Exercise Ameliorates Spinal Cord Injury by Changing DNA Methylation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Surgical Procedures
2.2. Treadmill Exercise
2.3. Histology
2.4. Immunohistochemistry (IHC)
2.5. DNA Dot Blot Assay
2.6. Real-Time PCR
2.7. Functional Assessments
2.8. Statistics
3. Results
3.1. Treadmill Exercise Reduces the Lesion Cavity and Inflammatory Response after SCI
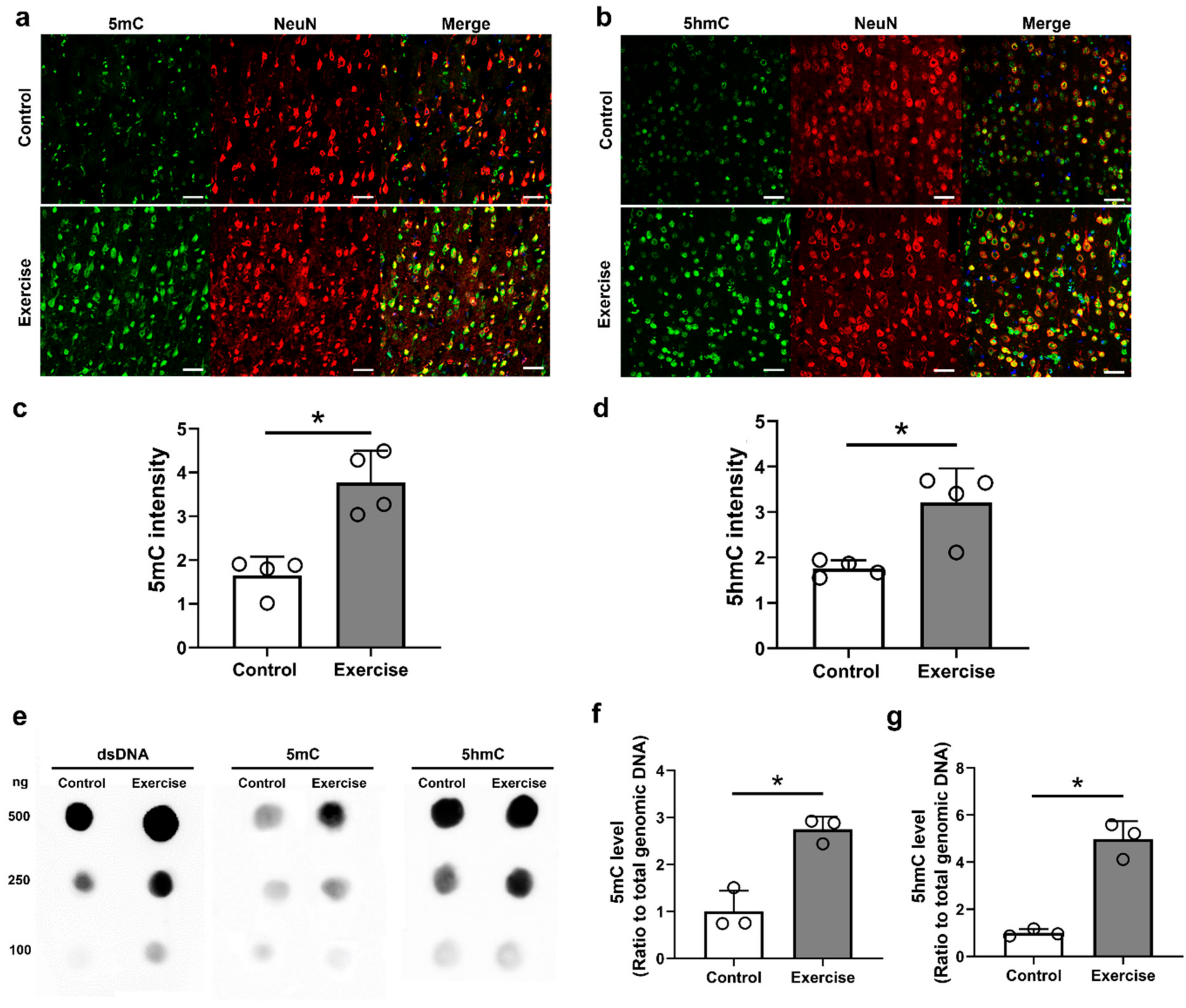
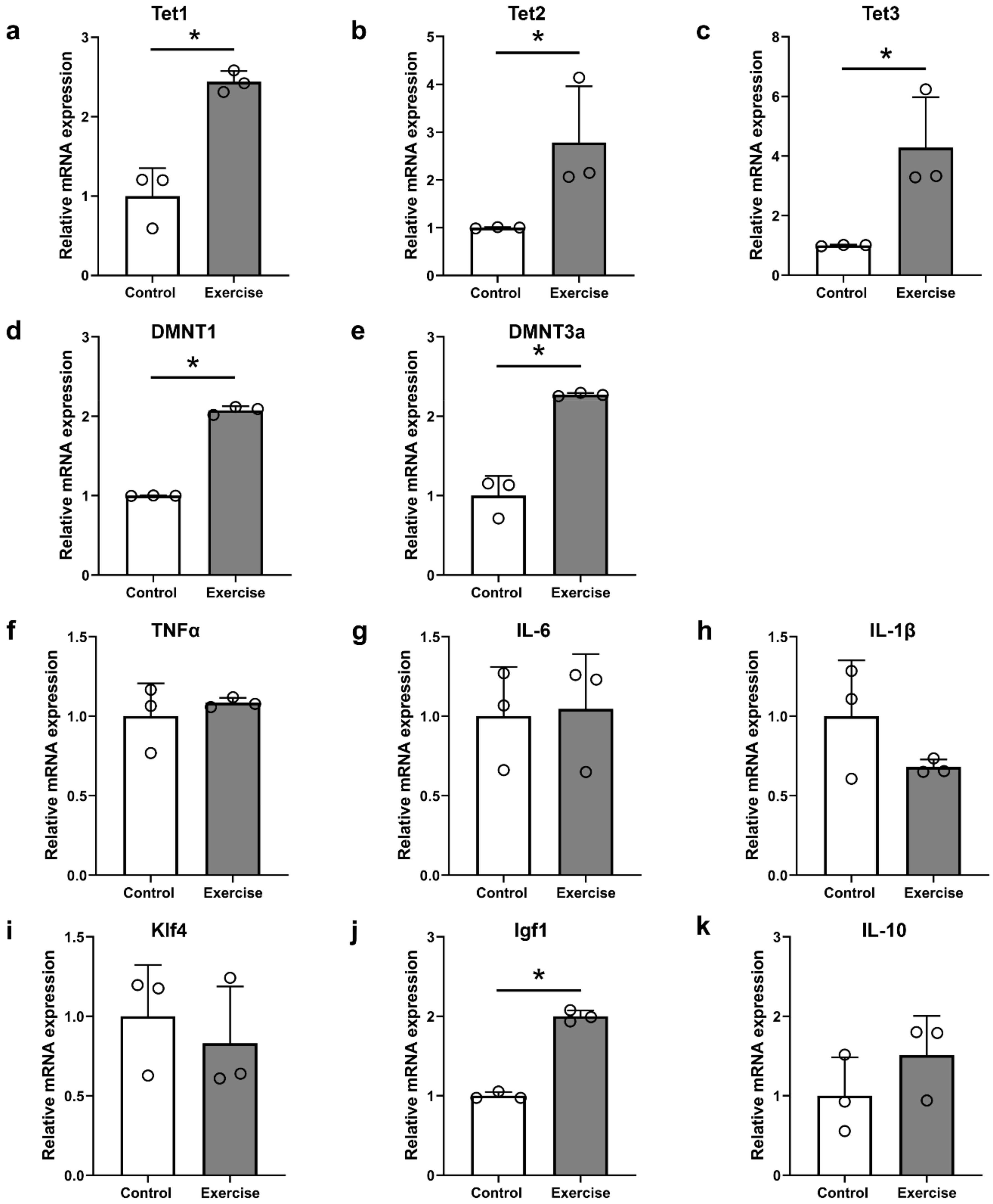
3.2. Treadmill Exercise Modulates DNA Methylation and Hydroxymethylation in the Brain Motor Cortex after SCI
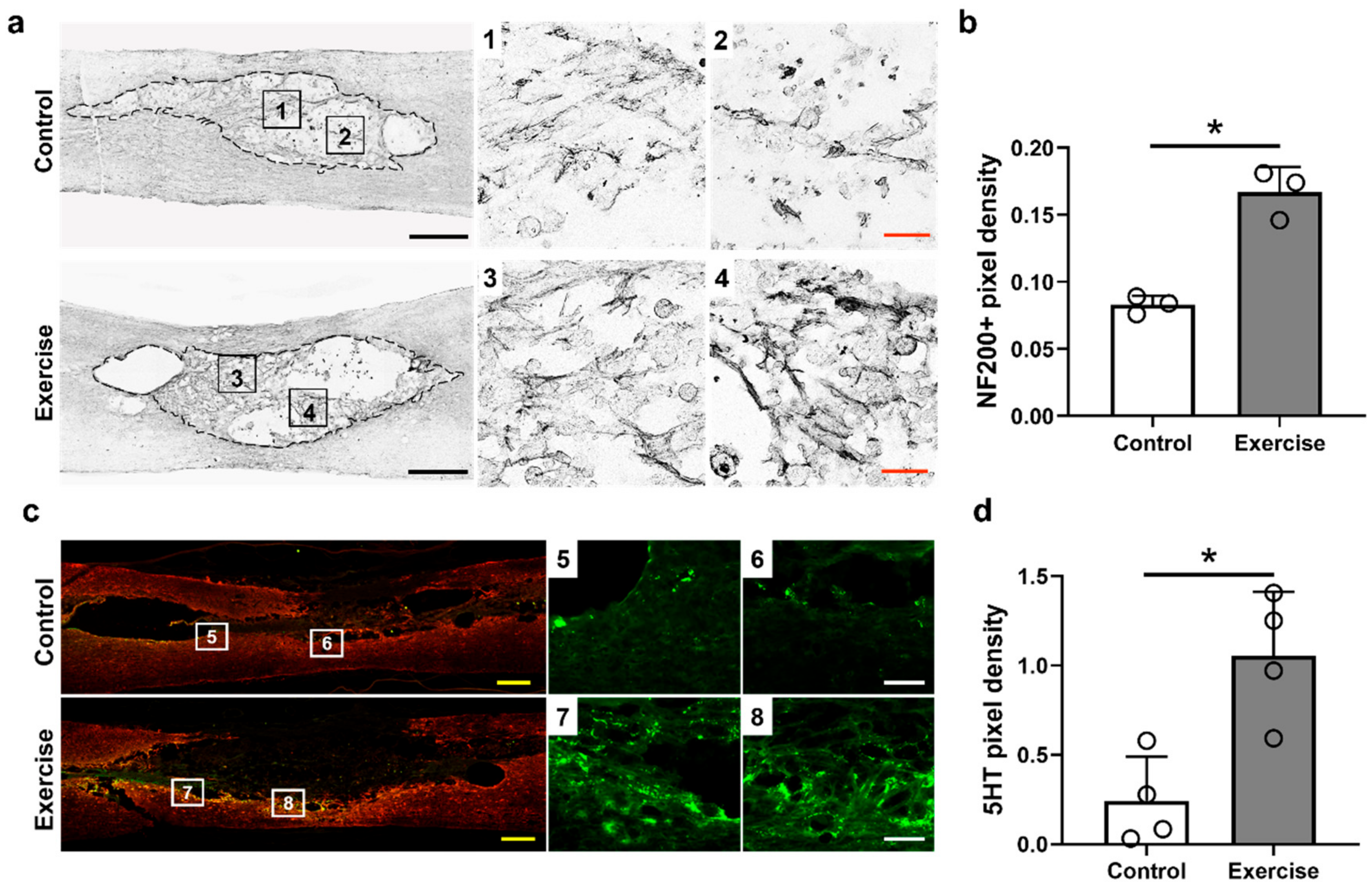
3.3. Treadmill Exercise Promotes Axonal Regeneration and Sprouting, but Do Not Affect Macrophage Polarization in the Lesion Cavity after SCI
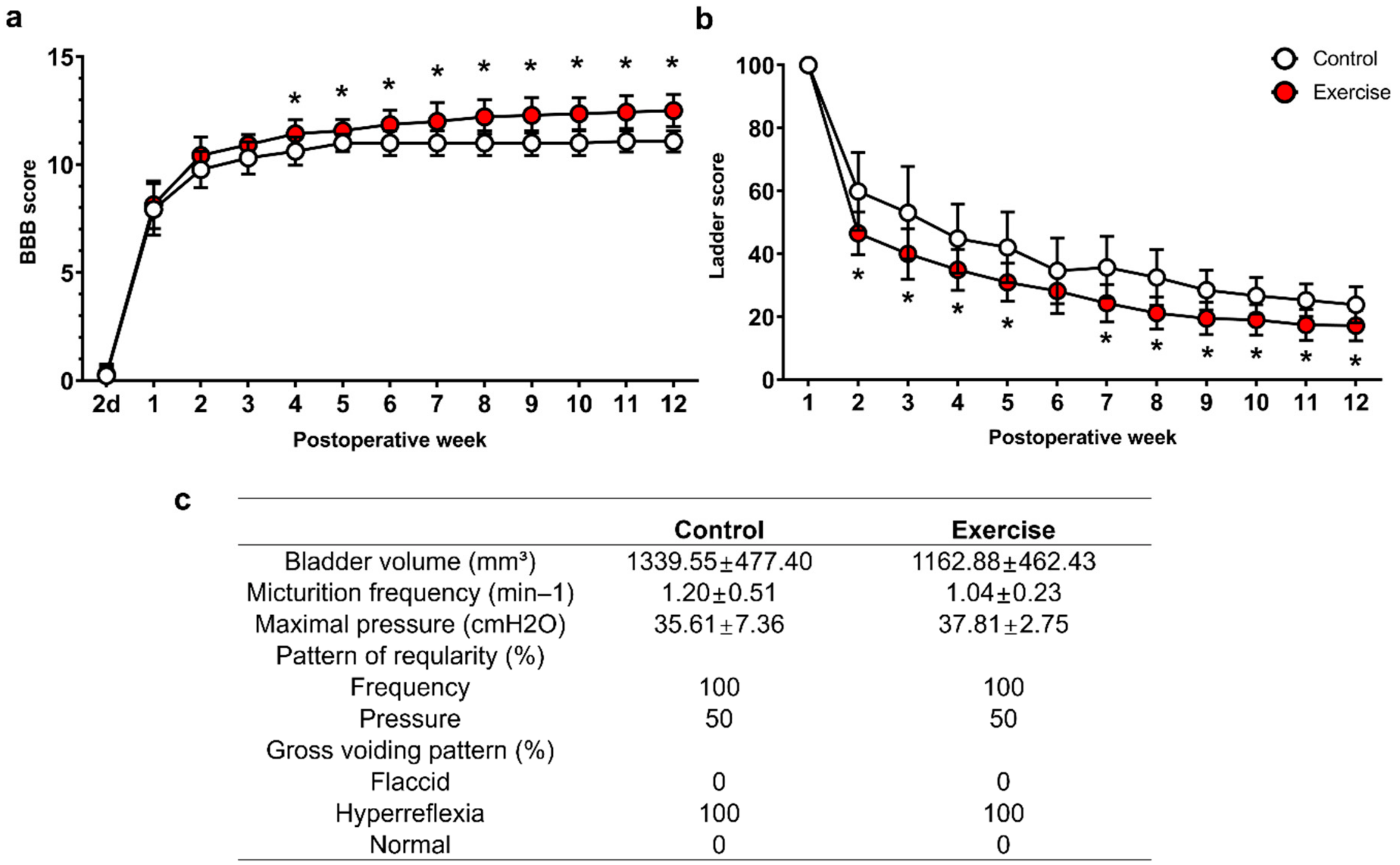
3.4. Treadmill Exercise Promotes Functional Improvement, but Do Not Modulate Neurogenic Bladder after SCI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barriere, G.; Leblond, H.; Provencher, J.; Rossignol, S. Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J. Neurosci. 2008, 28, 3976–3987. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Delivet-Mongrain, H.; Leblond, H.; Rossignol, S. Recovery of hindlimb locomotion after incomplete spinal cord injury in the cat involves spontaneous compensatory changes within the spinal locomotor circuitry. J. Neurophysiol. 2011, 106, 1969–1984. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.J.; Seo, T.B.; Kwon, K.B.; Yoon, S.J.; Elzi, D.J.; Kim, B.G.; Namgung, U. Axonal outgrowth and Erk1/2 activation by training after spinal cord injury in rats. J. Neurotrauma 2009, 26, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Keeler, B.E.; Liu, G.; Siegfried, R.N.; Zhukareva, V.; Murray, M.; Houle, J.D. Acute and prolonged hindlimb exercise elicits different gene expression in motoneurons than sensory neurons after spinal cord injury. Brain Res. 2012, 1438, 8–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, H.; Liu, N.K.; Zhang, Y.P.; Deng, L.; Lu, Q.B.; Shields, C.B.; Walker, M.J.; Li, J.; Xu, X.M. Treadmill training induced lumbar motoneuron dendritic plasticity and behavior recovery in adult rats after a thoracic contusive spinal cord injury. Exp. Neurol. 2015, 271, 368–378. [Google Scholar] [CrossRef]
- Ilha, J.; Meireles, A.; de Freitas, G.R.; do Espirito Santo, C.C.; Machado-Pereira, N.; Swarowsky, A.; Santos, A.R.S. Overground gait training promotes functional recovery and cortical neuroplasticity in an incomplete spinal cord injury model. Life Sci. 2019, 232, 116627. [Google Scholar] [CrossRef]
- Liu, G.; Detloff, M.R.; Miller, K.N.; Santi, L.; Houle, J.D. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Exp. Neurol. 2012, 233, 447–456. [Google Scholar] [CrossRef]
- Wahane, S.; Halawani, D.; Zhou, X.; Zou, H. Epigenetic Regulation of Axon Regeneration and Glial Activation in Injury Responses. Front Genet. 2019, 10, 640. [Google Scholar] [CrossRef]
- Hutson, T.H.; Kathe, C.; Palmisano, I.; Bartholdi, K.; Hervera, A.; De Virgiliis, F.; McLachlan, E.; Zhou, L.; Kong, G.; Barraud, Q.; et al. Cbp-dependent histone acetylation mediates axon regeneration induced by environmental enrichment in rodent spinal cord injury models. Sci. Transl. Med. 2019, 11, eaaw2064. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhou, Y.; Ye, L.; Lu, Q.; Zhang, K.; Zhang, J.; Xie, L.; Wu, Y.; Xu, K.; Zhang, H.; et al. Histone deacetylase 6 inhibition restores autophagic flux to promote functional recovery after spinal cord injury. Exp. Neurol. 2020, 324, 113138. [Google Scholar] [CrossRef]
- Hong, J.Y.; Davaa, G.; Yoo, H.; Hong, K.; Hyun, J.K. Ascorbic Acid Promotes Functional Restoration after Spinal Cord Injury Partly by Epigenetic Modulation. Cells 2020, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Hargreaves, M. Epigenetics and Exercise. Trends Endocrinol. Metab. 2019, 30, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Widmann, M.; Niess, A.M.; Munz, B. Physical Exercise and Epigenetic Modifications in Skeletal Muscle. Sports Med. 2019, 49, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Jacques, M.; Hiam, D.; Craig, J.; Barres, R.; Eynon, N.; Voisin, S. Epigenetic changes in healthy human skeletal muscle following exercise- a systematic review. Epigenetics 2019, 14, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Lovatel, G.A.; Elsner, V.R.; Bertoldi, K.; Vanzella, C.; Moyses Fdos, S.; Vizuete, A.; Spindler, C.; Cechinel, L.R.; Netto, C.A.; Muotri, A.R.; et al. Treadmill exercise induces age-related changes in aversive memory, neuroinflammatory and epigenetic processes in the rat hippocampus. Neurobiol. Learn. Mem. 2013, 101, 94–102. [Google Scholar] [CrossRef]
- Spindler, C.; Cechinel, L.R.; Basso, C.; Moyses, F.; Bertoldi, K.; Roesler, R.; Lovatel, G.A.; Rostirola Elsner, V.; Siqueira, I.R. Treadmill exercise alters histone acetyltransferases and histone deacetylases activities in frontal cortices from wistar rats. Cell. Mol. Neurobiol. 2014, 34, 1097–1101. [Google Scholar] [CrossRef]
- Jessop, P.; Toledo-Rodriguez, M. Hippocampal TET1 and TET2 Expression and DNA Hydroxymethylation are Affected by Physical Exercise in Aged Mice. Front. Cell Dev. Biol. 2018, 6, 45. [Google Scholar] [CrossRef]
- Sandrow-Feinberg, H.R.; Houle, J.D. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 2015, 1619, 12–21. [Google Scholar] [CrossRef]
- Massoto, T.B.; Santos, A.C.R.; Ramalho, B.S.; Almeida, F.M.; Martinez, A.M.B.; Marques, S.A. Mesenchymal stem cells and treadmill training enhance function and promote tissue preservation after spinal cord injury. Brain Res. 2020, 1726, 146494. [Google Scholar] [CrossRef]
- Tashiro, S.; Nishimura, S.; Iwai, H.; Sugai, K.; Zhang, L.; Shinozaki, M.; Iwanami, A.; Toyama, Y.; Liu, M.; Okano, H.; et al. Functional Recovery from Neural Stem/Progenitor Cell Transplantation Combined with Treadmill Training in Mice with Chronic Spinal Cord Injury. Sci. Rep. 2016, 6, 30898. [Google Scholar] [CrossRef]
- Ganzer, P.D.; Beringer, C.R.; Shumsky, J.S.; Nwaobasi, C.; Moxon, K.A. Serotonin receptor and dendritic plasticity in the spinal cord mediated by chronic serotonergic pharmacotherapy combined with exercise following complete SCI in the adult rat. Exp. Neurol. 2018, 304, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Foffani, G.; Shumsky, J.; Knudsen, E.B.; Ganzer, P.D.; Moxon, K.A. Interactive Effects Between Exercise and Serotonergic Pharmacotherapy on Cortical Reorganization After Spinal Cord Injury. Neurorehabil. Neural Repair 2016, 30, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Ajiki, T.; Inoue, H.; Kikuchi, M.; Yashiro, T.; Nakama, S.; Hoshino, Y.; Murakami, T.; Kobayashi, E. Early exercise in spinal cord injured rats induces allodynia through TrkB signaling. Biochem. Biophys. Res. Commun. 2009, 381, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.R.; Brown, E.H.; Shum-Siu, A.; Whelan, A.; Burke, D.A.; Benton, R.L.; Magnuson, D.S. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J. Neurotrauma 2009, 26, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Lee, S.H.; Lee, S.C.; Kim, J.W.; Kim, K.P.; Kim, S.M.; Tapia, N.; Lim, K.T.; Kim, J.; Ahn, H.S.; et al. Therapeutic potential of induced neural stem cells for spinal cord injury. J. Biol. Chem. 2014, 289, 32512–32525. [Google Scholar] [CrossRef]
- Hwang, D.H.; Park, H.H.; Shin, H.Y.; Cui, Y.; Kim, B.G. Insulin-like Growth Factor-1 Receptor Dictates Beneficial Effects of Treadmill Training by Regulating Survival and Migration of Neural Stem Cell Grafts in the Injured Spinal Cord. Exp. Neurobiol. 2018, 27, 489–507. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, B.; Liu, C.; Zhao, D. Molecular mechanisms underlying the positive role of treadmill training in locomotor recovery after spinal cord injury. Spinal Cord 2017, 55, 441–446. [Google Scholar] [CrossRef]
- Kim, J.W.; Mahapatra, C.; Hong, J.Y.; Kim, M.S.; Leong, K.W.; Kim, H.W.; Hyun, J.K. Functional Recovery of Contused Spinal Cord in Rat with the Injection of Optimal-Dosed Cerium Oxide Nanoparticles. Adv. Sci. (Weinh) 2017, 4, 1700034. [Google Scholar] [CrossRef]
- Hong, J.Y.; Seo, Y.; Davaa, G.; Kim, H.W.; Kim, S.H.; Hyun, J.K. Decellularized brain matrix enhances macrophage polarization and functional improvements in rat spinal cord injury. Acta Biomater. 2020, 101, 357–371. [Google Scholar] [CrossRef]
- DePaul, M.A.; Lin, C.Y.; Silver, J.; Lee, Y.S. Combinatory repair strategy to promote axon regeneration and functional recovery after chronic spinal cord injury. Sci. Rep. 2017, 7, 9018. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996, 139, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Fouad, K.; Klusman, I.; Schwab, M.E. Regenerating corticospinal fibers in the Marmoset (Callitrix jacchus) after spinal cord lesion and treatment with the anti-Nogo-A antibody IN-1. Eur. J. Neurosci. 2004, 20, 2479–2482. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.K.; Lee, Y.I.; Son, Y.J.; Park, J.S. Serial changes in bladder, locomotion, and levels of neurotrophic factors in rats with spinal cord contusion. J. Neurotrauma 2009, 26, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Park, W.B.; Kim, S.Y.; Lee, S.H.; Kim, H.W.; Park, J.S.; Hyun, J.K. The effect of mesenchymal stem cell transplantation on the recovery of bladder and hindlimb function after spinal cord contusion in rats. BMC Neurosci. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Veron, N.; Peters, A.H. Epigenetics: Tet proteins in the limelight. Nature 2011, 473, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Carey, N.; Marques, C.J.; Reik, W. DNA demethylases: A new epigenetic frontier in drug discovery. Drug Discov. Today 2011, 16, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Arida, R.M.; Gomez-Pinilla, F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 2017, 80, 443–456. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Li, S.; Wang, X.; Wilson, J.X.; Huang, G. Periconceptional Folic Acid Supplementation Benefit to Development of Early Sensory-Motor Function through Increase DNA Methylation in Rat Offspring. Nutrients 2018, 10, 292. [Google Scholar] [CrossRef]
- Madrid, A.; Borth, L.E.; Hogan, K.J.; Hariharan, N.; Papale, L.A.; Alisch, R.S.; Iskandar, B.J. DNA methylation and hydroxymethylation have distinct genome-wide profiles related to axonal regeneration. Epigenetics 2020, 1–15. [Google Scholar] [CrossRef]
- Bretzner, F.; Drew, T. Contribution of the motor cortex to the structure and the timing of hindlimb locomotion in the cat: A microstimulation study. J. Neurophysiol. 2005, 94, 657–672. [Google Scholar] [CrossRef]
- DiGiovanna, J.; Dominici, N.; Friedli, L.; Rigosa, J.; Duis, S.; Kreider, J.; Beauparlant, J.; van den Brand, R.; Schieppati, M.; Micera, S.; et al. Engagement of the Rat Hindlimb Motor Cortex across Natural Locomotor Behaviors. J. Neurosci. 2016, 36, 10440–10455. [Google Scholar] [CrossRef] [PubMed]
- Bonizzato, M.; Pidpruzhnykova, G.; DiGiovanna, J.; Shkorbatova, P.; Pavlova, N.; Micera, S.; Courtine, G. Brain-controlled modulation of spinal circuits improves recovery from spinal cord injury. Nat. Commun. 2018, 9, 3015. [Google Scholar] [CrossRef] [PubMed]
- Hollis, E.R., 2nd; Ishiko, N.; Yu, T.; Lu, C.C.; Haimovich, A.; Tolentino, K.; Richman, A.; Tury, A.; Wang, S.H.; Pessian, M.; et al. Ryk controls remapping of motor cortex during functional recovery after spinal cord injury. Nat. Neurosci. 2016, 19, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Lindner, R.; Puttagunta, R.; Di Giovanni, S. Epigenetic regulation of axon outgrowth and regeneration in CNS injury: The first steps forward. Neurotherapeutics 2013, 10, 771–781. [Google Scholar] [CrossRef]
- Sun, H.; Miao, Z.; Wang, H.; Tao, Y.; Yang, J.; Cai, J.; Wang, J.; Wang, Y. DNA hydroxymethylation mediated traumatic spinal injury by influencing cell death–related gene expression. J. Cell. Biochem. 2018, 119, 9295–9302. [Google Scholar] [CrossRef]
- Chhaya, S.J.; Quiros-Molina, D.; Tamashiro-Orrego, A.D.; Houle, J.D.; Detloff, M.R. Exercise-Induced Changes to the Macrophage Response in the Dorsal Root Ganglia Prevent Neuropathic Pain after Spinal Cord Injury. J. Neurotrauma 2019, 36, 877–890. [Google Scholar] [CrossRef]
- Goldshmit, Y.; Lythgo, N.; Galea, M.P.; Turnley, A.M. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J. Neurotrauma 2008, 25, 449–465. [Google Scholar] [CrossRef]
- Field-Fote, E.C.; Roach, K.E. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: A randomized clinical trial. Phys. Ther. 2011, 91, 48–60. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Ding, J.; Wang, S.; Dong, C.; Wu, Q. Exercise training modulates glutamic acid decarboxylase-65/67 expression through TrkB signaling to ameliorate neuropathic pain in rats with spinal cord injury. Mol. Pain 2020, 16, 1744806920924511. [Google Scholar] [CrossRef]
- Rank, M.M.; Flynn, J.R.; Battistuzzo, C.R.; Galea, M.P.; Callister, R.; Callister, R.J. Functional changes in deep dorsal horn interneurons following spinal cord injury are enhanced with different durations of exercise training. J. Physiol. 2015, 593, 331–345. [Google Scholar] [CrossRef]
- Tognini, P.; Napoli, D.; Pizzorusso, T. Dynamic DNA methylation in the brain: A new epigenetic mark for experience-dependent plasticity. Front. Cell Neurosci. 2015, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Munoz, P.; Estay, C.; Diaz, P.; Elgueta, C.; Ardiles, A.O.; Lizana, P.A. Inhibition of DNA Methylation Impairs Synaptic Plasticity during an Early Time Window in Rats. Neural Plast. 2016, 2016, 4783836. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.E.; Noristani, H.N. Serotonergic mechanisms in spinal cord injury. Exp. Neurol. 2019, 318, 174–191. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.T.; Cregg, J.M.; DePaul, M.A.; Tran, A.P.; Xu, K.; Dyck, S.M.; Madalena, K.M.; Brown, B.P.; Weng, Y.L.; Li, S.; et al. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature 2015, 518, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Zai, L.; Liang, P.; Schaffling, C.; Ahlborn, D.; Benowitz, L.I. Inosine enhances axon sprouting and motor recovery after spinal cord injury. PLoS ONE 2013, 8, e81948. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Joshi, M. Urodynamic patterns after traumatic spinal cord injury. J. Spinal Cord Med. 2015, 38, 128–133. [Google Scholar] [CrossRef]
- Hubscher, C.H.; Montgomery, L.R.; Fell, J.D.; Armstrong, J.E.; Poudyal, P.; Herrity, A.N.; Harkema, S.J. Effects of exercise training on urinary tract function after spinal cord injury. Am. J. Physiol. Renal Physiol. 2016, 310, F1258–F1268. [Google Scholar] [CrossRef]
- Zlatev, D.V.; Shem, K.; Elliott, C.S. How many spinal cord injury patients can catheterize their own bladder? The epidemiology of upper extremity function as it affects bladder management. Spinal Cord 2016, 54, 287–291. [Google Scholar] [CrossRef]
- Shin, H.Y.; Kim, H.; Kwon, M.J.; Hwang, D.H.; Lee, K.; Kim, B.G. Molecular and cellular changes in the lumbar spinal cord following thoracic injury: Regulation by treadmill locomotor training. PLoS ONE 2014, 9, e88215. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Lou, Y.; Xu, J.; Feng, Z.; Chen, Y.; Tang, Q.; Zheng, G.; Zhang, Z.; Wu, Y.; et al. Salidroside attenuates neuroinflammation and improves functional recovery after spinal cord injury through microglia polarization regulation. J. Cell. Mol. Med. 2018, 22, 1148–1166. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kim, D.Y.; Yune, T.Y.; Shin, D.H.; Baek, S.B.; Kim, C.J. Treadmill exercise reduces spinal cord injury-induced apoptosis by activating the PI3K/Akt pathway in rats. Exp. Ther. Med. 2014, 7, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Li, W.; Zhang, Q.; Li, Y.; Zhang, Z.; Zhu, J.; Chen, B.; Williams, P.R.; Zhang, Y.; et al. A Sensitized IGF1 Treatment Restores Corticospinal Axon-Dependent Functions. Neuron 2017, 95, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, C.; Zhou, X.; Wang, J.; Xia, K.; Yang, B.; Gong, Z.; Ying, L.; Yu, C.; Shi, K.; et al. Overexpression of the transcription factors OCT4 and KLF4 improves motor function after spinal cord injury. CNS Neurosci. 2020, 26, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.L.; Blackmore, M.G.; Hu, Y.; Kaestner, K.H.; Bixby, J.L.; Lemmon, V.P.; Goldberg, J.L. KLF family members regulate intrinsic axon regeneration ability. Science 2009, 326, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Liu, Z.; Zhang, J.; Xiang, Z.; Sun, T. Honokiol downregulates Kruppel-like factor 4 expression, attenuates inflammation, and reduces histopathology after spinal cord injury in rats. Spine 2015, 40, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Qin, X.Z.; Zhang, H.N.; Ma, Y.X.; Qi, S.B.; Zhang, H.C.; Ma, J.J.; Fu, X.Y.; Xie, J.L.; Saijilafu, S. Deletion of Kruppel-like factor-4 promotes axonal regeneration in mammals. Neural Regen Res. [CrossRef]




| Gene | Forward (5′-3′) | Reverse |
|---|---|---|
| Tet1 | GGCTTGCAGACACTGATGAA | GAAACACAGTCGCCTCTTCC |
| Tet2 | GGGGTTGGAGCAAGTACAAA | CGGGTGTGTGTCATTTGAAG |
| Tet3 | AGTGGGTGATCCGAAGACAC | GCCAGGATCAAGATGACGAT |
| DNMT1 | GTGTGCGGGAATGTGCTCGCT | CAGTGGTGGTGGCACAGCGT |
| DNMT3a | AGCAAAGTGAGGACCATTACCACCA | TGTGTAGTGGACAGGGAAGCCA |
| TNF-α | CTCAAGCCCTGGTATGAGCC | GGCTGGGTAGAGAACGGATG |
| IL-6 | ACCACCCACAACAGACCAGT | CAGAATTGCCATTGCACAAC |
| IL-1β | CACCTCTCAAGCAGAGCACAG | GGGTTCCATGGTGAAGTCAAC |
| Klf4 | CTTTCCTGCCAGACCAGATG | GGTTTCTCGCCTGTGTGAGT |
| Igf1 | CACACTGACATGCCCAAGAC | GGGAGGCTCCTCCTACATTC |
| IL-10 | CAGCTGCGACGCTGTCATCG | GCAGTCCAGTAGATGCCGGGT |
| 18 s rRNA | CACTGAGCATCTCCCTCACA | GAGGGTGCAGCGAACTTTAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davaa, G.; Hong, J.Y.; Kim, T.U.; Lee, S.J.; Kim, S.Y.; Hong, K.; Hyun, J.K. Exercise Ameliorates Spinal Cord Injury by Changing DNA Methylation. Cells 2021, 10, 143. https://doi.org/10.3390/cells10010143
Davaa G, Hong JY, Kim TU, Lee SJ, Kim SY, Hong K, Hyun JK. Exercise Ameliorates Spinal Cord Injury by Changing DNA Methylation. Cells. 2021; 10(1):143. https://doi.org/10.3390/cells10010143
Chicago/Turabian StyleDavaa, Ganchimeg, Jin Young Hong, Tae Uk Kim, Seong Jae Lee, Seo Young Kim, Kwonho Hong, and Jung Keun Hyun. 2021. "Exercise Ameliorates Spinal Cord Injury by Changing DNA Methylation" Cells 10, no. 1: 143. https://doi.org/10.3390/cells10010143
APA StyleDavaa, G., Hong, J. Y., Kim, T. U., Lee, S. J., Kim, S. Y., Hong, K., & Hyun, J. K. (2021). Exercise Ameliorates Spinal Cord Injury by Changing DNA Methylation. Cells, 10(1), 143. https://doi.org/10.3390/cells10010143





