Evaluation of Mobile Heat Treatment System for Treating In-Field HLB-Affected Trees by Analyzing Survival Rate of Surrogate Bacteria
Abstract
:1. Introduction
2. Materials and Methods
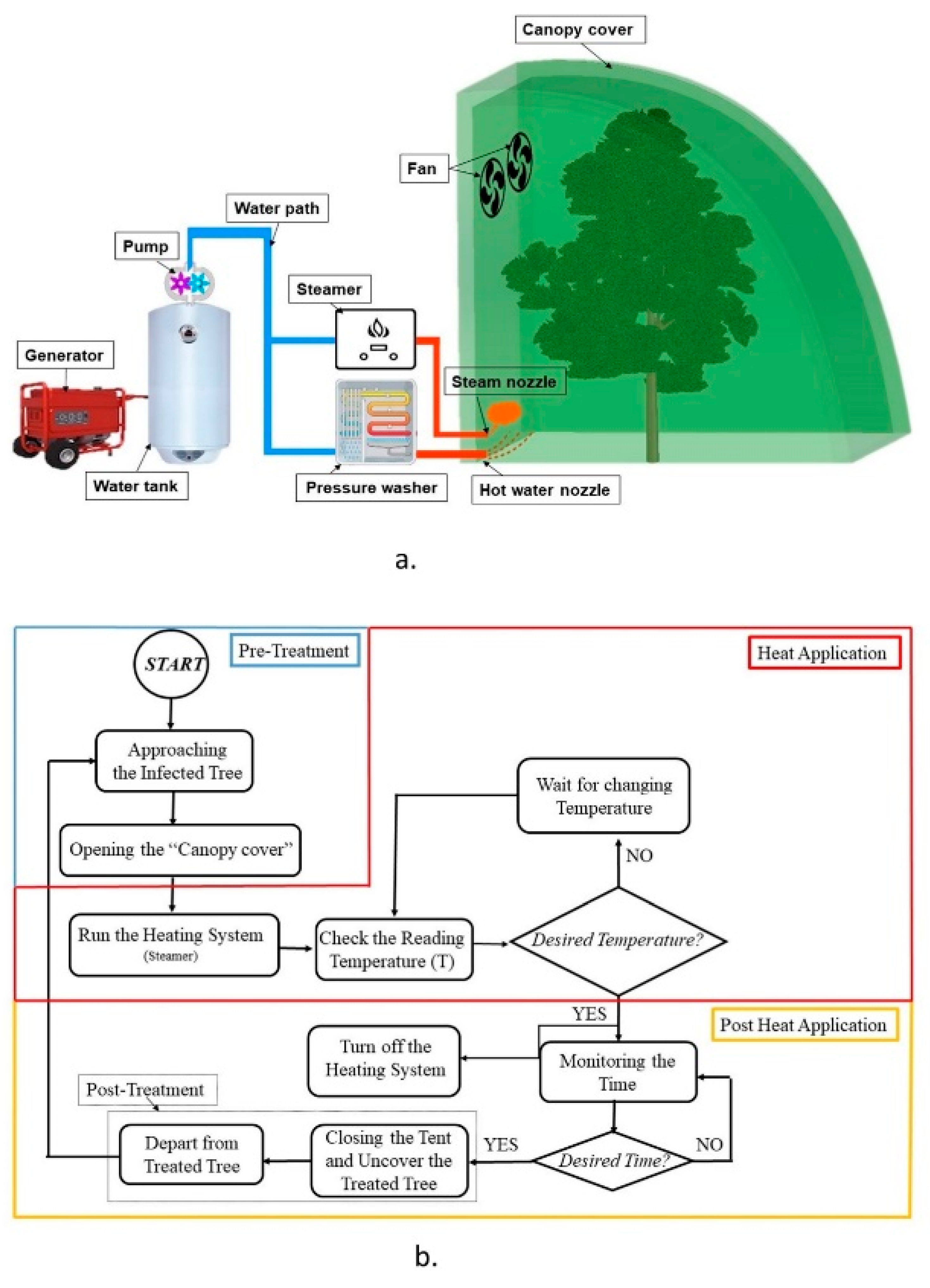
2.1. Mobile Heat Treatment System
2.2. Temperature Acquisition System
2.3. Biosensors
2.4. Experimental Design
3. Results and Discussion
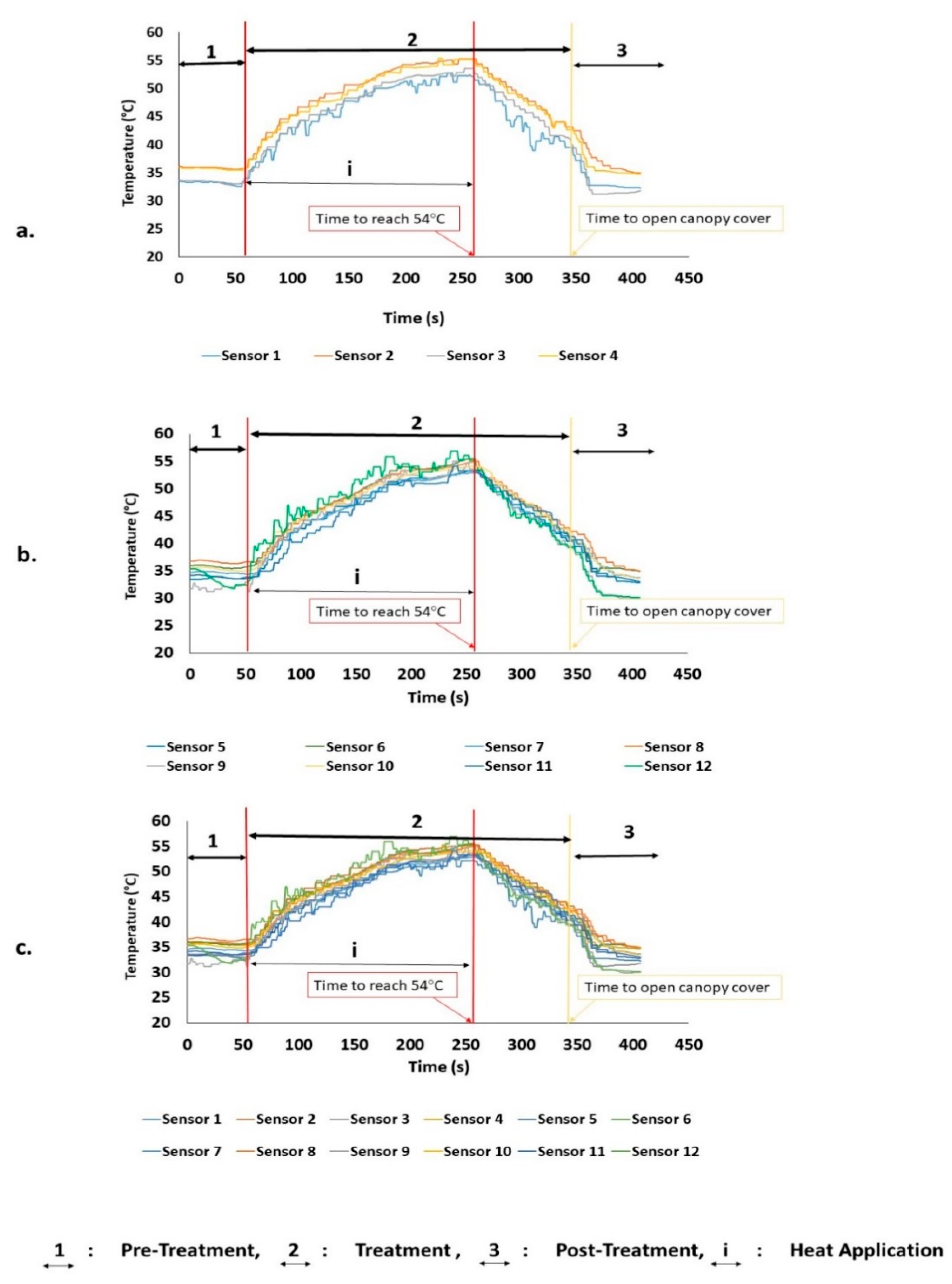
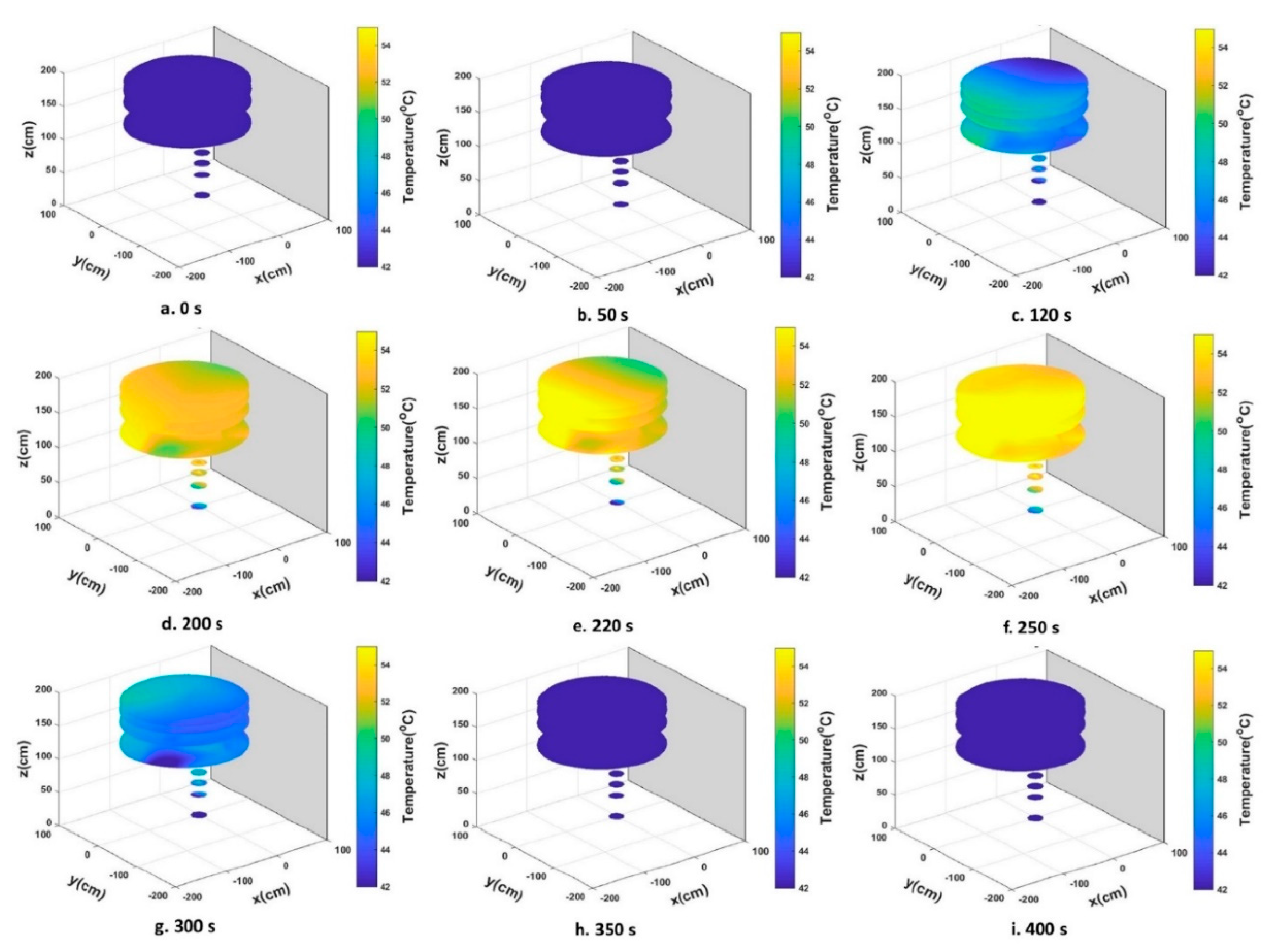
3.1. Heat Distribution and Uniformity During Treatment
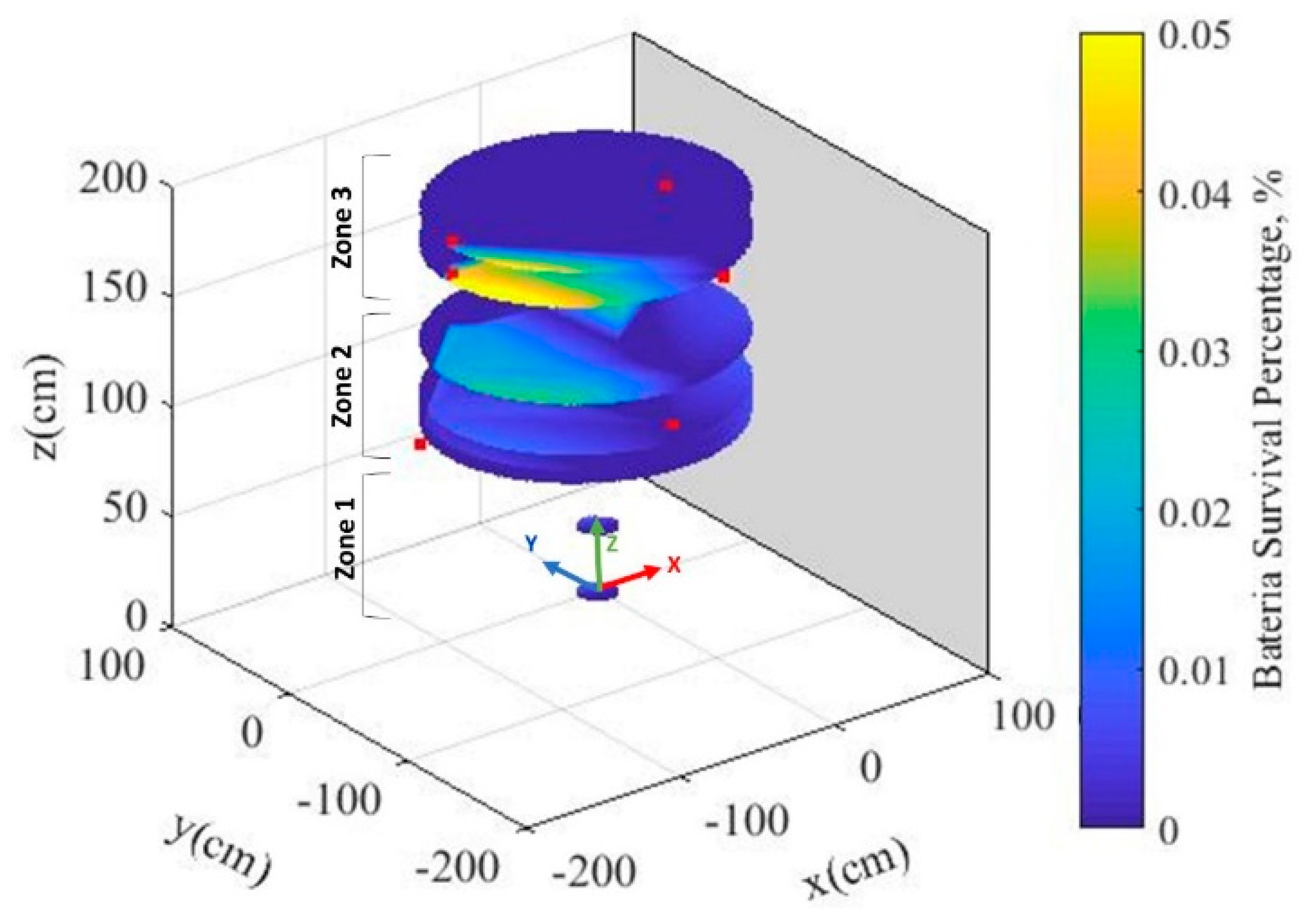
3.2. Biosensors Survival Rate after Heat Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grondeau, C.; Samson, R.; Sands, D.C. A Review of Thermotherapy to Free Plant Materials from Pathogens, Especially Seeds from Bacteria. Crit. Rev. Plant Sci. 1994, 13, 57–75. [Google Scholar] [CrossRef]
- Hoffman, M.T.; Doud, M.S.; Williams, L.; Zhang, M.-Q.; Ding, F.; Stover, E.; Hall, D.; Zhang, S.; Jones, L.; Gooch, M.; et al. Heat Treatment Eliminates ‘Candidatus Liberibacter asiaticus’ from Infected Citrus Trees Under Controlled Conditions. Am. Phytopathol. Soc. 2013, 103, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Sands, D.C.; Fourest, E.; Rehms, L.D. Dry heat seed treatment for Xanthomonas campestris pv. Translucens. In Proceedings of the 7th International Conference on Plant Pathogenic Bacteria, Budapest, Hungary, 11–16 June 1989; Klement, Z., Ed.; AkademiaiKiado: Budapest, Hungary, 1989. [Google Scholar]
- Kunkel, L.O. Heat treatment for the control of yellows and other virus diseases of peach. Phytopathology 1936, 26, 809–830. [Google Scholar]
- Grant, T.J. Heat treatments for obtaining sources of virus-free citrus budwood. Fla. State Hortic. Soc. 1957, 70, 51–53. [Google Scholar]
- Grant, T.J.; Jones, J.W.; Norman, G.C. Present status of heat treatment of citrus viruses. Fla. State Hortic. Soc. 1959, 72, 45–48. [Google Scholar]
- Nyland, G.; Goheen, A.C. Heat therapy of virus diseases of perennial plants. Annu. Rev. Phytopathol. 1969, 7, 331–354. [Google Scholar] [CrossRef]
- Verma, J.P.; Nayak, M.L.; Singh, R.P. Elimination of Xanthomonas malvacearum from cotton seeds by hot water. Indian Phytopathol. 1978, 30, 73–76. [Google Scholar]
- Leben, C. Chemical plus heat as seed treatments for control of angular leaf spot of cucumber seedlings. Plant Dis. 1983, 65, 991–993. [Google Scholar] [CrossRef]
- Shekhawat, G.S.; Srivastava, D.N. Control of bacterial leafleafstreak of rice (Oryza sativa L.). Indian J. Agric. Sci. 1971, 41, 1098–1101. [Google Scholar]
- Devash, Y.; Okon, Y.; Henis, Y. Survival of Pseudomonas tomato in Soil and Seeds. J. Phytopathol. 1980, 99, 175–185. [Google Scholar] [CrossRef]
- Hankin, L. Microwave Treatment of Tobacco Seed to Eliminate Bacteria on the Seed Surface. Phytopathology 1977, 77, 794–795. [Google Scholar] [CrossRef]
- Hoy, J.W.; Flynn, J.L. Control of ratoon stunting disease of sugarcane in Louisiana with seedcane produced through micropropagation and resistant cultivars. Int. Soc. Sugar Cane Technol. 2001, 24, 417–421. [Google Scholar]
- Damann, K.E.J.; Benda, G.T.A. Evaluation of commercial heat-treatment methods for control of ratoon stunting disease of sugarcane. Plant Dis. 1983, 67, 966–967. [Google Scholar] [CrossRef]
- Viswanathan, R. Different aerated steam therapy (AST) regimes on the development of grassy shoot disease symptoms in sugarcane. Sugar Tech. 2001, 3, 83–91. [Google Scholar] [CrossRef]
- Arioli, S.; Montanari, C.; Magnani, M.; Tabanelli, G.; Patrignani, F.; Lanciotti, R.; Mora, D.; Gardini, F. Modelling of Listeria monocytogenes Scott A after a mild heat treatment in the presence of thymol and carvacrol: Effects on culturability and viability. J. Food Eng. 2019, 240, 73–82. [Google Scholar] [CrossRef]
- Hong, E.; Park, S.; Kang, D. Sequential treatment of hydrogen peroxide, vacuum packaging, and dry heat for inactivating Salmonella Typhimurium on alfalfa seeds without detrimental effect on seeds viability. Food Microbiol. 2019, 77, 130–136. [Google Scholar] [CrossRef]
- Hussain, M.S.; Tango, C.N.; Oh, D.H. Inactivation kinetics of slightly acidic electrolyzed water combined with benzalkonium chloride and mild heat treatment on vegetative cells, spores, and biofilms of Bacillus cereus. Food Res. Int. 2019, 116, 157–167. [Google Scholar] [CrossRef]
- Kirk, J.A.; Fagan, R.P. Heat shock increases conjugation efficiency in Clostridium difficile. Anaerobe 2016, 42, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.L.; Varma, A. Cure of seed transmitted cowpea banding mosaic virus. Phytopathol. Z. 1975, 83, 144–151. [Google Scholar] [CrossRef]
- Gottwald, T.R. Citrus Canker and Citrus Huanglongbing, two Exotic Bacterial Diseases Threatening the Citrus Industries of the Western Hemisphere. Outlooks Pest Manag. 2007, 18, 274–279. [Google Scholar] [CrossRef]
- Lopes, S.A.; Frare, G.F. Liberibacters Associated with Citrus Huanglongbing in Brazil: ‘Candidatus Liberibacter asiaticus’ Is Heat Tolerant, ‘Ca. L. americanus’ Is Heat Sensitive. Plant Dis. 2009, 93, 257–262. [Google Scholar] [CrossRef]
- Lin, K.H.; Zheng, R.Y. A preliminary study on citrus yellow shoot disease virus and resistance of the diseased citrus budwood to heat. Acta Phytopathol. Sin. 1964, 4, 169–174. [Google Scholar]
- Fan, G.-C.; Xia, Y.-L.; Lin, X.-J.; Hu, H.-Q.; Wang, X.-D.; Ruan, C.-Q.; Lu, L.-M.; Liu, B. Evaluation of thermotherapy against Huanglongbing (citrus greening) in the greenhouse. J. Integr. Agric. 2016, 15, 111–119. [Google Scholar] [CrossRef]
- Doud, A.; Wang, Y.; Hoffman, M.T.; Latza, C.L.; Luo, W.; Armstrong, C.M.; Gottwald, T.R.; Dai, L.; Luo, F.; Duan, Y. Solar thermotherapy reduces the titer of Candidatus Liberibacter asiaticus and enhances canopy growth by altering gene expression profiles in HLB-affected citrus plants. Hortic. Res. 2017, 4, 17054. [Google Scholar] [CrossRef]
- Abdulridha, J.; Ampatzidis, Y.; Ghatrehsamani, S.; Ehsani, R. Mobile thermotherapy system for treating HLB-infected citrus trees utilizing hot water and steam. In Proceedings of the 2018 ASABE Annual International Meeting (ASABE AIM), Detroit, MI, USA, 29 July–1 August 2018. [Google Scholar]
- Ghatrehsamani, S.; Abdulridha, J.; Balafoutis, A.; Zhang, X.; Ehsani, R.; Ampatzidis, Y. Development and evaluation of a mobile thermotherapy technology for in-field treatment of Huanglongbing (HLB) affected trees. Biosyst. Eng. 2019, 182, 1–15. [Google Scholar] [CrossRef] [Green Version]
- European Association of National Metrology Institutes. Technical Committee for Thermometry, Calibration of Thermocouples; EURAMET: Berlin, Germany, 2011; pp. 1–19. [Google Scholar]
- Wood, B.E.; Ingram, L.O. Ethanol Production from Cellobiose, Amorphous Cellulose, and Crystalline Celluloseby Recombinant Klebsiella oxytoca Containing Chromosomally Integrated Zymomonas mobilis Genes for Ethanol Production and Plasmids Expressing Thermostable Cellulase Genes from Clostridium thermocellumt. Appl. Environ. Microbiol. 1992, 58, 2103–2110. [Google Scholar]
- Ohta, K.; Beall, D.S.; Mejia, J.P.; Shanmugam, K.T.; Ingram, L.O. Metabolic engineering of Klebsiella oxytoca M5A1 for ethanol production from xylose and glucose. Appl. Environ. Microbiol. 1991, 57, 2810–2815. [Google Scholar] [Green Version]
- Guidance for Industry: Reducing Microbial Food Safety Hazards for Sprouted Seeds; C.U.S. Food and Drug Administration: Silver Spring, MD, USA, 1999.

| Thermal Sensor No. | Coordinate (cm) | |||
|---|---|---|---|---|
| x | y | z | ||
| Zone 1 | 1 | 0 | 0 | 30.50 |
| 2 | 0 | 0 | 48.25 | |
| 3 | 0 | 0 | 63.50 | |
| 4 | 0 | 0 | 91.45 | |
| Zone 2 | 5 | −101.60 | −81.28 | 114.30 |
| 6 | −43.18 | 76.20 | 106.68 | |
| 7 | 45.72 | 12.70 | 119.38 | |
| 8 | −29.94 | −12.70 | 109.22 | |
| Zone 3 | 9 | 7.62 | −83.82 | 152.40 |
| 10 | −53.34 | 17.78 | 172.72 | |
| 11 | 17.78 | 25.40 | 182.88 | |
| 12 | −88.90 | −53.34 | 170.18 | |
| No. of Biosensor | Coordinate (cm) | |||
|---|---|---|---|---|
| x | y | z | ||
| Zone 2 | 1 | −60 | −30 | 94 |
| 2 | 0 | 0 | 94 | |
| 3 | 66 | 0 | 90 | |
| 4 | −20 | −90 | 107 | |
| 5 | 54 | 15 | 174 | |
| 6 | 0 | −107 | 175 | |
| 7 | −116 | 0 | 94 | |
| Zone 3 | 8 | −38 | −45 | 100 |
| 9 | −110 | −20 | 176 | |
| 10 | −110 | −20 | 190 | |
| 11 | −50 | 62 | 90 | |
| 12 | 25 | 0 | 95 | |
| 13 | 17 | −35 | 190 | |
| 14 | −43 | 36 | 107 | |
| Variety | Hamlin |
|---|---|
| Species | Citrus Sinensis |
| Origin | Florida, USA |
| Age | 6 years |
| Average size | 2.3 m height × 2 m width |
| Disease | HLB (Citrus Greening) |
| Location | 26.4608° N, 81.4359° W |
| Experiment No. ** | Time (s) | Trunk (Zone 1) | Canopy (Zones 2 and 3) | Tree (Zones 1–3) | |||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | SD | Temperature (°C) | SD | Temperature (°C) | SD | ||
| 1.1 | 252.0 | 50.57 | 1.81 | 54.30 | 1.18 | 54.22 | 1.09 |
| 1.2 | 257.0 | 51.40 | 1.31 | 53.97 | 1.21 | 53.92 | 1.05 |
| 1.3 | 246.0 | 50.00 | 1.45 | 53.81 | 1.19 | 53.68 | 1.15 |
| Average | 251.6 | 50.65 | 54.03 | 53.94 | |||
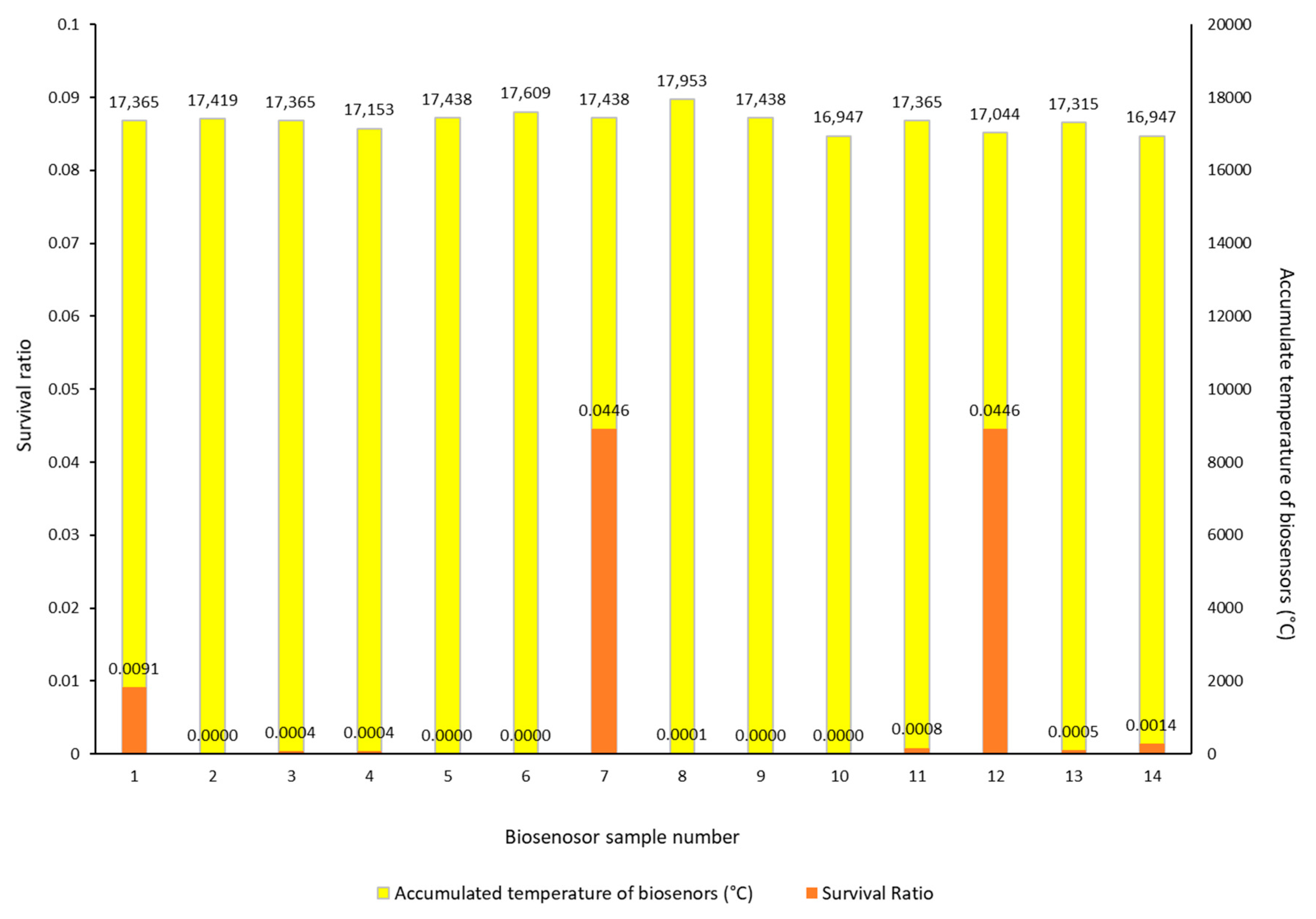
| Biosensor Sample No. | Survival Ratio | Cell Number | Colonies (Rep 1) | Colonies (Rep 2) | Colonies (Rep 3) | Dilution | |
|---|---|---|---|---|---|---|---|
| LC (Lab control) | 1.33 × 106 | 12 | 11 | 17 | 1.00 × 105 | ||
| FC (Field control) | 100 | 2.47 × 106 | 29 | 26 | 19 | 1.00 × 105 | |
| 1 | 0.0091 | −4.04 | 2.23 × 102 | 28 | 18 | 21 | 1.00 × 101 |
| 2 | 0.0000 | killed | 0.00 × 100 | 0 | 0 | 0 | 1.00 × 101 |
| 3 | 0.0004 | −5.39 | 1.00 × 101 | 1 | 1 | 1 | 1.00 × 101 |
| 4 | 0.0004 | −5.39 | 1.00 × 101 | 0 | 0 | 3 | 1.00 × 101 |
| 5 | 0.0000 | killed | 0.00 × 100 | 0 | 0 | 0 | 1.00 × 101 |
| 6 | 0.0000 | killed | 0.00 × 100 | 0 | 0 | 0 | 1.00 × 101 |
| 7 | 0.0446 | −3.35 | 1.10 × 103 | 12 | 11 | 10 | 1.00 × 101 |
| 8 | 0.0001 | −5.87 | 3.33 × 100 | 0 | 0 | 1 | 1.00 × 101 |
| 9 | 0.0000 | killed | 0.00 × 100 | 0 | 0 | 0 | 1.00 × 101 |
| 10 | 0.0000 | killed | 0.00 × 100 | 0 | 0 | 0 | 1.00 × 101 |
| 11 | 0.0008 | −5.09 | 2.00 × 102 | 0 | 4 | 2 | 1.00 × 101 |
| 12 | 0.0446 | −3.35 | 1.10 × 103 | 9 | 10 | 14 | 1.00 × 102 |
| 13 | 0.0005 | −5.27 | 1.33 × 101 | 2 | 2 | 0 | 1.00 × 101 |
| 14 | 0.0014 | −4.87 | 3.33 × 101 | 1 | 5 | 4 | 1.00 × 101 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghatrehsamani, S.; Czarnecka, E.; Verner, F.L.; Gurley, W.B.; Ehsani, R.; Ampatzidis, Y. Evaluation of Mobile Heat Treatment System for Treating In-Field HLB-Affected Trees by Analyzing Survival Rate of Surrogate Bacteria. Agronomy 2019, 9, 540. https://doi.org/10.3390/agronomy9090540
Ghatrehsamani S, Czarnecka E, Verner FL, Gurley WB, Ehsani R, Ampatzidis Y. Evaluation of Mobile Heat Treatment System for Treating In-Field HLB-Affected Trees by Analyzing Survival Rate of Surrogate Bacteria. Agronomy. 2019; 9(9):540. https://doi.org/10.3390/agronomy9090540
Chicago/Turabian StyleGhatrehsamani, Shirin, Eva Czarnecka, F. Lance Verner, William B. Gurley, Reza Ehsani, and Yiannis Ampatzidis. 2019. "Evaluation of Mobile Heat Treatment System for Treating In-Field HLB-Affected Trees by Analyzing Survival Rate of Surrogate Bacteria" Agronomy 9, no. 9: 540. https://doi.org/10.3390/agronomy9090540







