1. Introduction
Significant progress has been made in the understanding of fundamental processes that occur after spinal cord injury (SCI), including the involvement of multiple cell types, inflammatory reactions and the expression of trophic factors [
1,
2]. The irreversible functional impairment after SCI is caused by the disruption of neuronal connections across the lesion and the generation of refractory substrates that inhibit spontaneous recovery [
3,
4]. However, the discovery of the regenerative capacity of central nervous system (CNS) neurons in the proper environment, and the beneficial effects of their electrical stimulation, gives reasonable optimism that solutions for repair could soon be found. Indeed, despite the limited neuronal regeneration after SCI, some sprouting of axon collaterals does ensue [
5,
6,
7,
8]. Axons undergo remarkable structural rearrangements, and this property may underlie the recovery of lost functions that occur in some incomplete injuries [
4,
9,
10]. Even then, axonal sprouting does not replicate the exact connectivity of axons prior to injury, and targeting defects occur. These ectopic axonal projections produce improper rewiring, which may give rise to maladaptive plasticity and severe functional deficits [
8]. Furthermore, the lesion that is formed, and its refractory environment, lack the necessary substrate for axon regrowth and guidance. A major aim of SCI research is to repair the disrupted neural network to close to its former status. Moreover, the regenerative capabilities of electrical stimulation on CNS tissue have been well studied [
11] and the acceleration of axon outgrowth has been reported [
5,
12]. Therefore, it is becoming increasingly more frequent to use combined strategies that enhance regrowth and guidance.
Biomaterial scaffolds that provide guidance and a growth-permissive substrate for axons are logical therapeutic options. Many different types of scaffolds have been developed for the treatment of SCI, which provide biophysical and/or biochemical cues that support the regenerative process [
13,
14]. The ideal scaffold would have a simple design that allows for smooth manufacturing, low immunogenicity, mechanical properties that prevent mismatching with the neural tissue, biochemical cues to favor cell adhesion and axonal regeneration, and would be easy to transplant into the injured spinal cord not to entail further damage [
13,
15]. In particular, scaffolds made of conducting polymers, such as poly 3,4-ethylenedioxythiophene (PEDOT) have been shown to be suitable candidates for the fabrication of devices for neural repair [
16]. Indeed, PEDOT doped upon carbon microfibres (MFs) increased their electrical conductivity and provided a substrate to attach bioactive molecules that promote longitudinal alignment of glial cells and axonal elongation [
17,
18]. Since the microenvironment of the SCI lesion inhibits regeneration, we hypothesised that when coated, these MFs may also provide a chemical environment that favours growth.
In the present study, we implemented a partial unilateral dorsal quadrant lesion (PUDQL) model of SCI in mice with a glass window-based
in vivo imaging methodology. The lesion is compatible with the implantation of carbon MFs, functionalized with PLL/heparin/bFGF/fibronectin. We used Thy1-CFP//LysM-EGFP//CD11c-EYFP triple transgenic reporter animals [
19,
20] that allowed simultaneous imaging of axons, myeloid cells and microglial cells over time in the same animal to monitor the effects of the grafted MFs on the dynamics of axon regrowth and inflammation. We found that functionalized MFs accelerated and optimized the recovery of axonal network after PUDQL, likely by dampening chronic inflammation and promoting the accumulation of monocytes derived dendritic cells (moDCs).
2. Materials and Methods
2.1. Animals
We operated on a total of n = 43 Thy1-CFP//LysM-EGFP//CD11c-EYFP triple heterozygous transgenic adult mice (more than nine weeks old) with multiple fluorescent cell populations. These mice display a subpopulation of neurons with cytoplasmic expression of CFP (blue), neutrophils and monocytes with cytoplasmic expression of Enhanced Green Fluorescent Protein (EGFP), as well as activated resident microglia and peripheral dendritic cells with cytoplasmic expression of Enhanced Yellow Fluorescent Protein EYFP (see 20 for details). Mice were housed in cages with food and water ad libitum in a 12 h light/dark cycle at 22 ± 1 °C. Until the end of the protocols, food was supplemented with 4% agarose jelly containing 4%glucose. All experimental procedures were performed in accordance with the French legislation and in compliance with the European Community Council Directive of 24 November 1986 (86/609/EEC) for the care and use of laboratory animals. The research on animals was authorised by the Direction Départementale des Services Vétérinaires des Bouches-du-Rhône (license D-13-055-21) and approved by the National Committee for Ethic in Animal Experimentation (Section N°14, project APAFIS #4828-20 1504131125423 v2).
2.2. Preparation and Biofunctionalization of Carbon MFs
The protocol for the preparation and bio-functionalization of carbon MFs was described previously [
17,
18,
21]. Briefly, poly(3,4-ethylenedioxythiophene)/poly-(styrenesulfonate)-co-maleic acid) (PEDOT:PSS-co-MA) were electrodeposited on the surface of 7-µm-diameter carbon fibres (Goodfellow) by applying a constant anodic current of 1 µA/mm
2 and a polymerization charge of 96 mC/cm
2. Functionalisation with bioactive molecules was performed on PEDOT:PSS-co-MA-coated carbon MFs (hereafter referred to as bare-MFs). Poly-L-lysine (PLL; Sigma-Aldrich) was bonded covalently to the carboxylic groups of the dopant. Heparin (10 mM; Sigma-Aldrich) was dissolved in Phosphate Buffer Solution (PBS) was then assembled on the PLL layer for 4 min. Subsequently, recombinant human basic fibroblast growth factor (bFGF, PeproTech 100-18B) was applied at 1 µg/mL in PBS for 1 h. Finally, the MFs were incubated at 37 °C for 4 days with bovine fibronectin at 40 µg/mL (FN; Invitrogen, 33010-018) dissolved in PBS, as illustrated in Collazos Castro et al. [
17]. PEDOT/PLL/Heparin/bFGF/FN-MFs are hereafter referred to as MFs, or eventually as bf-MFs (for biofunctionalized-bf-MFs) when these were compared to bare MFs in
Section 3.2.
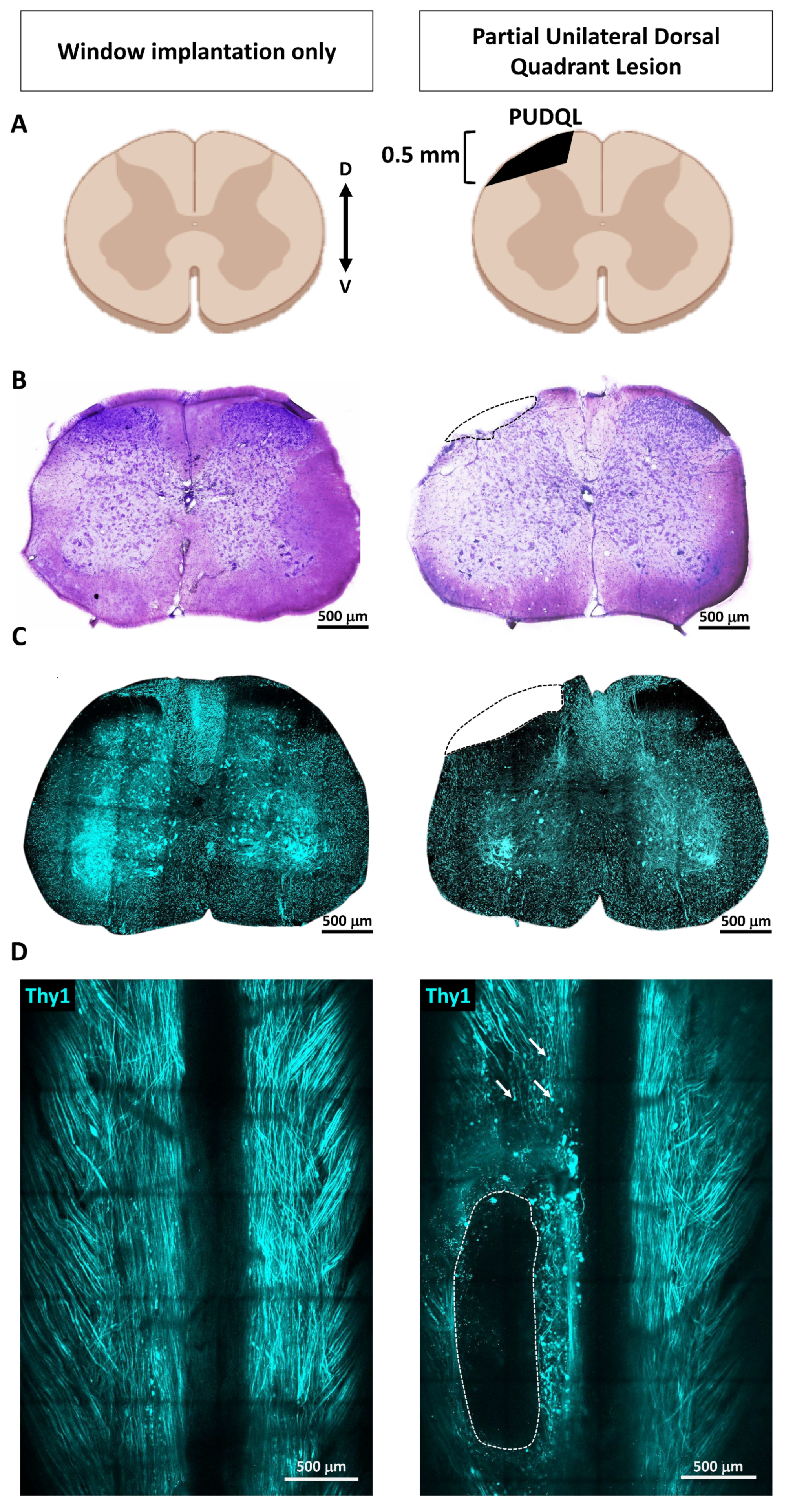
2.3. Partial Unilateral Dorsal Quadrant Lesion (PUDQL) and Glass Window Implantation
A glass window was cemented and sealed on the exposed spinal parenchyma [
19]. Briefly, mice were anesthetized with intraperitoneal ketamine/xylazine (120 mg/kg; 12 mg/kg), and supplemented hourly with the same cocktail at a lower dose (40 mg/kg; 4 mg/kg). Following a dorsal midline incision over T12 to L2, the muscles between the spinal and transverse processes were resected using a scalpel. Animals were then suspended from a spinal-fork stereotaxic device (Harvard Apparatus). The dorsal musculature was further resected to expose the vertebrae and the tips of modified staples were inserted along the edges of the T12 and L2 vertebrae and glued in place with cyanoacrylate. A modified paperclip was fixed to the staples to serve as a handle for surgery and imaging, and a layer of dental cement was applied to form a rigid ring to hold the vertebrae, staples and paper clip together. A laminectomy was performed by removing two spinal processes from the exposed vertebrae.
A partial unilateral dorsal quadrant lesion (PUDQL) was made on the rostral edge of the exposed spinal cord. To do so, a 26G needle blade was used to make a 0.6 mm incision on the right side of the spinal cord as close as possible to the central vein and the spinal cord, as deep as the shaft of the needle. The spinal cord was then transected using a pair of fine-angled Vannas spring scissors (Fine Science Tools) from the rostral end of the incision to 0.4 mm lateral and approximately 0.5 mm deep from the dorsal surface. A second identical incision was made from the caudal end of the initial incision. A final horizontal cut was made from the injured side toward the dorsal vein and the tissue between the rostral and caudal transections was removed. The lesion was made so that the caudal incision reached the middle of the window and the rostral incision was 200 microns away from the most frontal edge of the window. Special care was taken to not cut the central vein so that bleeding was kept minimal. Animal were split into two groups: PUDQL only and PUDQL immediately followed by MF implantation.
In MF-implanted animals, four carbon bf-MFs aligned in parallel to the central vein were inserted into the spinal cord parenchyma to bridge the injury. To evaluate the influence of protein coating, a subset of animals were implanted with two bare-MFs and two bf-MFs in alternation. In this case, difference in biocompatibility and on axon sprouting were assessed.
A line of liquid Kwik-Sil (World Precision Instruments) was applied along the midline of the spinal cord, and the glass window was immediately glued and cemented over the spinal cord. Kwik-Sil was used to embed the MFs and seal the lesion site to the glass window in order to prevent fibroblastic invasion. Kwik-Sil was applied on the dried lesion site using the mixing application tip. The glass window had to be perfectly clean to allow adherence between the silicone and the glass. All the procedures were performed under sterile conditions. Postoperative analgesia was obtained by administration of cortamethasone (0.2 mg/kg) and rimadyl (5 mg/kg) immediately following surgery and every two days for 10 days after surgery. Mice were active in their cage already 18h postsurgery and did not require manual bladder emptying, since the lesion affected only the most dorsal tracts.
2.4. Intravital Imaging
The same mice were imaged three days (D3) after window implantation to target the peak of peripheral cell infiltration and then every two weeks for two to three months (D15, D28-30, D42-45, D58-60, D90). For each imaging session, mice were lightly anesthetized with 1.5% isofluorane (v/v) in air for 2 min, followed by intraperitoneal ketamine/xylazine (100 mg/kg; 10 mg/kg) administration. Animals were supplemented with 0.4–1.0% isofluorane (v/v) in air from 45 min after the start of the session until completion. Throughout imaging, animals were freely breathing and the microscope chamber was warmed to 32 °C to maintain the body temperature at 37 °C. Following each imaging session, the animals were returned to their cage with a piece of tissue for nesting and kept warm until they recovered from anesthesia.
A tunable femtosecond pulsed laser (Ultra II Chamaleon Coherent) was coupled to a Zeiss two-photon (2P) microscope (LSM 780) equipped with a 20× water immersion objective lens (NA = 1.0) and five non-descanned detectors. The laser was tuned to 940 nm to optimize the simultaneous excitation of the labeling fluorophore combination while minimizing the heat accumulation by implanted carbon MFs. Filter sets were designed to optimize the separation of the emission spectra of multiple fluorophores. For each image stack laser intensity was adjusted according to imaging depth in order to maximize signal intensity while minimizing saturation throughout the image stack.
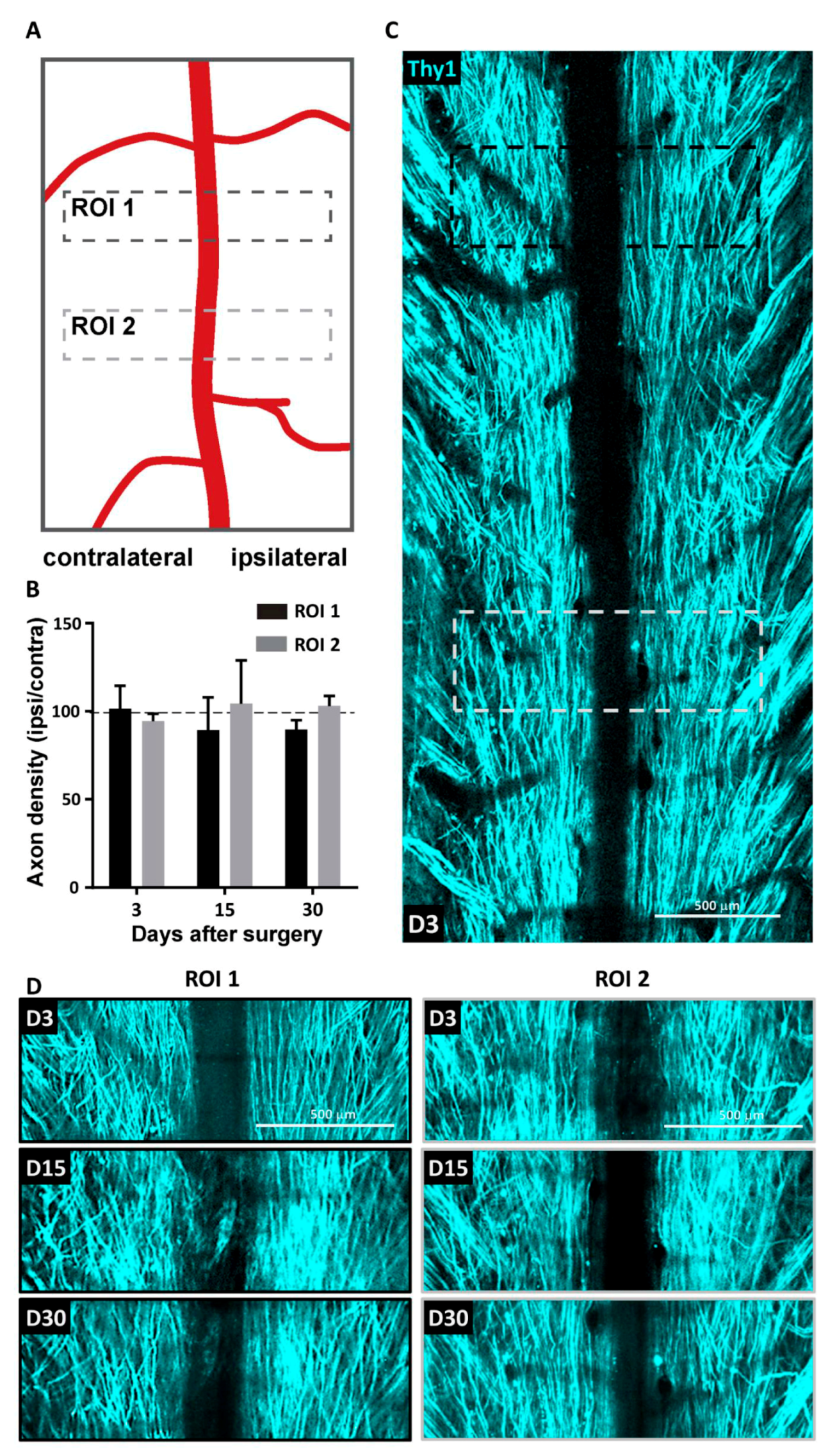
A second harmonic signal reflected back by superficial collagen fibres was used to identify meninges. Blood vessels and remarkable axons patterns were used as anatomical markers to find the region of interest for each animal. Tiled-stack images were acquired with a field of view of 424 × 424 µm with an optical sectioning of 3 µm over a depth of ~80 to 100 µm below the meninges. Microadjustments during mouse positioning allowed extraction of a conserved volume of interest throughout imaging sessions. The volume mainly lying between 20 to 50 µm was used for quantitative analysis.
2.5. Image Analysis
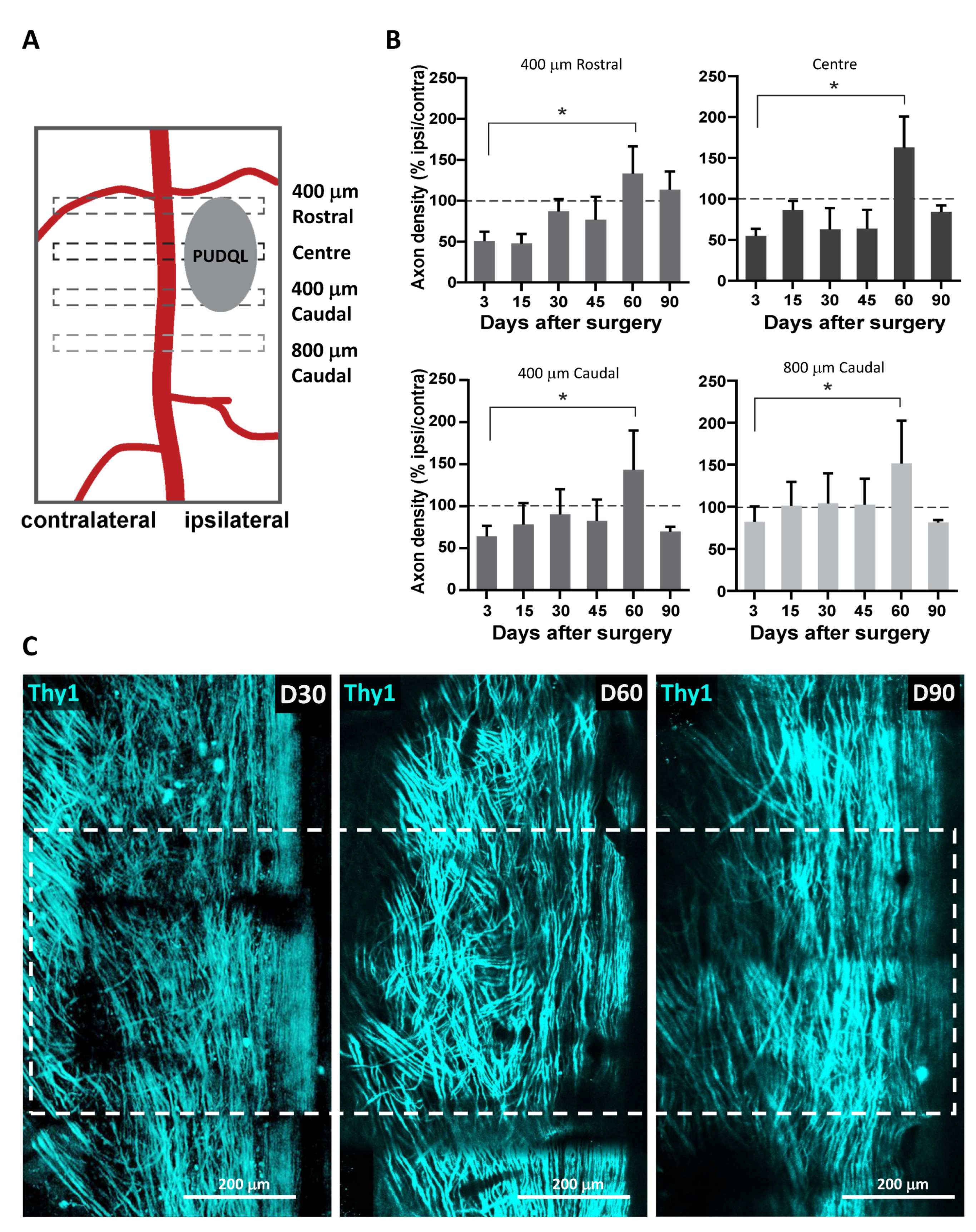
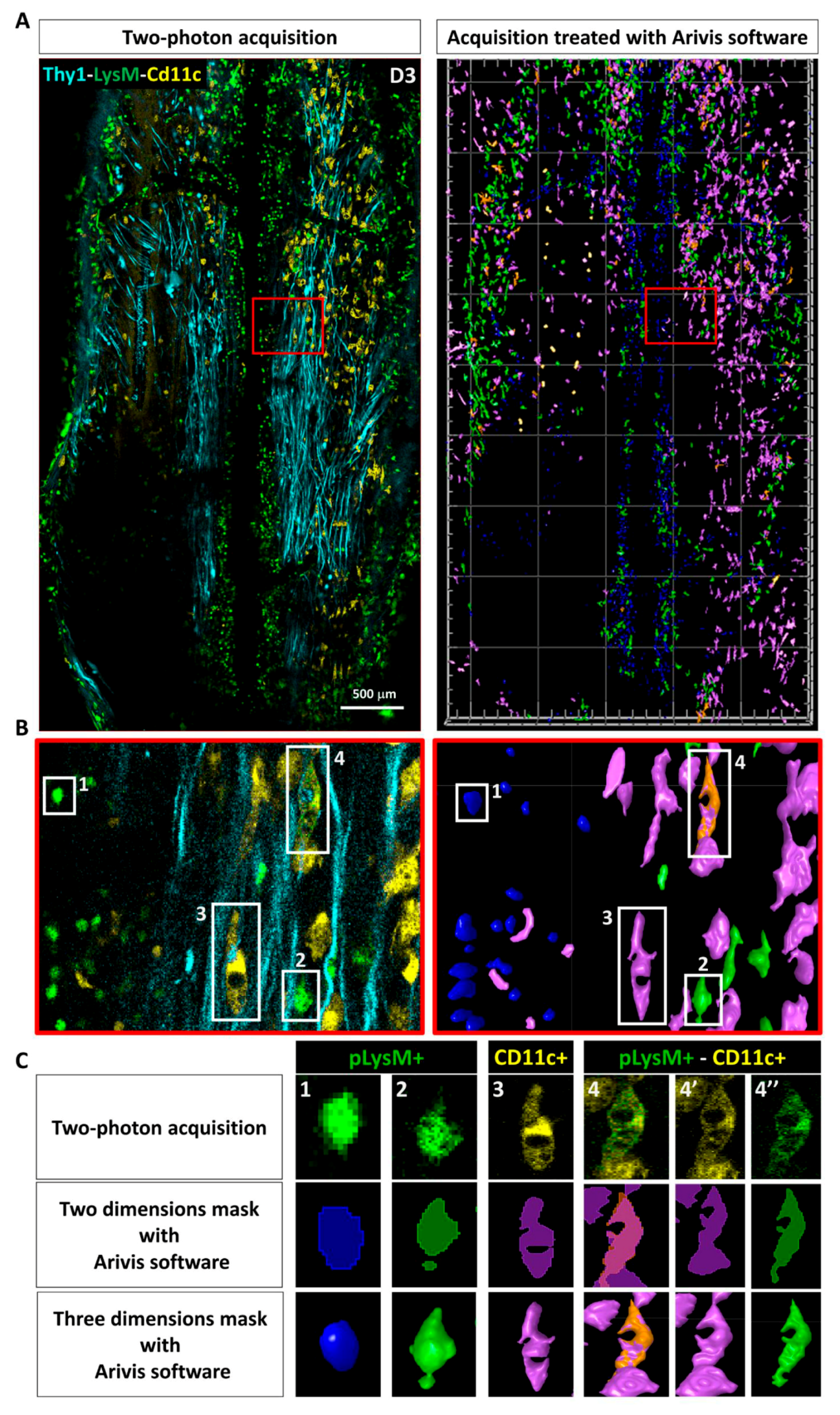
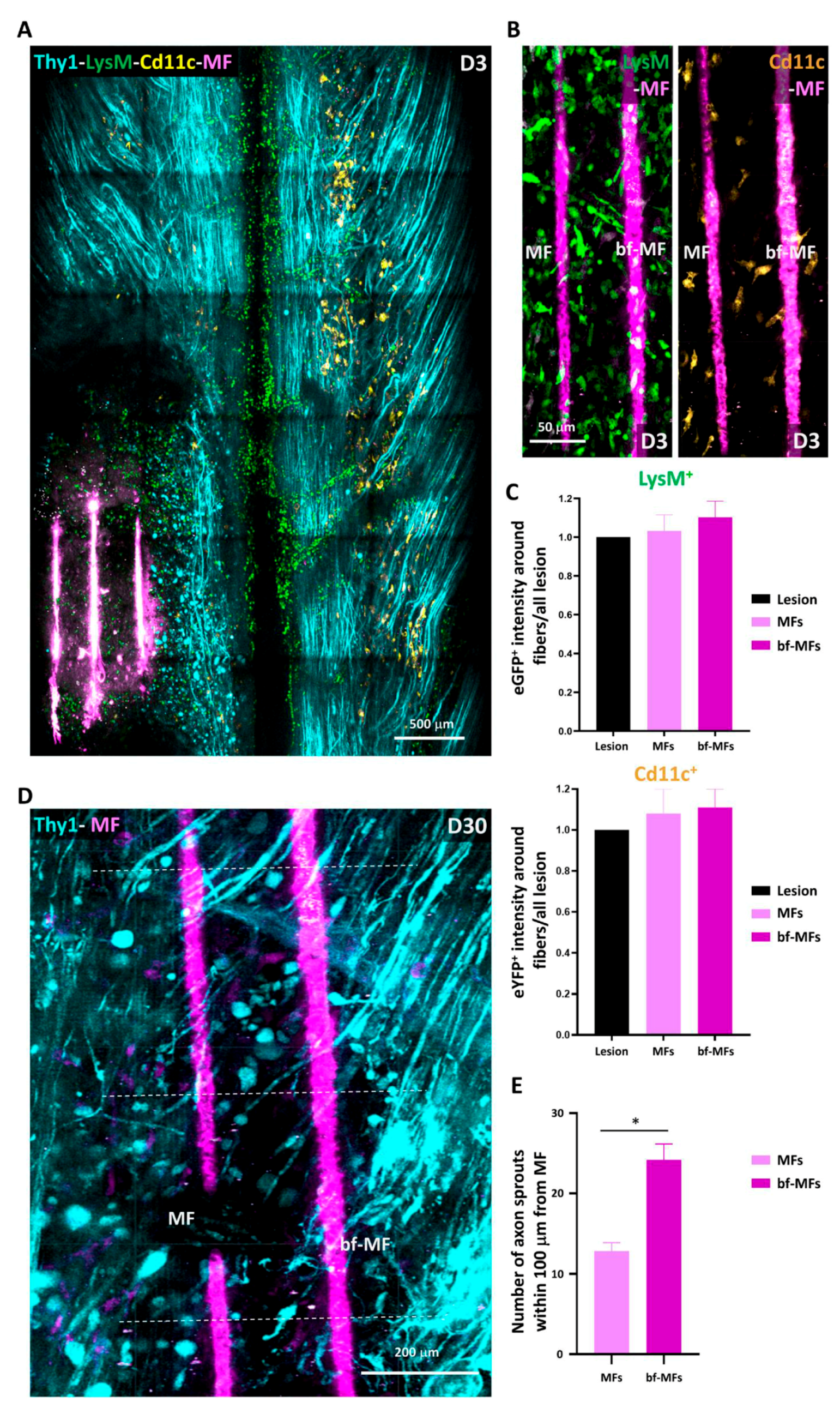
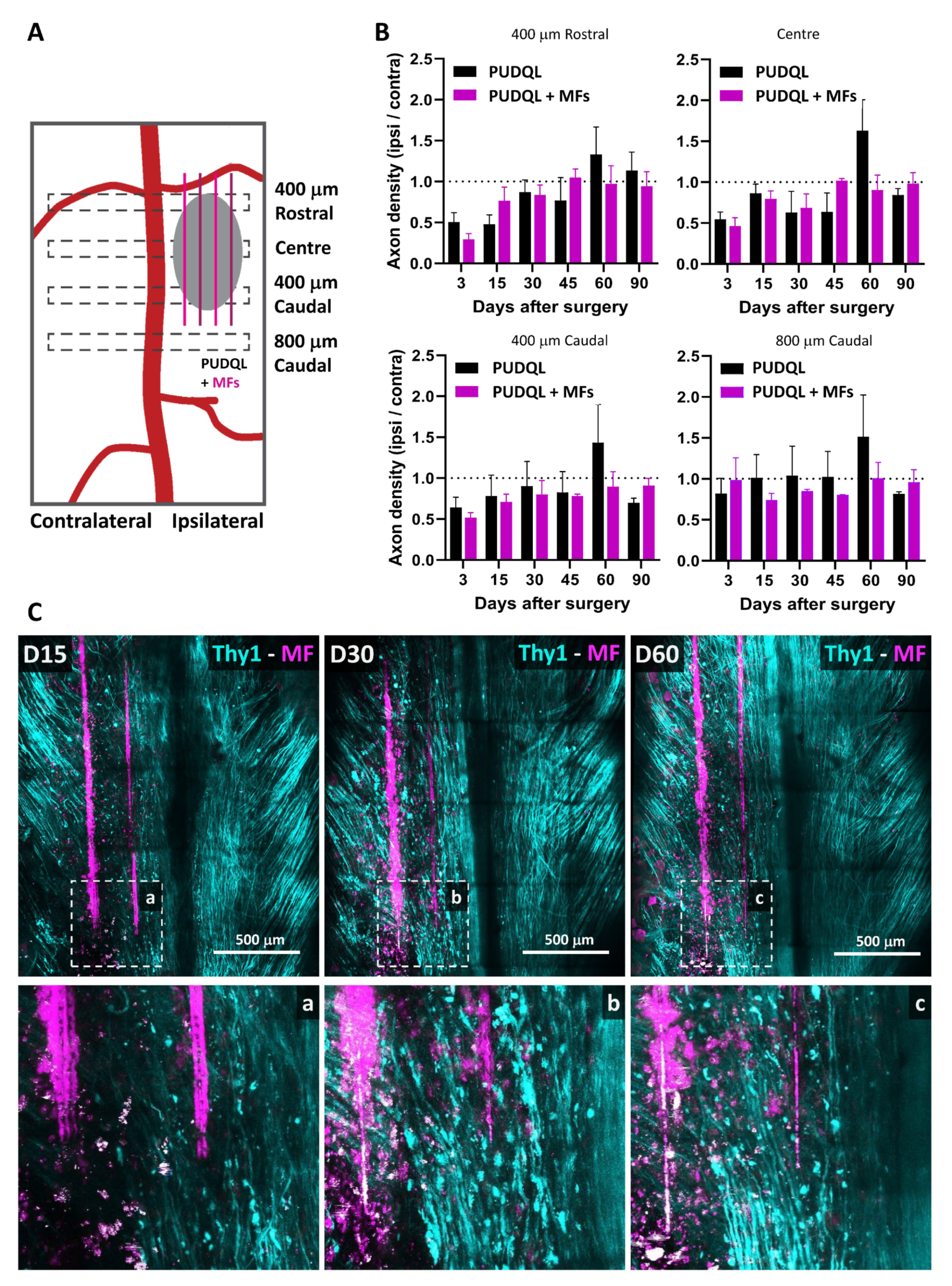
Images were analyzed using ZEN 2.1 (Zeiss, Jena, Germany), ImageJ software and Arivis Vision 4D software (Arivis AG, Berlin, Germany). ZEN 2.1 was used for post-acqusition spectral unmixing and analysis was performed on the resulting data. Presented images are pseudocolored and contrast-enhanced for clarity. For every mouse, the volumes of interest imaged at every time point were manually registered to account for small inaccuracy in repositioning both in the XY plane and in the Z position from one session to the next. For every time point in this registered volume of interest, axons were counted manually in 3D at five locations along the rostro-caudal axis, at the level of the lesion epicenter and respectively 400 µm and 800 µm in the caudal direction as well as 400 µm in the rostral direction. Counting was done on both sides of the dorsal vein. The numbers obtained on the lesioned ipsilateral side were normalized by the corresponding numbers of axons on the uninjured contralateral side. To study the coating dependance of the density of axons found around MFs, we evaluated the number of axons intercepting three different 100 µm segments running perpendicularly to the MF surface at three different positions along the MF segment visible in the centre of the lesion. Axons enclosed in the area between bare MF and bf-MFs were likely submitted to a mixed influence of both MF and were therefore not included in the counting.
Immune cells were counted in the total 3D volume of the 2P acquisitions using the object detection algorithm in the Arivis software, and their average densities were evaluated per volume unit (of about 0.01 mm3). Cells were distinguished using EGFP and EYFP labeling and morphological features. To validate our automated procedure, automated results were compared to manual counts of cellular densities obtained by two independent investigators on selected images stacks; these never differed by more than a few percent.
2.6. Statistics
All data are expressed as mean ± SEM. Statistical analysis was performed using Graph Pad Prism software for Kruskal-Wallis or Mann-Whitney tests, as required. p < 0.05 was considered for statistical significance.
2.7. Nissl Staining
Mice were transcardially perfused with 4% paraformaldehyde. Spinal cords were removed, postfixed overnight, and cut into 50-µm thickness coronal sections using a vibratome (Leica Microsysteme, Rueil-Malmaison, France). We used a standard Nissl staining protocol. Briefly, spinal cord sections were first dehydrated in standard series of increasing concentrations of alcohol (75%, 80%, 95%, 100%; 2 min each) and placed in distilled water for 5 mins. This step was followed by incubation of sections in a filtered solution containing 0.5% Cresyl violet (Sigma Aldrich) in distilled water, where they remained for approximately 1 min. Next, tissue was rehydrated by incubation in alcohol solutions of decreasing concentrations (100%, 95%, 70%, 50%; 2 min each) and cover-slipped.
4. Discussion
The treatment strategies for SCI are shifting toward combinatorial approaches. The characteristics provided by biomaterial scaffolds [
18,
25,
26,
27,
28] have led many studies to incorporate scaffolds into their treatment paradigms ( [
14] for review). Among them, bio-electronic microsystems hold strong promise for repairing neural damage and enhancing functional recovery. However, their development has been delayed in part because such devices should be biocompatible and their implantation requires procedures that inflict additional lesion to the neural tissue. Therefore, animal models and technical approaches are needed to precisely evaluate at the cellular level their effects over time. We designed a preclinical model of SCI in mice to evaluate in vivo the dynamic effects on neurons and inflammatory cells upon insertion of carbon MFs. The PUDQL lesion we used in this study was compatible with MF implantation in the lesion while avoiding major motor deficits in mice. The carbon MFs were coated with the conducting polymer PEDOT: PSS-co-MA and functionalized with the multimolecular complex PLL/heparin/bFGF/FN. Our results showed that these fibres are biocompatible as their insertion into the lesion did not trigger the acute inflammatory response beyond that observed in the lesion alone. Interestingly, they impinged upon the profile of immune cells that were recruited at later phases after the lesion, which may have resulted from the modified axon behaviour. We implanted MFs with an orientation parallel to the rostro-caudal axis and observed that they acted as scaffolds, resulting in a better axon alignment compared to controls. Furthermore, the protein-functionalized-MFs at least transiently supported and enhanced the endogenous potential of axons to sprout during the early (acute and subacute) phases. Therefore, functionalized carbon MFs provide an encouraging strategy for repair after SCI. Moreover, our experimental settings proved to be instrumental to longitudinally visualize the effect of biomaterial insertion at the cellular and circuit level in individual animals.
Regardless of the pathophysiological relevance of mice models [
29], we used these animals due to the availability of the transgenic Thy1-CFP//LysM-EGFP//CD11c-EYFP triple heterozygous animals that allow to concomitantly image several fluorescent cell populations. Compared to most of the biocompatibility studies reported so far, our 2P intravital approach allowed us to observe live cells and the same area in a given animal over time. We found that MF types were well-integrated in the spinal cord tissue and did not exacerbate inflammation in their vicinity irrespective of the presence of protein coating. Quantification of the density of subsets of innate immune cells could not evidence any significant differences or accumulation along fibres. Therefore, PEDOT:PSS-co-MA is a relatively inert material and is not rejected by the mouse immune system. Furthermore, PLL/heparin/bFGF/fibronectin coating did not specifically attract innate myeloid cells or activated microglia. On the other hand, we observed a differential effect of the fibres on the density of fluorescent sensory axons at early phases (D3 to D30 after lesion). Since neuron survival and axon sprouting depend on molecular cues in the surrounding environment, it is likely that the coated proteins on the bf-MFs directly or indirectly modified the signals addressed to the receptors of the severed axons [
30,
31].
It is well known that mouse axons regenerate spontaneously within the first month after SCI [
6,
32]. Even if axons with enhanced regenerative capacity are able to grow across a lesion using astrocytes as support [
33] they fail to grow across a large lesion. While we observed significant sprouting of axons after the lesion, their trajectories greatly differed from their normal rostro-caudal orientation. MFs may then serve as bridges for axon elongation either directly or in association with glial cells, as MFs functionalized with PLL/Heparin/bFGF/FN have been shown to promote glial migration along the fibres and thus provide environmental cues for aligned growth [
18]. This effect may be particularly important at early time points when the association of regrowing axons with MFs must be ensured. Afterwards, we could not exclude the possibility that the proteins coating the MFs were released or degraded. Nevertheless, the physical support they provided to the tissue at later time points seemed enough to guide axons in the right direction during regeneration, as has been shown for other biomaterial scaffolds [
26,
27]. It should be noted that MFs can be coated with very different combinations of proteins and thus offer an extensive therapeutic flexibility. Besides proteins associated to cell adhesion and growth factors, the MF coating could also provide modulators of molecular pathways involved in intrinsic growth, thereby extending the range of manipulation of the local environment, especially at early time points [
33,
34,
35,
36].
Inflammation plays a predominant role in the aftermath of SCI and subsequent axonal regeneration. Having characterized the phenotype of fluorescent cells subpopulations in our Thy1-CFP//LysM-EGFP//CD11c-EYFP mice [
20,
23], we showed that the neuroinflammatory response is generally mediated by various myeloid cell types including infiltrating neutrophils, monocytes and their progeny, as well as by resident microglia [
20,
32]. Here our glass window preparation in triple transgenic mice allowed us to follow the dynamic evolution of immune cells, along with axonal plasticity, as well as the changes in these dynamics induced by MF implantation. We observed that the early phase of PUDQL (D0-3) was governed by a predominance of peripheral pLysM+ inflammatory cells over resident CD11c+ microglia. This dominance progressively declined over time until the lesion environment reached an inverted state dominated by CD11c+microglia in later stages of the trauma. Resident CD11c+ and peripheral pLysM+ immune cell densities thus appeared to balance each other out, as observed in other neuropathological conditions [
20,
23,
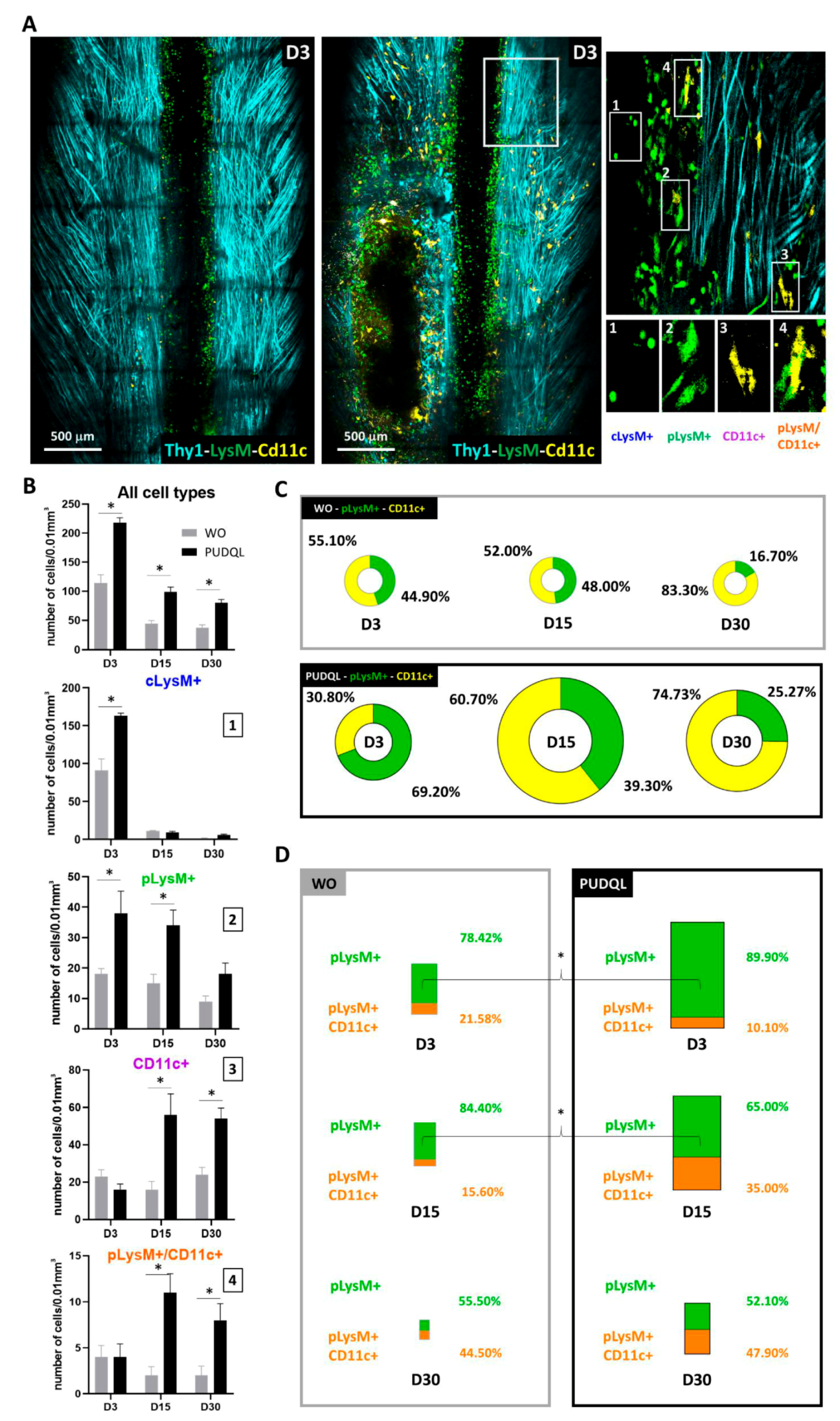
32]. In all these cases moreover, the highest relative densities of pLysM+ immune cells coincided with conditions of maximal neural degeneration, while the highest relative densities of CD11c+ microglia coincided with conditions of regeneration and healing of the neural tissue.
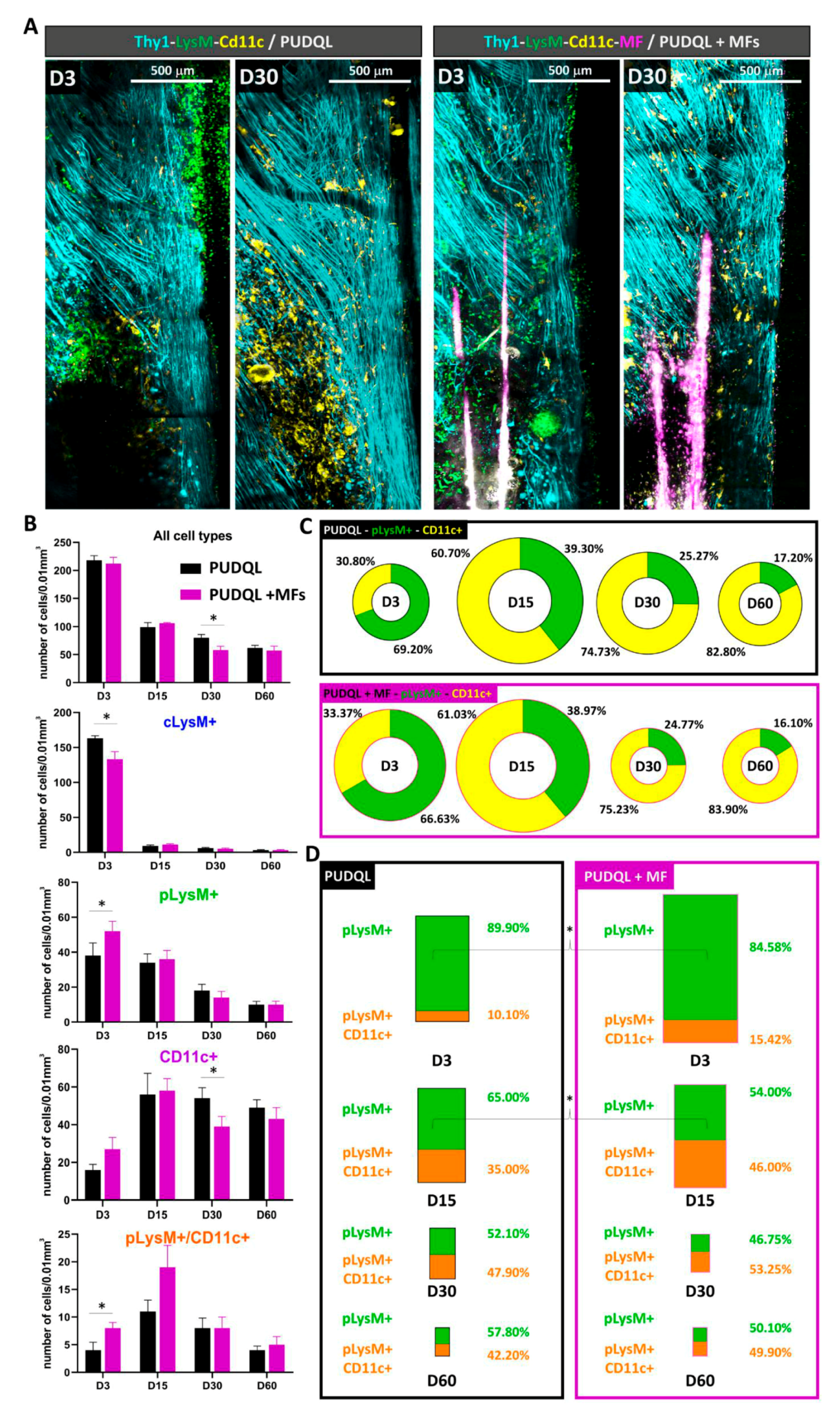
Upon MF implantation at early stages (D0-3), the participation of pLysM+ to the inflammatory response was higher than the one observed in PUDQL-only mice. Whether this early mobilisation could account for the accelerated reduction of inflammatory cell densities on D30 remains to be clarified. Noteworthy, CD11c+ microglial contribution stabilized to a level three times larger than the one of peripheral immune cells at the time of axonal network recovery. Altogether these observations suggested that post-traumatic immune response switched from a deleterious proinflammatory acute state mediated by the early recruitment of pLysM+ cells to a prohealing anti-inflammatory chronic state involving resident CD11c+ microglia.
Part of these pLysM+ cells are neutrophils which are the first reported peripheral immune cells to infiltrate the spinal cord by 3–6 h. They peak around 1-day post injury and their presence is short lived ([
37] for review). The other part are monocytes which at later times represent the vast majority of pLysM+ cells [
20].
In the presence of MFs, the decrease of cLysM+ cells was coincident with a slight increase of pLysM+ cells in the spinal cord tissue. Mechanical constraints exerted by MFs and subsequent release of damage associated molecular patterns along implantation tracks most likely accelerated the recruitment of peripheral immune cells. In response to the factors present in the microenvironment, monocytes then differentiated locally towards moDC as indicated by their double (EGFP+/EYFP+) labelling [
20]. MoDCs subsequently regulate the adaptive immune response and are key in secondary events such as the recruitment of T- and B-lymphocytes [
38]. In particular, they can exhibit anti-inflammatory properties through the education of regulatory T cells. For example, their adoptive transfer was shown to dampen autoimmune deficits in a model of Experimental Autoimmune Encephalomyelitis (EAE) [
39]. In our PUDQL model, these cells represented ~10% of the overall pLysM+ population in the first few days post-trauma, but their contribution increased progressively to reach ~40% on D60, a time of presumed anti-inflammatory condition. Importantly, MF implantation was responsible for increasing their contribution by half as early as D3, while a ~50% contribution was already observed from D15 onwards. In all PUDQL animals, their proportion increased coincidently with the contribution of CD11c+ microglia, supporting the idea of mutual interactions that might induce resident microglia to express growth promoting factors associated with a prohealing phenotype such as neurotrophin-3 and thrombospondin ([
29] for review).