MRGPRX2 Activation by Rocuronium: Insights from Studies with Human Skin Mast Cells and Missense Variants
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Mice
2.3. Cell Line Cultures
2.4. Human Skin-Derived Mast Cell Isolation and Culture
2.5. Peritoneal Mast Cell Isolation and Culture
2.6. Mast Cell Staining
2.7. RNA Isolation and PCR
2.8. Generation of RBL-2H3 Cells Transiently Expressing MRGPRX2 and Its Variants
- M196I: Forward: 5′-TTTTATTCATCGTTCTCTGTGGGTCC-3′
- Reverse: 5′-AAATCAGCCACGCTGCAG-3′;
- L226P: Forward: 5′-CTGACCATCCCGCTCACAGTG-3′
- Reverse: 5′-GTACAGCCTGGTCAGTGG-3′;
- L237P: Forward: 5′-CTCTGCGGCCCGCCCTTTGGC-3′
- Reverse: 5′-GAGGAACACCAGCACTGTGAGC-3′;
2.9. MRGPRX2 Expression and Internalization Using Flow Cytometry
2.10. Degranulation Measured by β-Hexosaminidase Release Assay
2.11. Degranulation Measured by the Surface Expression of Lysosomal-Associated Membrane Protein 1 (LAMP-1)
2.12. Statistical Analysis
3. Results
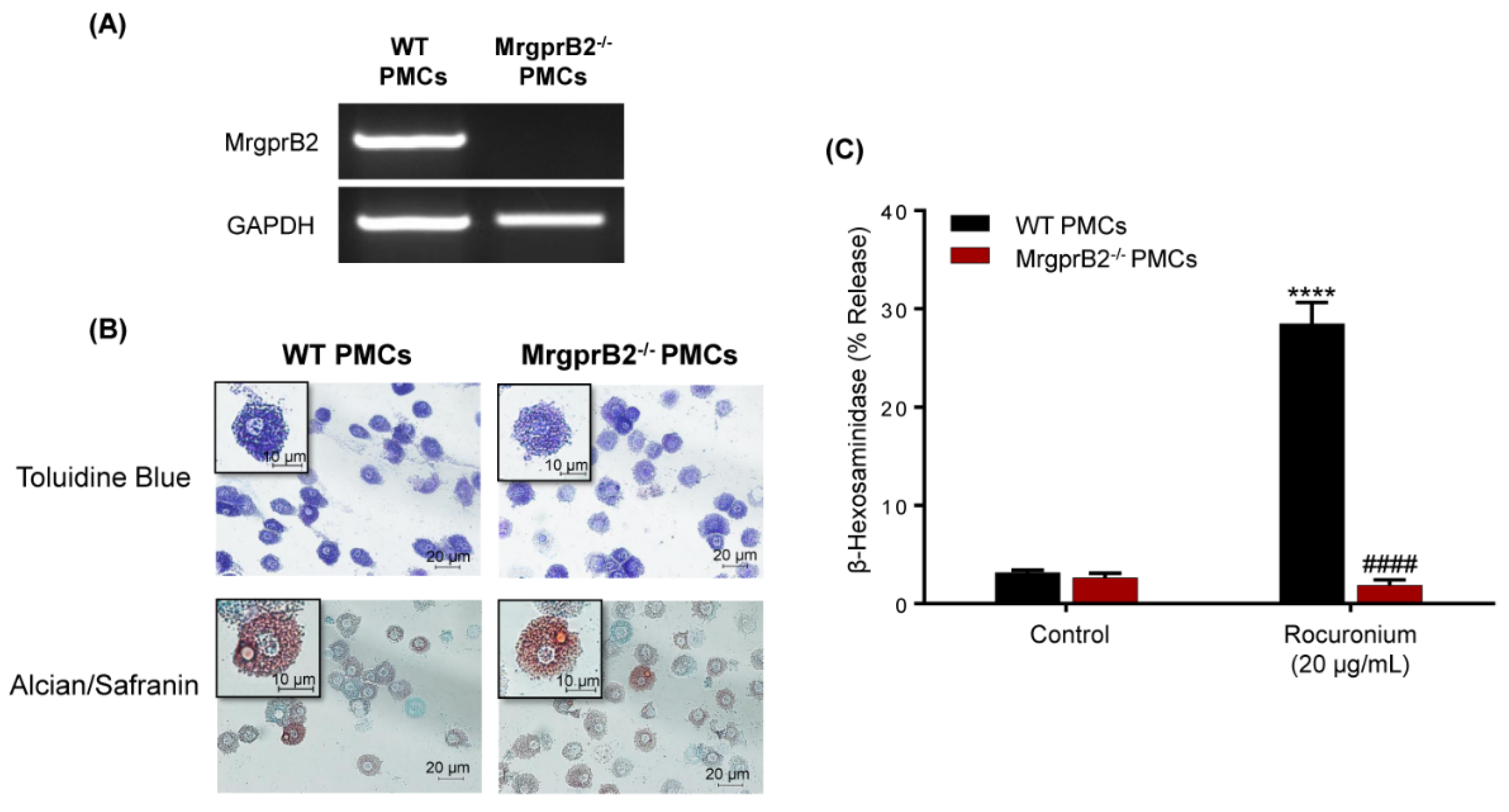
3.1. Rocuronium Activates Murine PMCs via MrgprB2
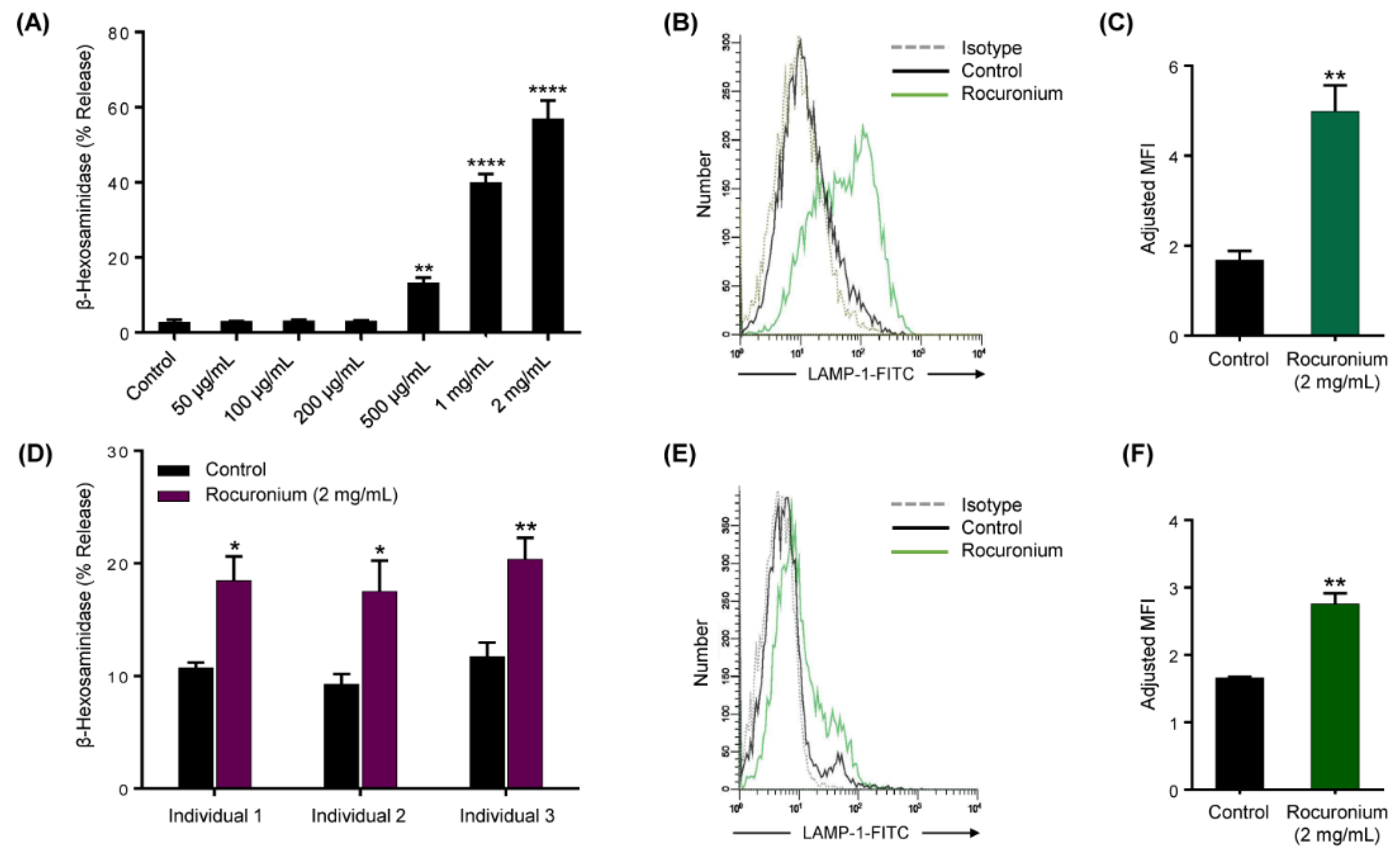
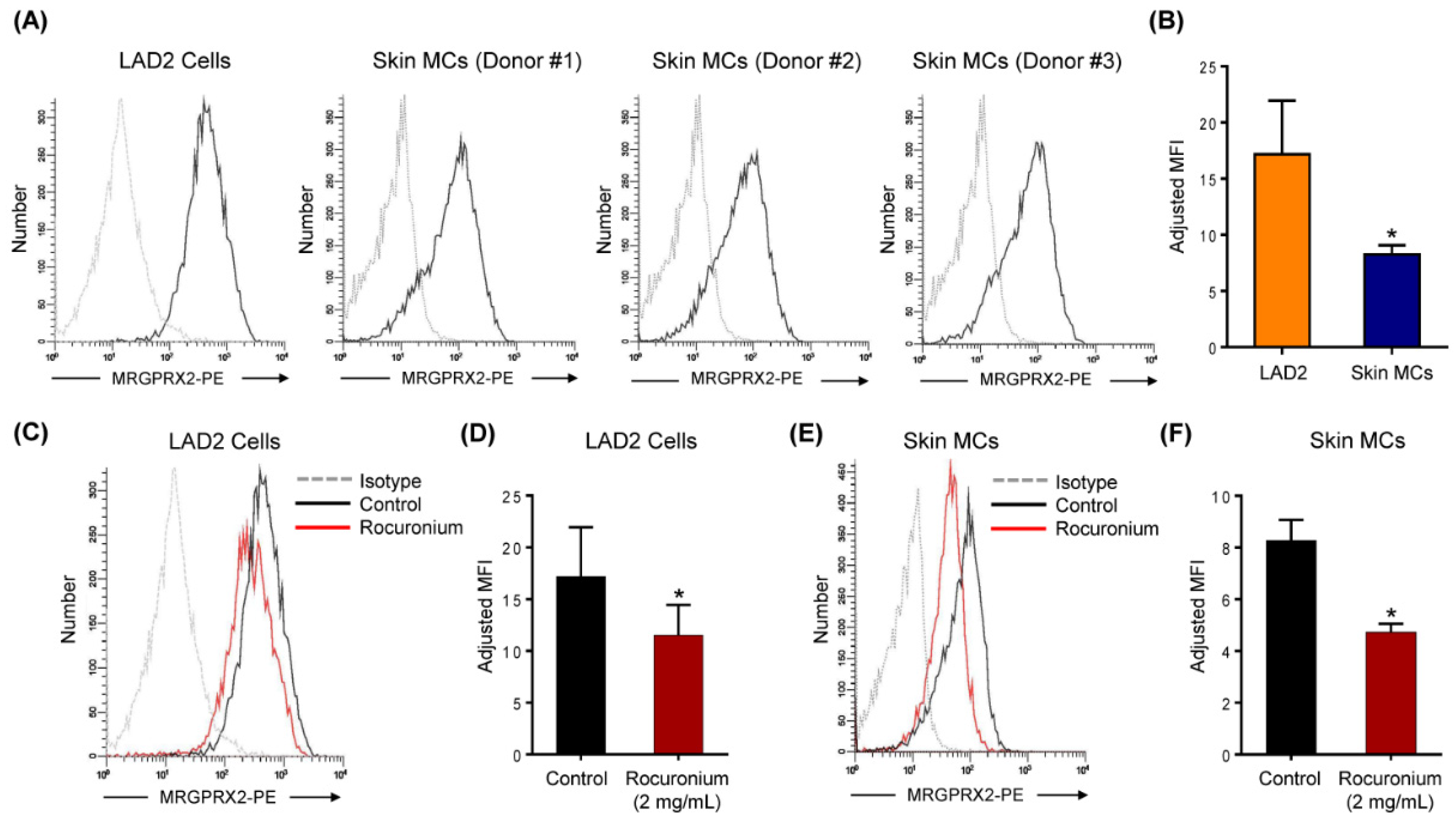
3.2. Rocuronium Induces MRGPRX2-Mediated Degranulation in Human MCs
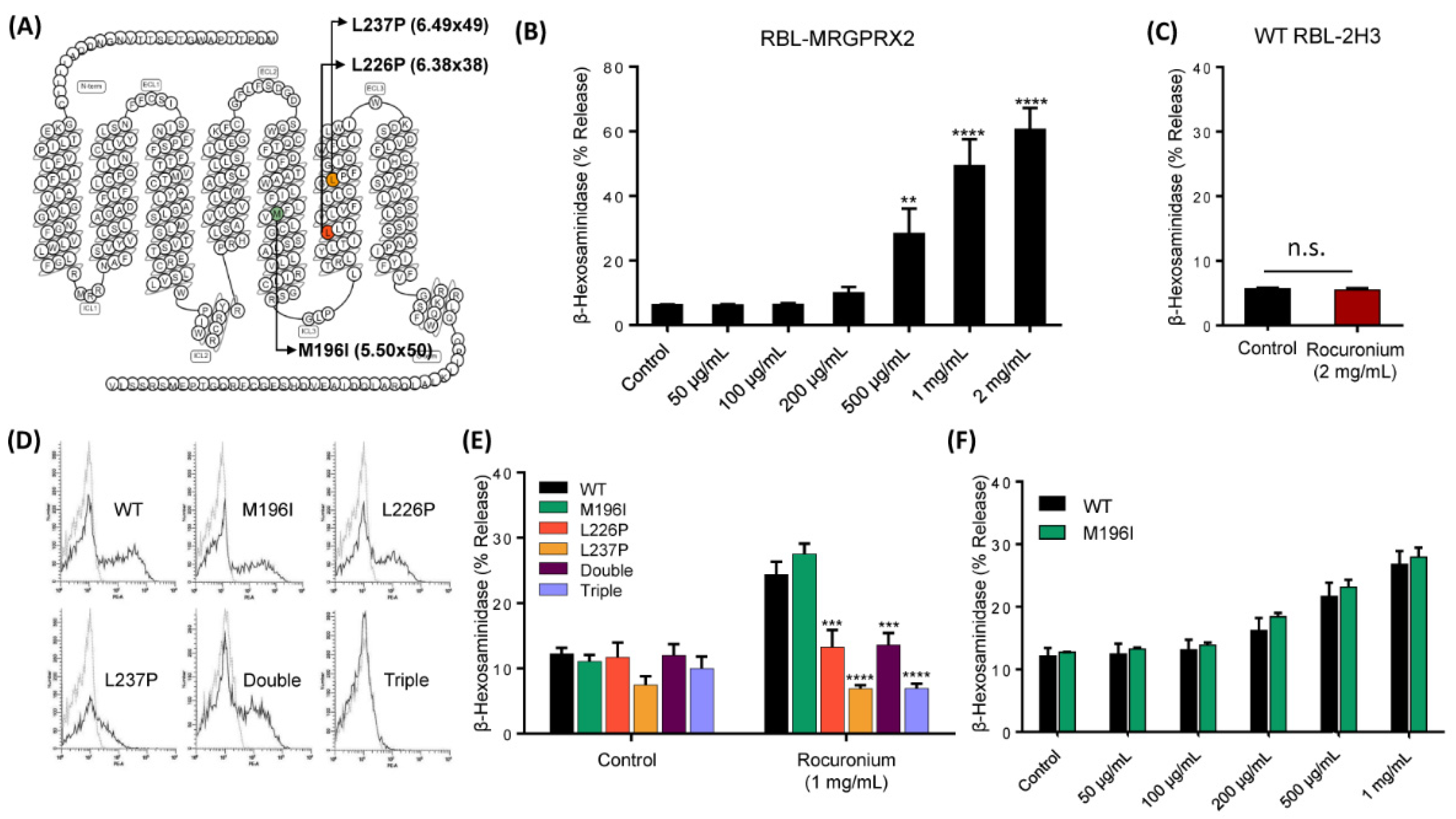
3.3. MRGPRX2 Variants Presented in a Patient with POH to Rocuronium Do Not Display Gain-of-Function Phenotype for Rocuronium-Induced MC Degranulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volcheck, G.W.; Hepner, D.L. Identification and Management of Perioperative Anaphylaxis. J. Allergy Clin. Immunol. Pract. 2019, 7, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Van Gasse, A.L.; Elst, J.; Bridts, C.H.; Mertens, C.; Faber, M.; Hagendorens, M.M.; De Clerck, L.S.; Sabato, V.; Ebo, D.G. Rocuronium Hypersensitivity: Does Off-Target Occupation of the MRGPRX2 Receptor Play a Role? J. Allergy Clin. Immunol. Pract. 2019, 7, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Spoerl, D.; D’Incau, S.; Roux-Lombard, P.; Harr, T.; Czarnetzki, C. Non-IgE-Dependent Hypersensitivity to Rocuronium Reversed by Sugammadex: Report of Three Cases and Hypothesis on the Underlying Mechanism. Int. Arch. Allergy Immunol. 2016, 169, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Liu, S.; Kadoya, F.; Takasaki, Y.; Yorozuya, T.; Mogi, M. Association between mutated Mas-related G protein-coupled receptor-X2 and rocuronium-induced intraoperative anaphylaxis. Br. J. Anaesth. 2020. [Google Scholar] [CrossRef] [PubMed]
- Spoerl, D.; Nigolian, H.; Czarnetzki, C.; Harr, T. Reclassifying Anaphylaxis to Neuromuscular Blocking Agents Based on the Presumed Patho-Mechanism: IgE-Mediated, Pharmacological Adverse Reaction or “Innate Hypersensitivity”? Int. J. Mol. Sci. 2017, 18, 1223. [Google Scholar] [CrossRef] [PubMed]
- Elst, J.; Sabato, V.; Faber, M.A.; Bridts, C.H.; Mertens, C.; Van Houdt, M.; Van Gasse, A.L.; Hagendorens, M.M.; Van Tendeloo, V.; Maurer, M.; et al. MRGPRX2 and Immediate Drug Hypersensitivity: Insights from Cultured Human Mast Cells. J. Investig. Allergol. Clin. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fernandopulle, N.A.; Zhang, S.S.; Soeding, P.F.; Mackay, G.A. MRGPRX2 activation in mast cells by neuromuscular blocking agents and other agonists: Modulation by sugammadex. Clin. Exp. Allergy 2020. [Google Scholar] [CrossRef]
- Navinés-Ferrer, A.; Serrano-Candelas, E.; Lafuente, A.; Muñoz-Cano, R.; Martín, M.; Gastaminza, G. MRGPRX2-mediated mast cell response to drugs used in perioperative procedures and anaesthesia. Sci. Rep. 2018, 8, 11628. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef]
- Alkanfari, I.; Freeman, K.B.; Roy, S.; Jahan, T.; Scott, R.W.; Ali, H. Small-Molecule Host-Defense Peptide Mimetic Antibacterial and Antifungal Agents Activate Human and Mouse Mast Cells via Mas-Related GPCRs. Cells 2019, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Kirshenbaum, A.S.; Akin, C.; Wu, Y.; Rottem, M.; Goff, J.P.; Beaven, M.A.; Rao, V.K.; Metcalfe, D.D. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk. Res. 2003, 27, 677–682. [Google Scholar] [CrossRef]
- Ali, H.; Richardson, R.M.; Tomhave, E.D.; DuBose, R.A.; Haribabu, B.; Snyderman, R. Regulation of stably transfected platelet activating factor receptor in RBL-2H3 cells. Role of multiple G proteins and receptor phosphorylation. J. Biol. Chem. 1994, 269, 24557–24563. [Google Scholar] [PubMed]
- Subramanian, H.; Kashem, S.W.; Collington, S.J.; Qu, H.; Lambris, J.D.; Ali, H. PMX-53 as a dual CD88 antagonist and an agonist for Mas-related gene 2 (MrgX2) in human mast cells. Mol. Pharmacol. 2011, 79, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Gupta, K.; Guo, Q.; Price, R.; Ali, H. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: Resistance to receptor phosphorylation, desensitization, and internalization. J. Biol. Chem. 2011, 286, 44739–44749. [Google Scholar] [CrossRef] [PubMed]
- Kambe, N.; Kambe, M.; Kochan, J.P.; Schwartz, L.B. Human skin-derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes. Blood 2001, 97, 2045–2052. [Google Scholar] [CrossRef]
- Roy, S.; Gupta, K.; Ganguly, A.; Ali, H. β-Arrestin2 expressed in mast cells regulates ciprofloxacin-induced pseudoallergy and IgE-mediated anaphylaxis. J. Allergy Clin. Immunol. 2019, 144, 603–606. [Google Scholar] [CrossRef]
- Malbec, O.; Roget, K.; Schiffer, C.; Iannascoli, B.; Dumas, A.R.; Arock, M.; Daëron, M. Peritoneal cell-derived mast cells: An in vitro model of mature serosal-type mouse mast cells. J. Immunol. 2007, 178, 6465–6475. [Google Scholar] [CrossRef]
- Babina, M.; Wang, Z.; Roy, S.; Guhl, S.; Franke, K.; Artuc, M.; Ali, H.; Zuberbier, T. MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through β-Arrestin and Lack of Correlation with the FcεRI Pathway. J. Investig. Dermatol. 2020. [Google Scholar] [CrossRef]
- Alkanfari, I.; Gupta, K.; Jahan, T.; Ali, H. Naturally Occurring Missense MRGPRX2 Variants Display Loss of Function Phenotype for Mast Cell Degranulation in Response to Substance P, Hemokinin-1, Human β-Defensin-3, and Icatibant. J. Immunol. 2018, 201, 343–349. [Google Scholar] [CrossRef]
- Chompunud Na Ayudhya, C.; Roy, S.; Alkanfari, I.; Ganguly, A.; Ali, H. Identification of Gain and Loss of Function Missense Variants in MRGPRX2’s Transmembrane and Intracellular Domains for Mast Cell Activation by Substance P. Int. J. Mol. Sci. 2019, 20, 5247. [Google Scholar] [CrossRef] [PubMed]
- Elst, J.; Sabato, V.; Mertens, C.; Garvey, L.H.; Ebo, D.G. Association between mutated Mas-related G protein-coupled receptor-X2 and rocuronium-induced intraoperative anaphylaxis. Comment on Br J Anaesth 2020, 125, e446–e448. Br. J. Anaesth. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Van der Poorten, M.L.; Elst, J.; Van Gasse, A.L.; Mertens, C.; Bridts, C.; Garvey, L.H.; Horiuchi, T.; Sabato, V. Immunoglobulin E cross-linking or MRGPRX2 activation: Clinical insights from rocuronium hypersensitivity. Br. J. Anaesth. 2020. [Google Scholar] [CrossRef]
- Pándy-Szekeres, G.; Munk, C.; Tsonkov, T.M.; Mordalski, S.; Harpsøe, K.; Hauser, A.S.; Bojarski, A.J.; Gloriam, D.E. GPCRdb in 2018: Adding GPCR structure models and ligands. Nucleic Acids Res. 2018, 46, D440–D446. [Google Scholar] [CrossRef]
- Porebski, G.; Kwiecien, K.; Pawica, M.; Kwitniewski, M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front. Immunol. 2018, 9, 3027. [Google Scholar] [CrossRef]
- Lansu, K.; Karpiak, J.; Liu, J.; Huang, X.P.; McCorvy, J.D.; Kroeze, W.K.; Che, T.; Nagase, H.; Carroll, F.I.; Jin, J.; et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat. Chem. Biol. 2017, 13, 529–536. [Google Scholar] [CrossRef]
- Reddy, V.B.; Graham, T.A.; Azimi, E.; Lerner, E.A. A single amino acid in MRGPRX2 necessary for binding and activation by pruritogens. J. Allergy Clin. Immunol. 2017, 140, 1726–1728. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chompunud Na Ayudhya, C.; Amponnawarat, A.; Roy, S.; Oskeritzian, C.A.; Ali, H. MRGPRX2 Activation by Rocuronium: Insights from Studies with Human Skin Mast Cells and Missense Variants. Cells 2021, 10, 156. https://doi.org/10.3390/cells10010156
Chompunud Na Ayudhya C, Amponnawarat A, Roy S, Oskeritzian CA, Ali H. MRGPRX2 Activation by Rocuronium: Insights from Studies with Human Skin Mast Cells and Missense Variants. Cells. 2021; 10(1):156. https://doi.org/10.3390/cells10010156
Chicago/Turabian StyleChompunud Na Ayudhya, Chalatip, Aetas Amponnawarat, Saptarshi Roy, Carole A. Oskeritzian, and Hydar Ali. 2021. "MRGPRX2 Activation by Rocuronium: Insights from Studies with Human Skin Mast Cells and Missense Variants" Cells 10, no. 1: 156. https://doi.org/10.3390/cells10010156
APA StyleChompunud Na Ayudhya, C., Amponnawarat, A., Roy, S., Oskeritzian, C. A., & Ali, H. (2021). MRGPRX2 Activation by Rocuronium: Insights from Studies with Human Skin Mast Cells and Missense Variants. Cells, 10(1), 156. https://doi.org/10.3390/cells10010156




