In-Season Diagnosis of Winter Wheat Nitrogen Status in Smallholder Farmer Fields Across a Village Using Unmanned Aerial Vehicle-Based Remote Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plot Experiments
2.3. UAV Remote Sensing Data Collection
2.4. Field Data Collection and Analysis
2.5. Different Approaches to Estimate NNI
2.6. Statistical Analysis
3. Results
3.1. Variability of Winter Wheat Nitrogen Status Indicators
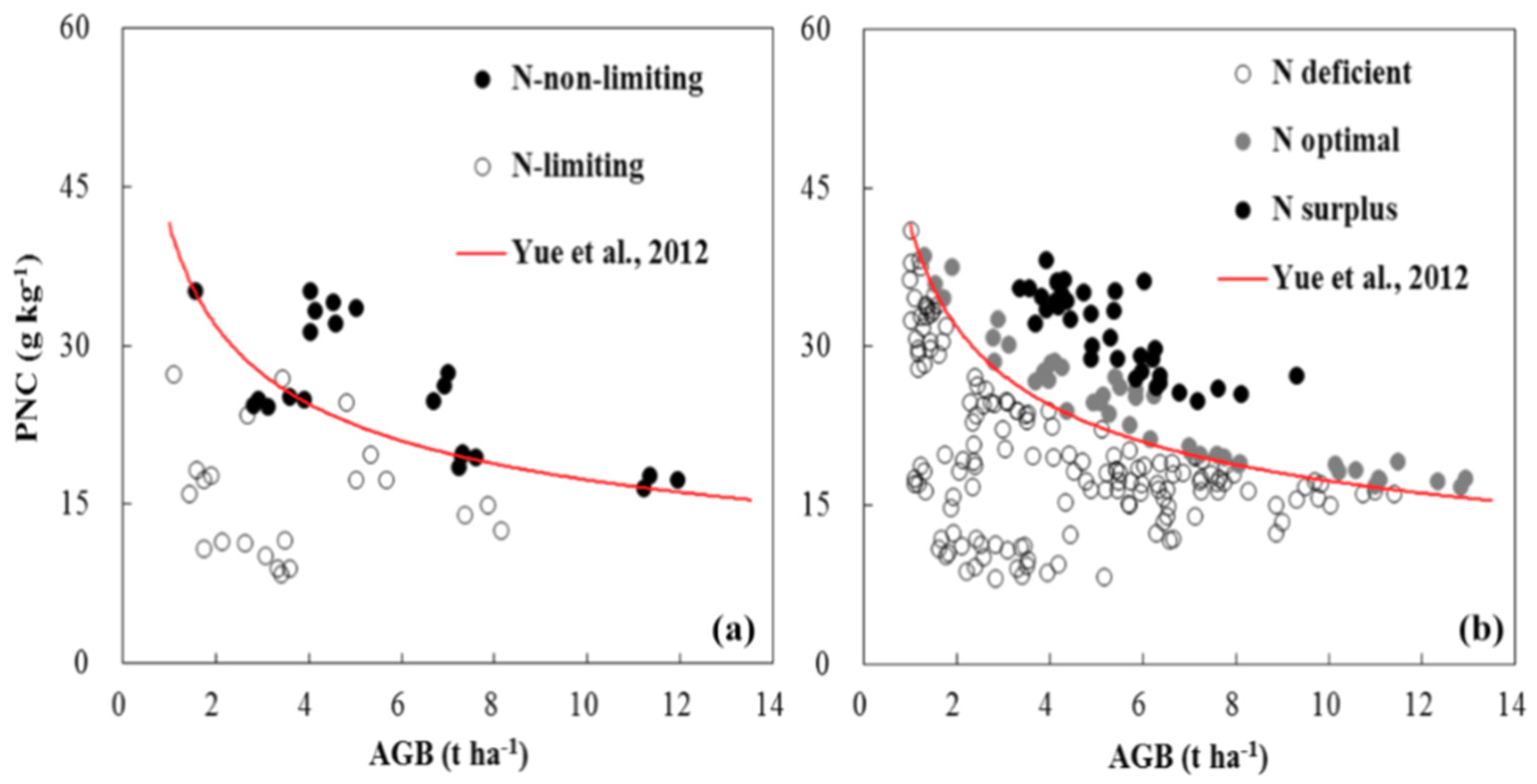
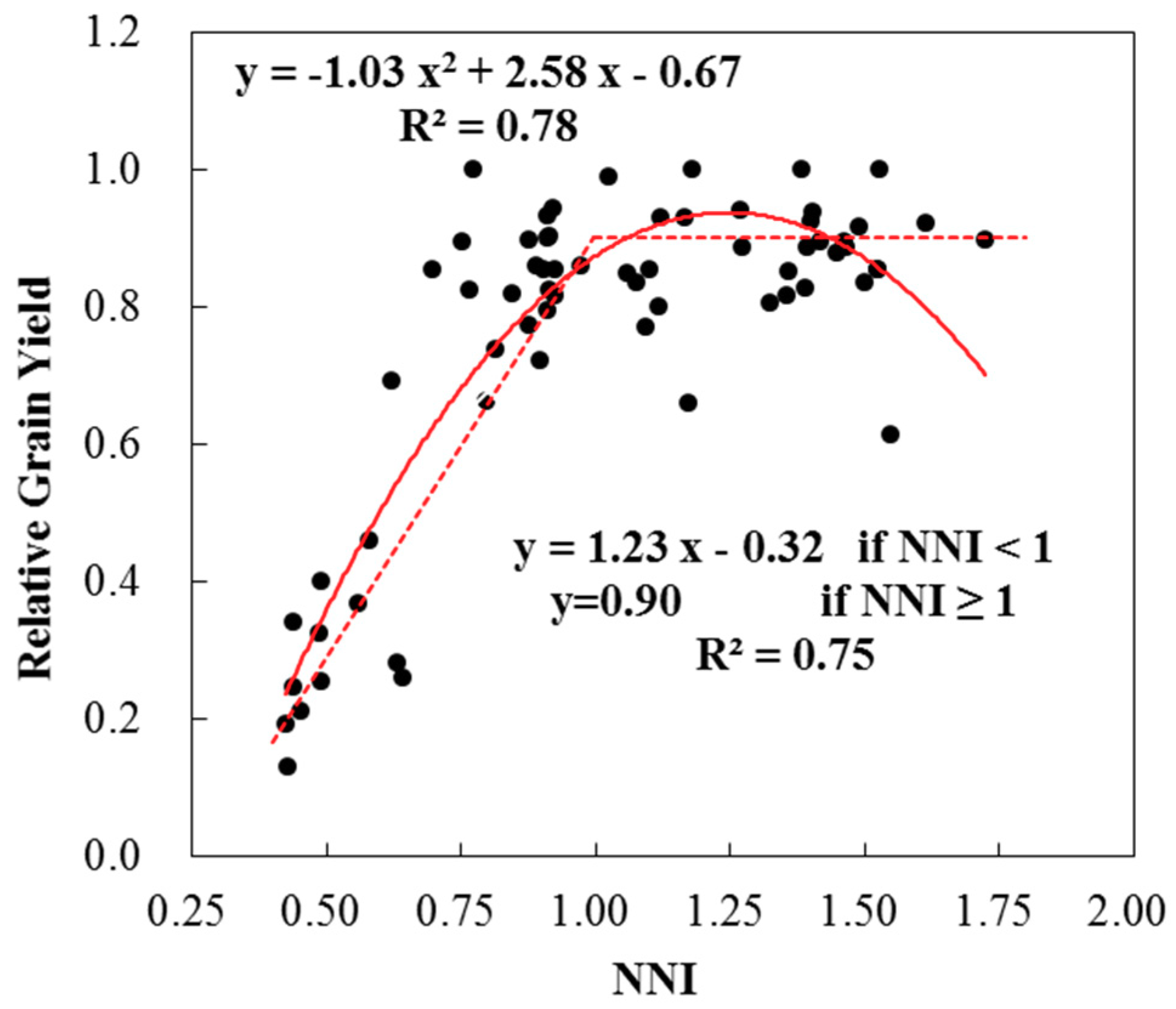
3.2. Evaluation of the Established Critical Nitrogen Dilution Curve
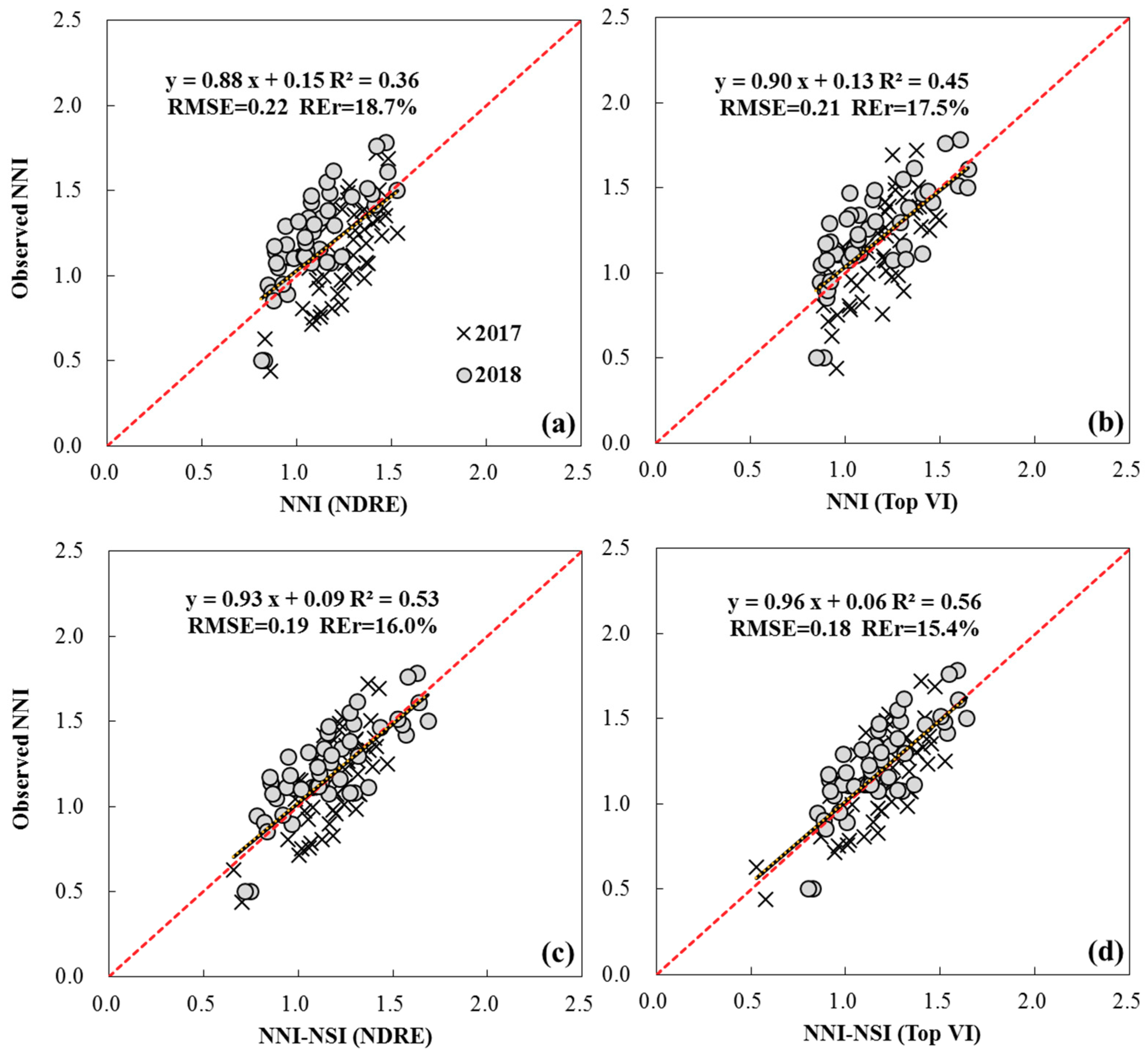
3.3. The Estimation of NNI Using Two Indirect Approaches
3.4. The Estimation of NNI Using Two Direct Approaches
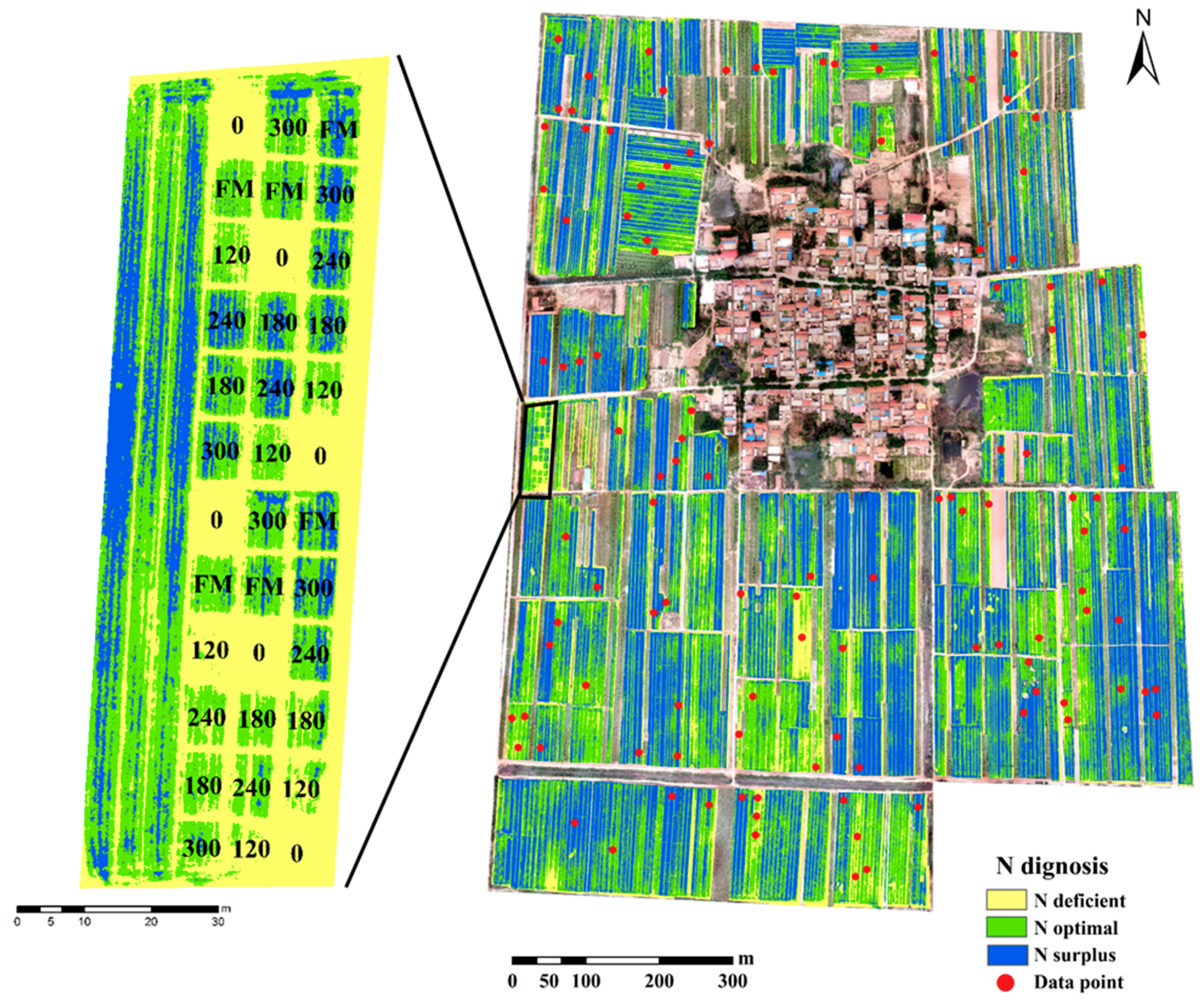
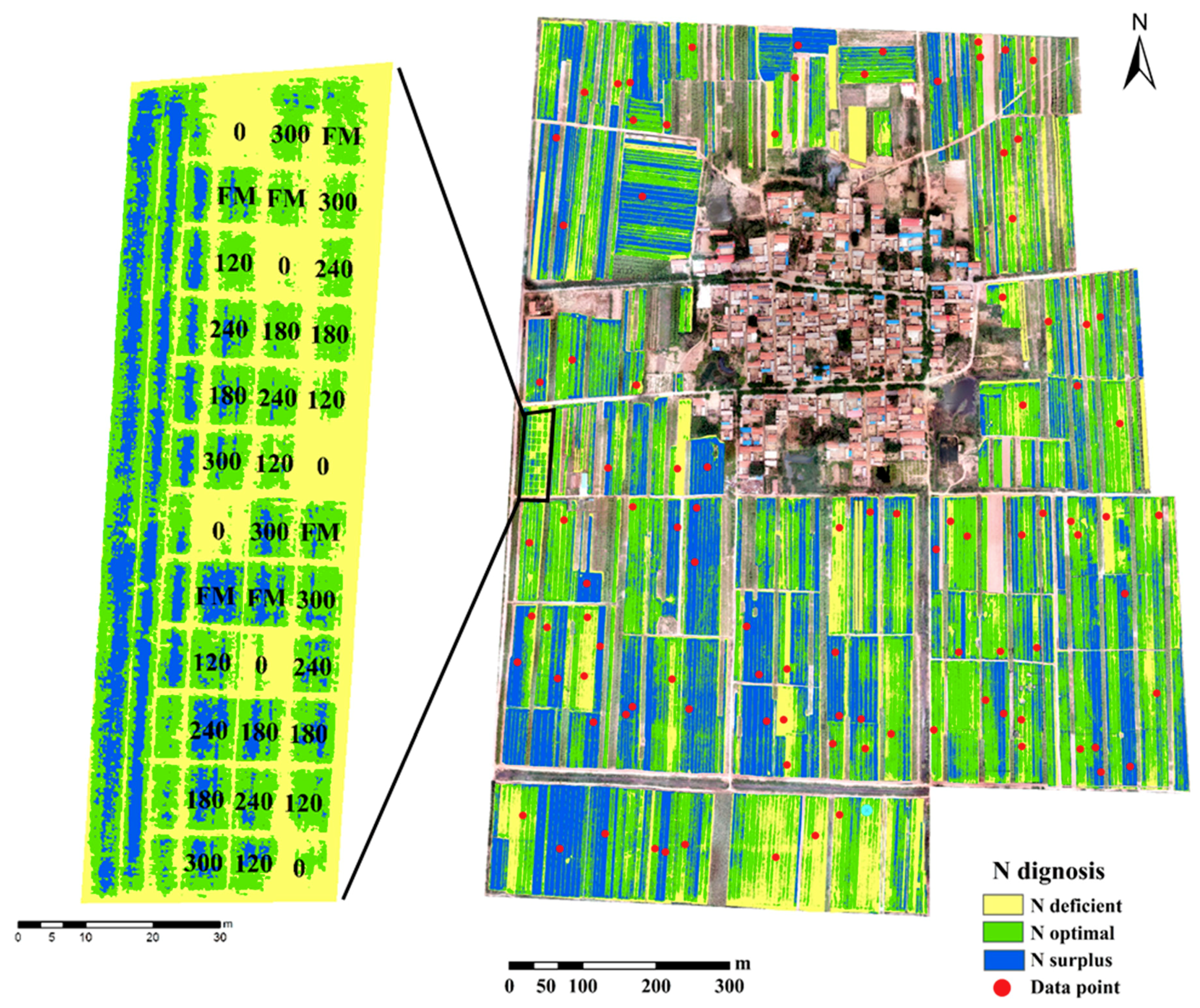
3.5. Nitrogen Status Diagnosis at the Village Scale
4. Discussion
4.1. The Accuracy of N Status Diagnosis Using eBee UAV Remote Sensing
4.2. The Performance of Normalized Difference VIs
4.3. The Suitability and Usability of the NNI Estimation Approach
4.4. Applications for N Status Diagnosis and Topdressing N Recommendation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Index | Formula | Reference |
|---|---|---|
| Normalized Difference Vegetation Index (NDVI) | (NIR − R)/(NIR + R) | [50] |
| Ratio Vegetation Index (RVI) | NIR/R | [51] |
| Difference Vegetation Index (DVI) | NIR − R | [52] |
| Renormalized Difference Vegetation Index (RDVI) | (NIR − R)/SQRT (NIR + R) | [53] |
| Wide Dynamic Range Vegetation Index (WDRVI) | (0.12 × NIR − R)/(0.12 × NIR + R) | [54] |
| Soil Adjusted Vegetation Index (SAVI) | 1.5 × (NIR − R)/(NIR + R + 0.5) | [55] |
| Optimized SAVI (OSAVI) | (1 + 0.16) × (NIR − R)/(NIR + R + 0.16) | [56] |
| Modified SAVI (MSAVI) | 0.5 × [2 × NIR + 1 − SQRT ((2 × NIR + 1)2 − 8 × (NIR − R))] | [57] |
| Modified Simple Ratio (MSR) | (NIR/R − 1)/SQRT (NIR/R + 1) | [58] |
| Transformed Normalized vegetation index (TNDVI) | SQRT ((NIR − R)/(NIR + R) + 0.5) | [59] |
| Optimal Vegetation Index (VIopt) | 1.45 × ((NIR2 + 1)/(R + 0.45)) | [60] |
| Red Edge Point Reflectance (REPR) | (R + NIR)/2 | [61] |
| Nonlinear Index (NLI) | (NIR2 − R)/(NIR2 + R) | [62] |
| Modified Nonlinear Index (MNLI) | 1.5 × (NIR2 − R)/(NIR2 + R + 0.5) | [63] |
| NDVI*RVI | (NIR2 − R)/(NIR + R2) | [63] |
| SAVI*SR | (NIR2 − R)/((NIR + R + 0.5) × R) | [63] |
| Normalized Difference Red Edge (NDRE) | (NIR − RE)/(NIR + RE) | [64] |
| Red Edge Ratio Vegetation Index (RERVI) | NIR/RE | [65] |
| Red Edge Difference Vegetation Index (REDVI) | NIR − RE | [66] |
| Red Edge Renormalized Different Vegetation Index (RERDVI) | (NIR − RE)/SQRT (NIR + RE) | [66] |
| Red Edge Wide Dynamic Range Vegetation Index (REWDRVI) | (0.12 × NIR − RE)/(0.12 × NIR + RE) | [66] |
| Red Edge Soil Adjusted Vegetation Index (RESAVI) | 1.5 × (NIR − RE)/(NIR + RE + 0.5) | [66] |
| Red Edge Optimized SAVI (REOSAVI) | (1 + 0.16) × (NIR − RE)/(NIR + RE + 0.16) | [66] |
| Modified Red Edge SAVI (MRESAVI) | 0.5 × (2 × NIR + 1 − SQRT ((2 × NIR + 1)2 − 8 × (NIR − RE))) | [66] |
| Modified Red Edge Simple Ratio (MSR_RE) | (NIR/RE − 1)/SQRT (NIR/RE + 1) | [66] |
| Optimized Red Edge Vegetation Index (REVIopt) | 100 × (lnNIR − lnRE) | [67] |
| Green Normalized Difference Vegetation Index (GNDVI) | (NIR − G)/(NIR + G) | [68] |
| Green Ratio Vegetation Index (GRVI) | NIR/G | [69] |
| Green Difference Vegetation Index (GDVI) | NIR − G | [17] |
| Green Renormalized Difference Vegetation Index (GRDVI) | (NIR − G)/SQRT (NIR + G) | [17] |
| Green Wide Dynamic Range Vegetation Index (GWDRVI) | (0.12 × NIR − G)/(0.12 × NIR + G) | [17] |
| Green Soil Adjusted Vegetation Index (GSAVI) | 1.5 × (NIR − G)/(NIR + G + 0.5) | [17] |
| Green Optimized SAVI (GOSAVI) | (1 + 0.16) × (NIR − G)/(NIR + G + 0.16) | [17] |
| Modified Green SAVI (MGSAVI) | 0.5 × (2 × NIR + 1 − SQRT ((2 × NIR + 1)2 − 8 × (NIR − G))) | [17] |
| Modified Green Simple Ratio (MSR_G) | (NIR/G − 1)/SQRT (NIR/G + 1) | [66] |
| Red Edge Normalized Difference Vegetation Index (RENDVI) | (RE − R)/(RE + R) | [70] |
| Red Edge Simple Ratio (RESR) | RE/R | [71] |
| Modified Red Edge Difference Vegetation Index (MREDVI) | RE − R | This study, modified from [52] |
| Modified Simple Ratio Green and Red (MSRGR) | SQRT(G/R) | [52] |
| Green and Red Difference (GRD) | G − R | [52] |
| Normalized Difference Green and Red (NDGR) | (G − R)/(G + R) | [52] |
| Greenness Index (GI) | G/R | [52] |
| Transformed Normalized Green and Red (TNDGR) | SQRT ((G − R)/(G + R) + 0.5) | [52] |
| MERIS terrestrial chlorophyll index (MTCI) | (NIR − RE)/(RE − R) | [61] |
| DATT index (DATT) | (NIR − RE)/(NIR − R) | [72] |
| Modified canopy chlorophyll content index (MCCCI) | NDRE/NDVI | [73] |
| Modified Normalized Difference Vegetation Index 1 (mNDVI1) | (NIR − R + 2 × G)/(NIR + R − 2 × G) | [74] |
| Plant Senescence Reflectance Index (PSRI) | (R − G)/NIR | [75] |
| Modified Chlorophyll Absorption in Reflectance Index (MCARI) | ((RE − R) − 0.2 × (RE − G)) × (RE/R) | This study, modified from [76] |
| Modified Chlorophyll Absorption in Reflectance Index 1 (MCARI1) | 1.2 × [2.5 × (NIR−R) − 1.3 × (NIR − G)] | [77] |
| Modified Chlorophyll Absorption in Reflectance Index 2 (MCARI2) | 1.5 × (2.5 × (NIR − R) − 1.3 × (NIR − G)) /SQRT ((2 × NIR + 1)2 − (6 × NIR − 5 × SQRT(R)) − 0.5) | [77] |
| Triangular Vegetation Index (TVI) | 0.5 × (120 × (NIR − G) − 200 × (R − G)] | [78] |
| Transformed Chlorophyll Absorption in Reflectance Index (TCARI) | 3 × ((RE − R) − 0.2 × (RE − G) × (RE/R)) | This study, modified from [79] |
| Triangular Chlorophyll Index (TCI) | 1.2 × (RE − G) − 1.5 × (R − G) × SQRT (RE/R) | This study, modified from [80] |
| TCARI/OSAVI | TCARI/OSAVI | [79] |
| TCARI/MSAVI | TCARI/MSAVI | [79] |
| TCI/OSAVI | TCI/OSAVI | [80] |
| Normalized Near Infrared Index (NNIRI) | NIR/(NIR + RE + R) | [31] |
| Red Edge Transformed Vegetation Index (RETVI) | 0.5 × (120 × (NIR − R) − 200 × (RE − R)) | [31] |
| Abbreviation | Full Name | Abbreviation | Full Name |
|---|---|---|---|
| AGB | aboveground biomass | NSI_NDRE | nitrogen sufficiency index calculated with normalized difference red edge |
| ASD | analytical spectral devices | NSI_NDVI | nitrogen sufficiency index calculated with normalized difference vegetation index |
| CV | coefficient of variation | NSI_REDVI | nitrogen sufficiency index calculated with red edge difference vegetation index |
| DM | dry matter | NSI_RERVI | nitrogen sufficiency index calculated with red edge ratio vegetation index |
| E | exponential fit | NSI_REWDRVI | nitrogen sufficiency index calculated with red edge wide dynamic range vegetation index |
| FM | farmer management | NSI_VIs | nitrogen sufficiency index calculated with vegetation indices |
| G | green | NUE | nitrogen use efficiency |
| GNDVI | green normalized difference vegetation index | P | power fit |
| GRD | green and red difference | PNC | plant nitrogen concentration |
| GRDVI | green renormalized difference vegetation index | PNM | precision nitrogen management |
| INS | indigenous nitrogen supply | PNU | plant nitrogen uptake |
| KCl | potassium chloride | PNUa | actual measured plant nitrogen uptake |
| MCCCI | modified canopy chlorophyll content index | PNUc | critical plant nitrogen uptake |
| MGSAVI | modified green soil adjusted vegetation index | PSRI | plant senescence reflectance index |
| MSR_RE | modified red edge simple ratio | Q | quadratic fit |
| N | nitrogen | R | red |
| Na | actual measured nitrogen concentration | RE | red edge |
| Nc | critical nitrogen concentration | REIP | red-edge inflection point |
| NCP | North China Plain | REr | relative error |
| NDRE | normalized difference red edge | REVIopt | optimized red edge vegetation index |
| NDVI | normalized difference vegetation index | REWDRVI | red edge wide dynamic range vegetation index |
| NIR | near infrared | RMSE | root mean square error |
| Nmin | mineral nitrogen | SD | standard deviation of the mean |
| NNI | nitrogen nutrition index | TNDGR | transformed normalized green and red |
| NNIRI | normalized near infrared index | UAV | unmanned aerial vehicle |
| NSI | nitrogen sufficiency index | VIs | vegetation indices |
| NSI_GNDVI | nitrogen sufficiency index calculated with green normalized difference vegetation index |
References
- Wang, J.; Wang, E.; Yang, X.; Zhang, F.; Yin, H. Increased yield potential of wheat-maize cropping system in the North China Plain by climate change adaptation. Clim. Chang. 2012, 113, 825–840. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, H.; Chen, X.; Zhang, C.; Ma, W.; Huang, C.; Zhang, W.; Mi, G.; Miao, Y.; Li, X. Pursuing sustainable productivity with millions of smallholder farmers. Nature 2018, 555, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Stewart, B.A.; Zhang, F. Long-term experiments for sustainable nutrient management in China. A review. Agron. Sustain. Dev. 2011, 31, 397–414. [Google Scholar] [CrossRef]
- Chen, G.; Cao, H.; Liang, J.; Ma, W.; Guo, L.; Zhang, S.; Jiang, R.; Zhang, H.; Goulding, K.W.T.; Zhang, F. Factors affecting nitrogen use efficiency and grain yield of summer maize on smallholder farms in the North China Plain. Sustainability 2018, 10, 363. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, G.; Li, X.; Zhang, H.; Wang, C.; Liu, Q.; Chen, X.; Cui, Z.; Shen, J.; Jiang, R.; et al. Closing yield gaps in China by empowering smallholder farmers. Nature 2016, 537, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cui, Z.; Fan, M.; Vitousek, P.; Zhao, M.; Ma, W.; Wang, Z.; Zhang, W.; Yan, X.; Yang, J. Producing more grain with lower environmental costs. Nature 2014, 514, 486–489. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Vitousek, P. Chinese agriculture: An experiment for the world. Nature 2013, 497, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Cui, Z.; Chen, X.; Khosla, R.; Dao, T.H.; Miao, Y. Quantifying spatial variability of indigenous nitrogen supply for precision nitrogen management in small scale farming. Precis. Agric. 2012, 13, 45–61. [Google Scholar] [CrossRef]
- Diacono, M.; Rubino, P.; Montemurro, F. Precision nitrogen management of wheat. A review. Agron. Sustain. Dev. 2013, 33, 219–241. [Google Scholar] [CrossRef]
- Miao, Y.; Mulla, D.J.; Hernandez, J.A.; Wiebers, M.; Robert, P.C. Potential impact of precision nitrogen management on corn yield, protein content, and test weight. Soil Sci. Soc. Am. J. 2007, 71, 1490–1499. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, F.; Chen, X.; Miao, Y.; Li, J.; Shi, L.; Xu, J.; Ye, Y.; Liu, C.; Yang, Z.; et al. On-farm evaluation of an in-season nitrogen management strategy based on soil Nmin test. Field Crops Res. 2008, 105, 48–55. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Feng, G.; Gao, X.; Liu, B.; Liu, Y.; Li, F.; Khosla, R.; Mulla, D.; Zhang, F. Improving nitrogen use efficiency with minimal environmental risks using an active canopy sensor in a wheat-maize cropping system. Field Crops Res. 2017, 214, 365–372. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, Y.; Huang, S.; Gao, L.; Ma, X.; Zhao, G.; Jiang, R.; Chen, X.; Zhang, F.; Yu, K. Active canopy sensor-based precision N management strategy for rice. Agron. Sustain. Dev. 2012, 32, 925–933. [Google Scholar] [CrossRef]
- Huang, S.; Miao, Y.; Yuan, F.; Gnyp, M.; Yao, Y.; Cao, Q.; Wang, H.; Lenzwiedemann, V.; Bareth, G. Potential of Rapideye and Worldview-2 satellite data for improving rice nitrogen status monitoring at different growth stages. Remote Sens. 2017, 9, 227. [Google Scholar] [CrossRef]
- Zhao, Q.; Lenzwiedemann, V.I.S.; Yuan, F.; Jiang, R.; Miao, Y.; Zhang, F.; Bareth, G. Investigating within-field variability of rice from high resolution satellite imagery in Qixing Farm County, Northeast China. ISPRS Int. J. Geo-Inf. 2015, 4, 236–261. [Google Scholar] [CrossRef]
- Gevaert, C.M.; Suomalainen, J.; Tang, J.; Kooistra, L. Generation of spectral–temporal response surfaces by combining multispectral satellite and hyperspectral UAV imagery for precision agriculture applications. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 3140–3146. [Google Scholar] [CrossRef]
- Huang, S.; Miao, Y.; Zhao, G.; Yuan, F.; Ma, X.; Tan, C.; Yu, W.; Gnyp, M.L.; Lenz-Wiedemann, V.I.; Rascher, U. Satellite remote sensing-based in-season diagnosis of rice nitrogen status in Northeast China. Remote Sens. 2015, 7, 10646–10667. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Zhao, C.; Li, Z.; Huang, Y.; Yu, H.; Xu, B.; Yang, X.; Zhu, D.; Zhang, X. Unmanned aerial vehicle remote sensing for field-based crop phenotyping: Current status and perspectives. Front. Plant Sci. 2017, 8, 1111. [Google Scholar] [CrossRef]
- Geipel, J.; Link, J.; Wirwahn, J.; Claupein, W. A programmable aerial multispectral camera system for in-season crop biomass and nitrogen content estimation. Agriculture 2016, 6, 4. [Google Scholar] [CrossRef]
- Perry, E.M.; Goodwin, I.; Cornwall, D. Remote sensing using canopy and leaf reflectance for estimating nitrogen status in red-blush pears. Hortscience 2018, 53, 78–83. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, T.; Li, D.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y. Combining unmanned aerial vehicle (UAV)-based multispectral imagery and ground-based hyperspectral data for plant nitrogen concentration estimation in rice. Front. Plant Sci. 2018, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Huang, M.; Zou, Y.; Jiang, P.; Cao, F.; Xie, X. Chlorophyll meter-based nitrogen management for no-till direct seeded rice. Res. Crop. 2012, 13, 809–821. [Google Scholar]
- Marcaccio, J.V.; Markle, C.E.; Chow-Fraser, P. Use of fixed-wing and multi-rotor unmanned aerial vehicles to map dynamic changes in a freshwater marsh. J. Unmanned Veh. Syst. 2016, 4, 193–202. [Google Scholar] [CrossRef]
- Roumenina, E.; Jelev, G.; Dimitrov, P.; Vassilev, V.; Krasteva, V.; Kamenova, I.; Nankov, M.; Kolchakov, V. Winter Wheat Crop State Assessment, Based on Satellite Data from the Experiment Spot-5 Take-5, Unmanned Airial Vehicle Sensefly Ebee Ag and Field Data in Zlatia Test Site, Bulgaria. In Proceedings of the Eleventh Scientific Conference with International Participation, Sofia, Bulgaria, 4–6 November 2015. [Google Scholar]
- Marino, S.; Alvino, A. Detection of homogeneous wheat areas using multi-temporal UAS images and ground truth data analyzed by cluster analysis. Eur. J. Remote Sens. 2018, 51, 266–275. [Google Scholar] [CrossRef]
- Greenwood, D.J.; Gastal, F.; Lemaire, G.; Draycott, A.; Millard, P.; Neeteson, J.J. Growth rate and % N of field grown crops: Theory and experiments. Ann. Bot. 1991, 67, 181–190. [Google Scholar] [CrossRef]
- Lemaire, G.; Jeuffroy, M.-H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage: Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Yuan, Z.; Ata-Ul-Karim, S.T.; Cao, Q.; Lu, Z.; Cao, W.; Zhu, Y.; Liu, X. Indicators for diagnosing nitrogen status of rice based on chlorophyll meter readings. Field Crops Res. 2016, 185, 12–20. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Z.; Ata-Ul-Karim, S.T.; Xiao, J.; Liu, Z.; Qi, A.; Ning, D.; Nan, J.; Duan, A. Rapid and nondestructive estimation of the nitrogen nutrition index in winter barley using chlorophyll measurements. Field Crops Res. 2016, 185, 59–68. [Google Scholar] [CrossRef]
- Chen, P. A comparison of two approaches for estimating the wheat nitrogen nutrition index using remote sensing. Remote Sens. 2015, 7, 4527–4548. [Google Scholar] [CrossRef]
- Lu, J.; Miao, Y.; Shi, W.; Li, J.; Yuan, F. Evaluating different approaches to non-destructive nitrogen status diagnosis of rice using portable RapidSCAN active canopy sensor. Sci. Rep. 2017, 7, 14073. [Google Scholar] [CrossRef]
- Xia, T.; Miao, Y.; Wu, D.; Shao, H.; Khosla, R.; Mi, G. Active optical sensing of spring maize for in-season diagnosis of nitrogen status based on nitrogen nutrition index. Remote Sens. 2016, 8, 605. [Google Scholar] [CrossRef]
- Samborski, S.M.; Tremblay, N.; Fallon, E. Strategies to make use of plant sensors-based diagnostic information for nitrogen recommendations. Agron. J. 2009, 101, 800–816. [Google Scholar] [CrossRef]
- Yue, X.L.; Hu, Y.; Zhang, H.Z.; Schmidhalter, U. Green Window Approach for improving nitrogen management by farmers in small-scale wheat fields. J. Agric. Sci. 2015, 153, 446–454. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, H.; Xu, X.; He, J.; Ge, X.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.; Tian, Y. Predicting grain yield in rice using multi-temporal vegetation indices from UAV-based multispectral and digital imagery. ISPRS J. Photogramm. 2017, 130, 246–255. [Google Scholar] [CrossRef]
- Yue, S.; Meng, Q.; Zhao, R.; Li, F.; Chen, X.; Zhang, F.; Cui, Z. Critical nitrogen dilution curve for optimizing nitrogen management of winter wheat production in the North China Plain. Agron. J. 2012, 104, 523–529. [Google Scholar] [CrossRef]
- Huang, S.; Miao, Y.; Cao, Q.; Yao, Y.; Zhao, G.; Yu, W.; Shen, J.; Kang, Y.; Bareth, G. A new critical nitrogen dilution curve for rice nitrogen status diagnosis in Northeast China. Pedosphere 2018, 28, 814–822. [Google Scholar] [CrossRef]
- Ziadi, N.; Bélanger, G.; Claessens, A.; Lefebvre, L.; Cambouris, A.N.; Tremblay, N.; Nolin, M.C.; Parent, L.É. Determination of a critical nitrogen dilution curve for spring wheat. Agron. J. 2010, 102, 241–250. [Google Scholar] [CrossRef]
- Campbell, J.B. Introduction to Remote Sensing, 3rd ed.; The Guilford Press: New York, NY, USA, 2002. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Feng, G.; Gao, X.; Li, F.; Liu, B.; Yue, S.; Cheng, S.; Ustin, S.L.; Khosla, R. Active canopy sensing of winter wheat nitrogen status: An evaluation of two sensor systems. Comput. Electron. Agric. 2015, 112, 54–67. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Shen, J.; Yuan, F.; Cheng, S.; Cui, Z. Evaluating two crop circle active canopy sensors for in-season diagnosis of winter wheat nitrogen status. Agronomy 2018, 8, 201. [Google Scholar] [CrossRef]
- Li, S.; Ding, X.; Kuang, Q.; Ata-Ul-Karim, S.T.; Cheng, T.; Liu, X.; Tian, Y.; Zhu, Y.; Cao, W.; Cao, Q. Potential of UAV-based active sensing for monitoring rice leaf nitrogen status. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Bonfil, D.J. Monitoring wheat fields by RapidScan: Accuracy and limitations. Adv. Anim. Biosci. 2017, 8, 333–337. [Google Scholar] [CrossRef]
- Lu, J.; Miao, Y.; Shi, W.; Li, J.; Wan, J.; Gao, X.; Zhang, J.; Zha, H. Using portable RapidSCAN active canopy sensor for rice nitrogen status diagnosis. Adv. Anim. Biosci. 2017, 8, 349–352. [Google Scholar] [CrossRef]
- Peng, S.; Buresh, R.J.; Huang, J.; Zhong, X.; Zou, Y.; Yang, J.; Wang, G.; Liu, Y.; Hu, R.; Tang, Q. Improving nitrogen fertilization in rice by site-specific N management. A review. Agron. Sustain. Dev. 2010, 30, 649–656. [Google Scholar] [CrossRef]
- Raun, W.R.; Solie, J.B.; Taylor, R.K.; Arnall, D.B.; Mack, C.J.; Edmonds, D.E. Ramp calibration strip technology for determining midseason nitrogen rates in corn and wheat. Agron. J. 2008, 100, 1088–1093. [Google Scholar] [CrossRef]
- Roberts, D.C.; Brorsen, B.W.; Taylor, R.K.; Solie, J.B.; Raun, W.R. Replicability of nitrogen recommendations from ramped calibration strips in winter wheat. Precis. Agric. 2011, 12, 653–665. [Google Scholar] [CrossRef]
- Zha, H.; Cammarano, D.; Wilson, L.; Li, Y.; Batchelor, W.D.; Miao, Y. Combining crop modelling and remote sensing to create yield maps for management zone delineation in small scale farming systems. Precis. Agric.’19 2019, 883–889. [Google Scholar]
- Rouse, J.W.; Haas, J.R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Third Earth Resources Technology Satellite-1 Symposium; NASA Special Publication: Washington, DC, USA, 1974; pp. 309–317. [Google Scholar]
- Jordan, C.F. Derivation of leaf-area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Roujean, J.L.; Breon, F.M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide Dynamic Range Vegetation Index for remote quantification of biophysical characteristics of vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.; Kerr, Y.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of vegetation indices and a modified simple ratio for boreal applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Sandham, L.; Zietsman, H. Surface temperature measurement from space: A case study in the south western Cape of South Africa. S. Afr. J. Enol. Vitic. 1997, 18, 25–30. [Google Scholar] [CrossRef][Green Version]
- Reyniers, M.; Walvoort, D.J.J.; Baardemaaker, J.D. A linear model to predict with a multi-spectral radiometer the amount of nitrogen in winter wheat. Int. J. Remote Sens. 2006, 27, 4159–4179. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Goel, N.S.; Qin, W. Influences of canopy architecture on relationships between various vegetation indices and LAI and FPAR. Remote Sens. Rev. 1994, 10, 309–347. [Google Scholar] [CrossRef]
- Gong, P.; Pu, R.; Biging, G.S.; Larrieu, M.R. Estimation of forest leaf area index using vegetation indices derived from Hyperion hyperspectral data. IEEE Trans. Geosci. Remote 2003, 41, 1355–1362. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T. Coincident Detection of Crop Water Stress, Nitrogen Status and Canopy Density Using Ground-based Multispectral Data. In Proceedings of the International Conference on Precision Agriculture and Other Resource Management, Bloomington, IN, USA, 16–19 July 2000. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Wang, H.; Huang, S.; Cheng, S.; Khosla, R.; Jiang, R. Non-destructive estimation of rice plant nitrogen status with Crop Circle multispectral active canopy sensor. Field Crops Res. 2013, 154, 133–144. [Google Scholar] [CrossRef]
- Jasper, J.; Reusch, S.; Link, A. Active Sensing of the N Status of Wheat using Optimized Wavelength Combination: Impact of Seed Rate, Variety and Growth Stage. Precis. Agric.’09 2009, 23–30. [Google Scholar]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Buschmann, C.; Nagel, E. In vivo spectroscopy and internal optics of leaves as basis for remote sensing of vegetation. Int. J. Remote Sens. 1993, 14, 711–722. [Google Scholar] [CrossRef]
- Elsayed, S.; Rischbeck, P.; Schmidhalter, U. Comparing the performance of active and passive reflectance sensors to assess the normalized relative canopy temperature and grain yield of drought-stressed barley cultivars. Field Crops Res. 2015, 177, 148–160. [Google Scholar] [CrossRef]
- Erdle, K.; Mistele, B.; Schmidhalter, U. Comparison of active and passive spectral sensors in discriminating biomass parameters and nitrogen status in wheat cultivars. Field Crops Res. 2011, 124, 74–84. [Google Scholar] [CrossRef]
- Datt, B. Visible/near infrared reflectance and chlorophyll content in Eucalyptus leaves. Int. J. Remote Sens. 1999, 20, 2741–2759. [Google Scholar] [CrossRef]
- Long, D.S.; Eitel, J.U.; Huggins, D.R. Assessing nitrogen status of dryland wheat using the canopy chlorophyll content index. Crop Manag. 2009, 8. [Google Scholar] [CrossRef]
- Wang, W.; Yao, X.; Yao, X.; Tian, Y.; Liu, X.; Ni, J.; Cao, W.; Zhu, Y. Estimating leaf nitrogen concentration with three-band vegetation indices in rice and wheat. Field Crops Res. 2012, 129, 90–98. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Daughtry, C.; Walthall, C.; Kim, M.; De Colstoun, E.B.; McMurtrey, J. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2000, 76, 156–172. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Haboudane, D.; Tremblay, N.; Miller, J.R.; Vigneault, P. Remote estimation of crop chlorophyll content using spectral indices derived from hyperspectral data. IEEE Trans. Geosci. Remote 2008, 46, 423–437. [Google Scholar] [CrossRef]


| Number of Samples | AGB (t ha−1) | PNC (g kg−1) | PNU (kg ha−1) | NNI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental Plots | Farmers’ Fields | Mean | SD | CV | Mean | SD | CV | Mean | SD | CV | Mean | SD | CV | |
| Calibration dataset | ||||||||||||||
| 2017 (n = 101) | 24 | 77 | 3.41 | 1.40 | 40.9 | 29.8 | 5.62 | 18.9 | 104.6 | 50.9 | 48.7 | 1.13 | 0.32 | 28.5 |
| 2018 (n = 91) | 24 | 67 | 2.50 | 1.14 | 45.5 | 36.8 | 5.92 | 16.1 | 91.5 | 41.5 | 45.4 | 1.23 | 0.27 | 21.7 |
| Across Both Years (n = 192) | 48 | 144 | 2.98 | 1.36 | 45.5 | 33.1 | 6.75 | 20.4 | 98.4 | 47.0 | 47.8 | 1.18 | 0.30 | 25.5 |
| Validation dataset | ||||||||||||||
| 2017 (n = 49) | 12 | 37 | 3.32 | 1.23 | 37.2 | 30.2 | 4.88 | 16.1 | 101.9 | 44.0 | 43.2 | 1.14 | 0.28 | 24.3 |
| 2018 (n = 47) | 12 | 35 | 2.54 | 1.17 | 46.1 | 36.4 | 4.76 | 13.1 | 92.9 | 44.9 | 48.4 | 1.23 | 0.27 | 22.1 |
| Across Both Years (n = 96) | 24 | 72 | 2.94 | 1.26 | 42.9 | 33.3 | 5.72 | 17.2 | 97.5 | 44.5 | 45.6 | 1.18 | 0.28 | 23.4 |
| Normalized Difference VIs | Top 3 VIs | |||||
|---|---|---|---|---|---|---|
| Index | Model | R2 | Index | Model | R2 | |
| AGB (t ha−1) | NDVI | E | 0.70 | NNIRI | P | 0.72 |
| NDRE | P | 0.70 | MSR | P | 0.71 | |
| GNDVI | E | 0.62 | TNDVI | E | 0.70 | |
| PNC (g kg−1) | NDVI | Q | 0.02 | MCCCI | Q | 0.15 |
| NDRE | P | 0.01 | PSRI | Q | 0.09 | |
| GNDVI | Q | 0.03 | GRD | Q | 0.09 | |
| PNU (kg ha−1) | NDVI | Q | 0.58 | REVIopt | P | 0.64 |
| NDRE | P | 0.64 | MSR_RE | P | 0.64 | |
| GNDVI | E | 0.46 | TNDGR | P | 0.64 | |
| NNI | NDVI | Q | 0.31 | TNDGR | Q | 0.46 |
| NDRE | Q | 0.39 | NDGR | Q | 0.46 | |
| GNDVI | Q | 0.20 | GI | Q | 0.46 | |
| NNI-NSI | NSI_NDVI | Q | 0.44 | NSI_REWDRVI | Q | 0.57 |
| NSI_NDRE | Q | 0.52 | NSI_REDVI | Q | 0.57 | |
| NSI_GNDVI | Q | 0.44 | NSI_RERVI | Q | 0.56 | |
| Index | Approach | Areal Agreement (%) | Kappa Statistics |
|---|---|---|---|
| Normalized Difference VIs | NNI-PNC | 54 | 0.30 |
| NNI-PNU | 54 | 0.30 | |
| NNI-VI | 53 | 0.29 | |
| NNI-NSI | 57 | 0.34 | |
| The top VIs | NNI-PNC | 54 | 0.29 |
| NNI-PNU | 52 | 0.28 | |
| NNI-VI | 57 | 0.34 | |
| NNI-NSI | 59 | 0.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Miao, Y.; Lu, J.; Zhou, L.; Li, Y.; Zhang, H.; Lou, W.; Zhang, Z.; Kusnierek, K.; Liu, C. In-Season Diagnosis of Winter Wheat Nitrogen Status in Smallholder Farmer Fields Across a Village Using Unmanned Aerial Vehicle-Based Remote Sensing. Agronomy 2019, 9, 619. https://doi.org/10.3390/agronomy9100619
Chen Z, Miao Y, Lu J, Zhou L, Li Y, Zhang H, Lou W, Zhang Z, Kusnierek K, Liu C. In-Season Diagnosis of Winter Wheat Nitrogen Status in Smallholder Farmer Fields Across a Village Using Unmanned Aerial Vehicle-Based Remote Sensing. Agronomy. 2019; 9(10):619. https://doi.org/10.3390/agronomy9100619
Chicago/Turabian StyleChen, Zhichao, Yuxin Miao, Junjun Lu, Lan Zhou, Yue Li, Hongyan Zhang, Weidong Lou, Zheng Zhang, Krzysztof Kusnierek, and Changhua Liu. 2019. "In-Season Diagnosis of Winter Wheat Nitrogen Status in Smallholder Farmer Fields Across a Village Using Unmanned Aerial Vehicle-Based Remote Sensing" Agronomy 9, no. 10: 619. https://doi.org/10.3390/agronomy9100619
APA StyleChen, Z., Miao, Y., Lu, J., Zhou, L., Li, Y., Zhang, H., Lou, W., Zhang, Z., Kusnierek, K., & Liu, C. (2019). In-Season Diagnosis of Winter Wheat Nitrogen Status in Smallholder Farmer Fields Across a Village Using Unmanned Aerial Vehicle-Based Remote Sensing. Agronomy, 9(10), 619. https://doi.org/10.3390/agronomy9100619







