21.5 | 85.6 ± 19.8 | 71.4 ± 11.4 |
| ALT | 104.9 ± 43.4 | 94 ± 28.7 | 42.8 ± 6.9 | 52 ± 15.6 | 68.6 ± 24.0 |
| TB | 0.68 ± 0.2 | 0.86 ± 0.2 | 2.08 ± 0.8 | 2.42 ± 1.0 | 2.78 ± 0.9 a |
| γGT | 330 ± 110 | 133 ± 70.2 | 383 ± 228 | 744 ± 335 | 346 ± 231 |
| PT | 0.92 ± 0.04 | 0.92 ± 0.02 | 1.04 ± 0.04 | 0.93 ± 0.03 | 1.31 ± 0.07 b |
| PLT | 349,400 ± 98,522 | 349,800 ± 55,757 | 181,400 ± 55,275 | 160,200 ± 40,786 | 98,000 ± 14,638 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.H.; Park, S.; Kim, G.D.; Kim, J.Y.; Jun, J.H.; Bae, S.H.; Baik, S.K.; Hwang, S.-G.; Kim, G.J. Increased Phosphatase of Regenerating Liver-1 by Placental Stem Cells Promotes Hepatic Regeneration in a Bile-Duct-Ligated Rat Model. Cells 2021, 10, 2530. https://doi.org/10.3390/cells10102530
Choi JH, Park S, Kim GD, Kim JY, Jun JH, Bae SH, Baik SK, Hwang S-G, Kim GJ. Increased Phosphatase of Regenerating Liver-1 by Placental Stem Cells Promotes Hepatic Regeneration in a Bile-Duct-Ligated Rat Model. Cells. 2021; 10(10):2530. https://doi.org/10.3390/cells10102530
Chicago/Turabian StyleChoi, Jong Ho, Sohae Park, Gi Dae Kim, Jae Yeon Kim, Ji Hye Jun, Si Hyun Bae, Soon Koo Baik, Seong-Gyu Hwang, and Gi Jin Kim. 2021. "Increased Phosphatase of Regenerating Liver-1 by Placental Stem Cells Promotes Hepatic Regeneration in a Bile-Duct-Ligated Rat Model" Cells 10, no. 10: 2530. https://doi.org/10.3390/cells10102530
APA StyleChoi, J. H., Park, S., Kim, G. D., Kim, J. Y., Jun, J. H., Bae, S. H., Baik, S. K., Hwang, S.-G., & Kim, G. J. (2021). Increased Phosphatase of Regenerating Liver-1 by Placental Stem Cells Promotes Hepatic Regeneration in a Bile-Duct-Ligated Rat Model. Cells, 10(10), 2530. https://doi.org/10.3390/cells10102530







