Foliar Applications of Biostimulants Promote Growth, Yield and Fruit Quality of Strawberry Plants Grown under Nutrient Limitation
Abstract
1. Introduction
2. Materials and Methods
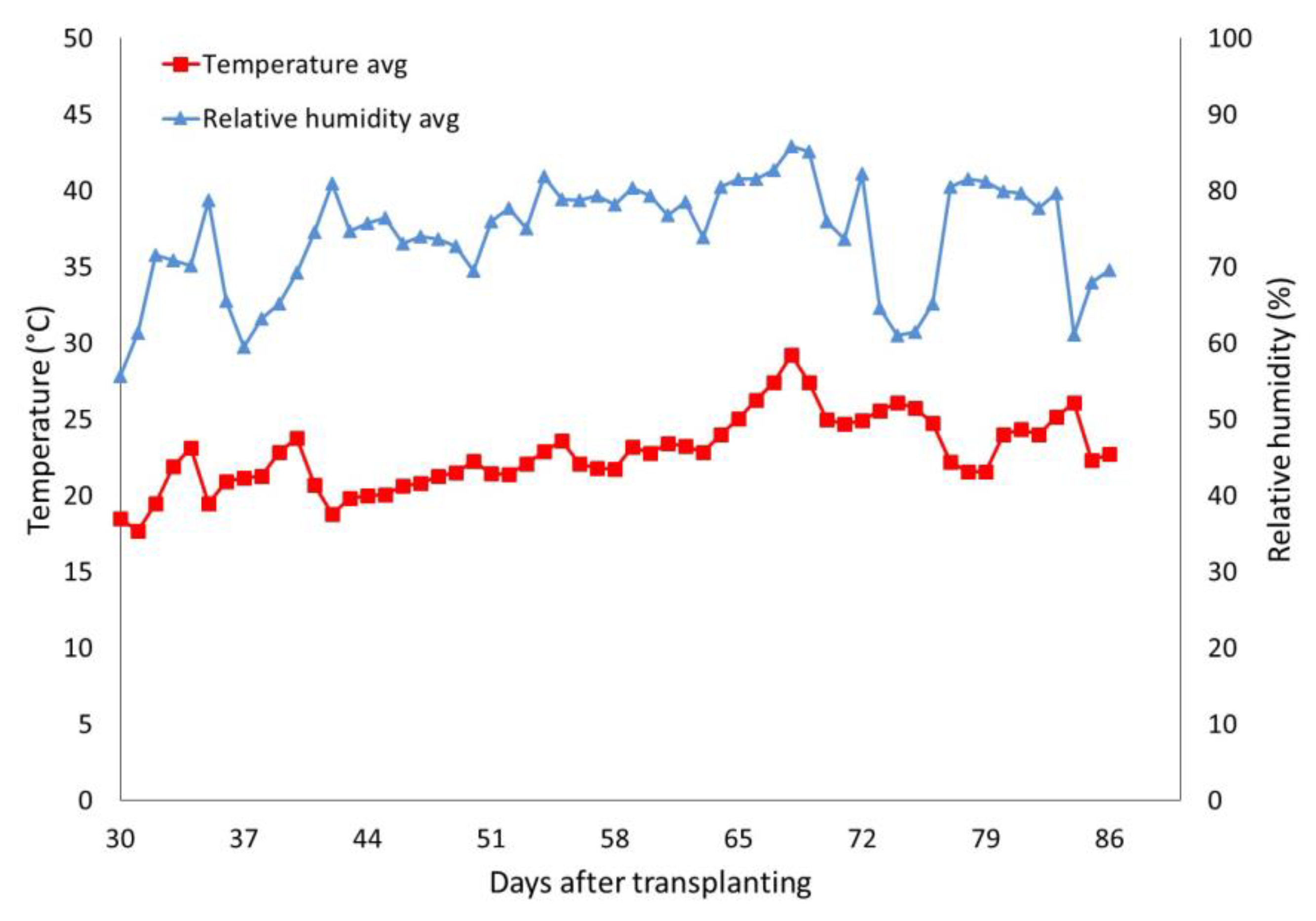
2.1. Experimental Site and Biostimulant Applications
2.2. Vegetative Growth and Leaf Gas Exchanges
2.3. Yield and Fruit Quality
2.4. Biochemical Analysis of Strawberry Fruits
2.4.1. Sample Preparation and Extraction Procedure
2.4.2. Total Polyphenols Content (TPC)
2.4.3. Determination of Total Anthocyanin Content (TAC)
2.4.4. Antioxidant Activity (ABTS)
2.4.5. Ascorbic Acid Quantification
2.4.6. Chemicals
2.5. Mineral Element Analysis in Plant Tissues
2.6. Statistical Analysis
3. Results
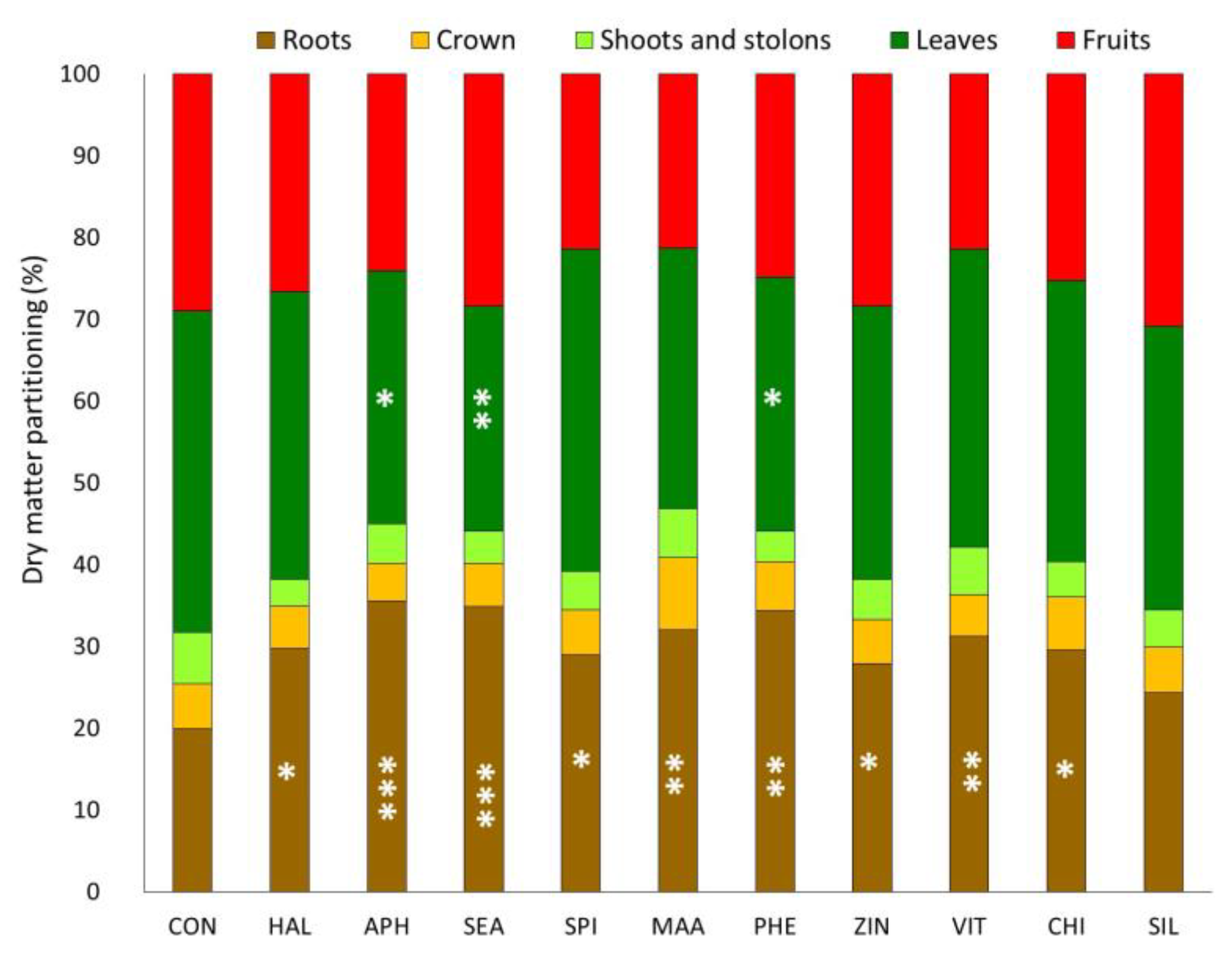
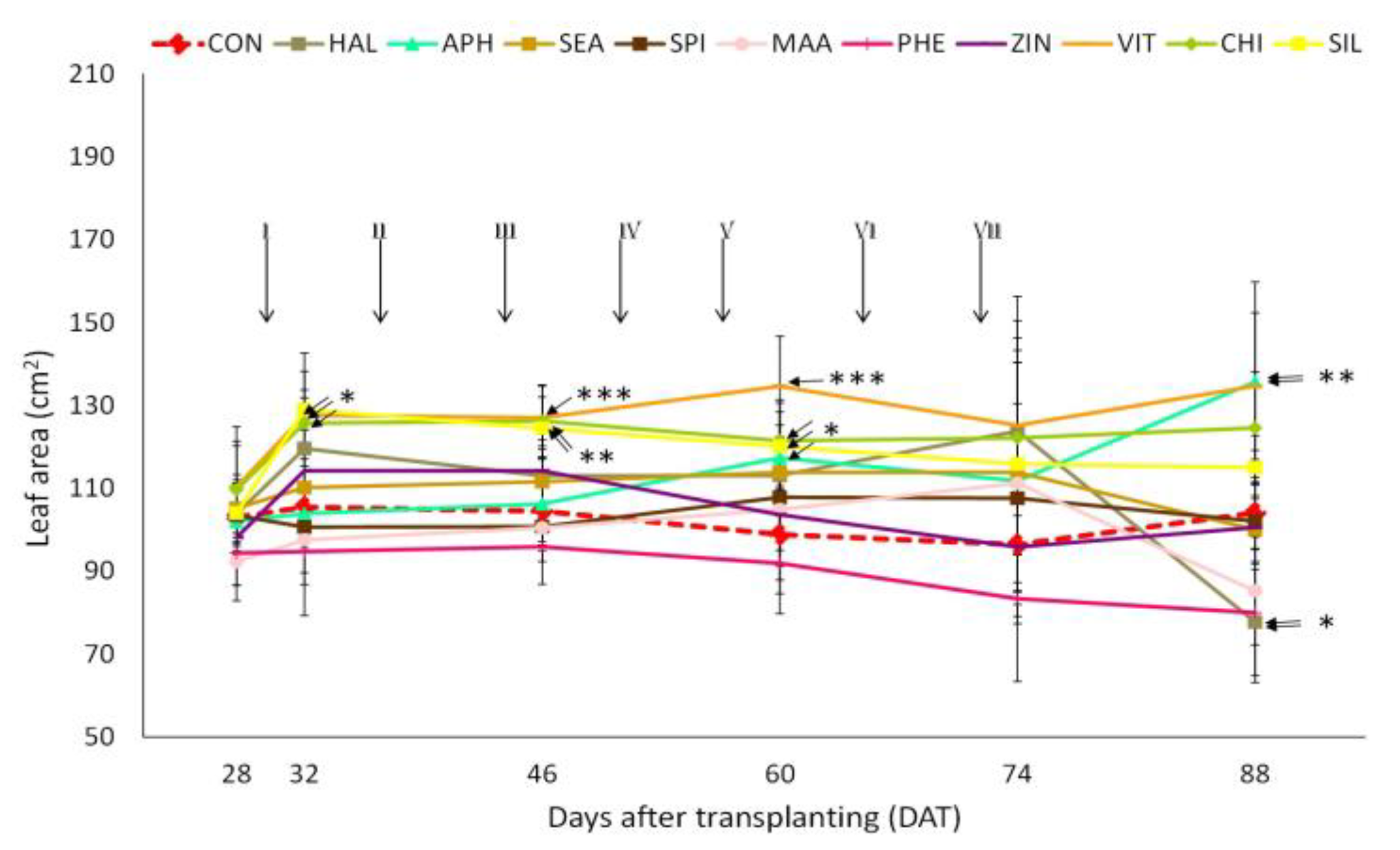
3.1. Plant Growth Parameters
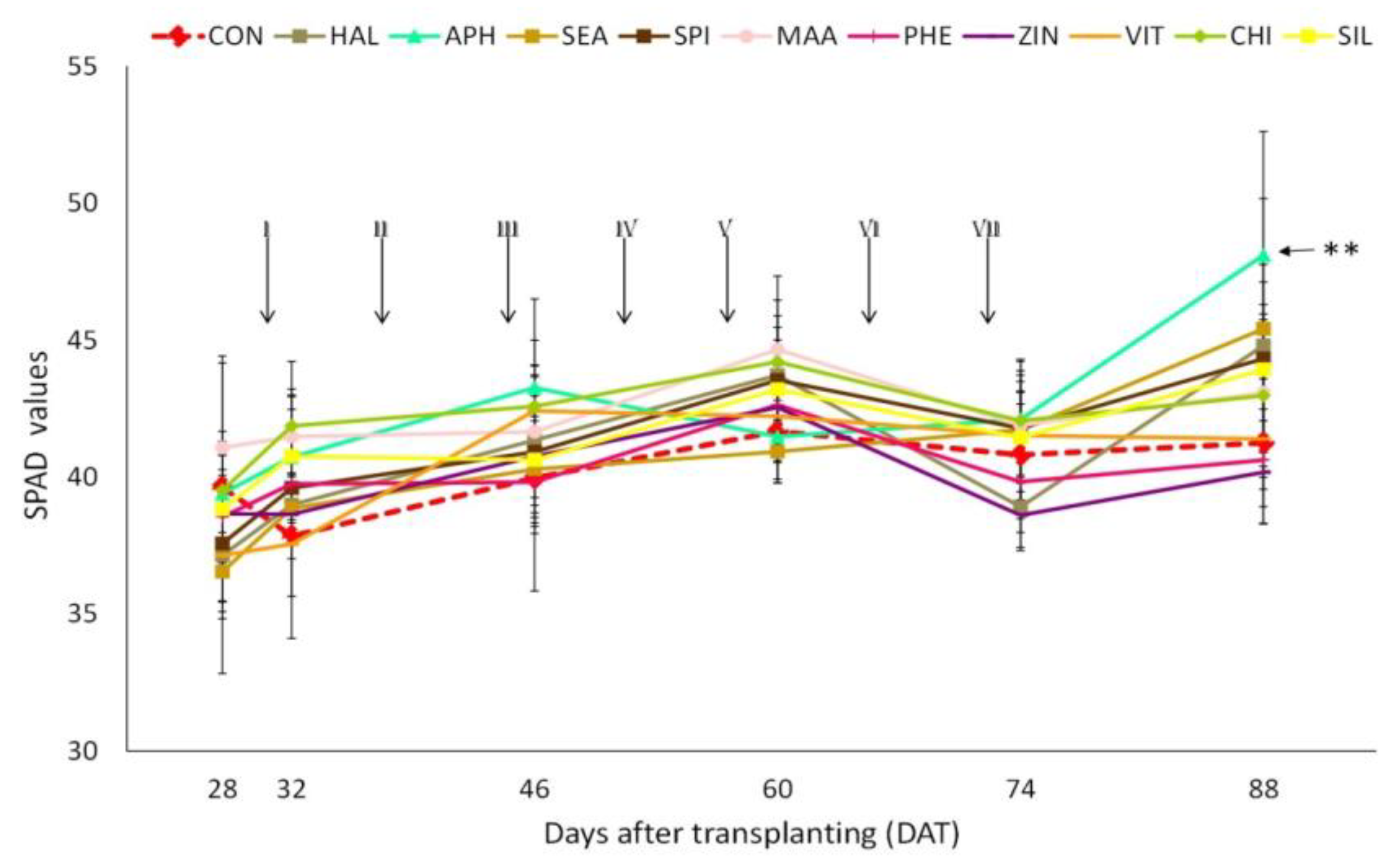
3.2. Nutrient Concentration in Plant Tissues and SPAD Values
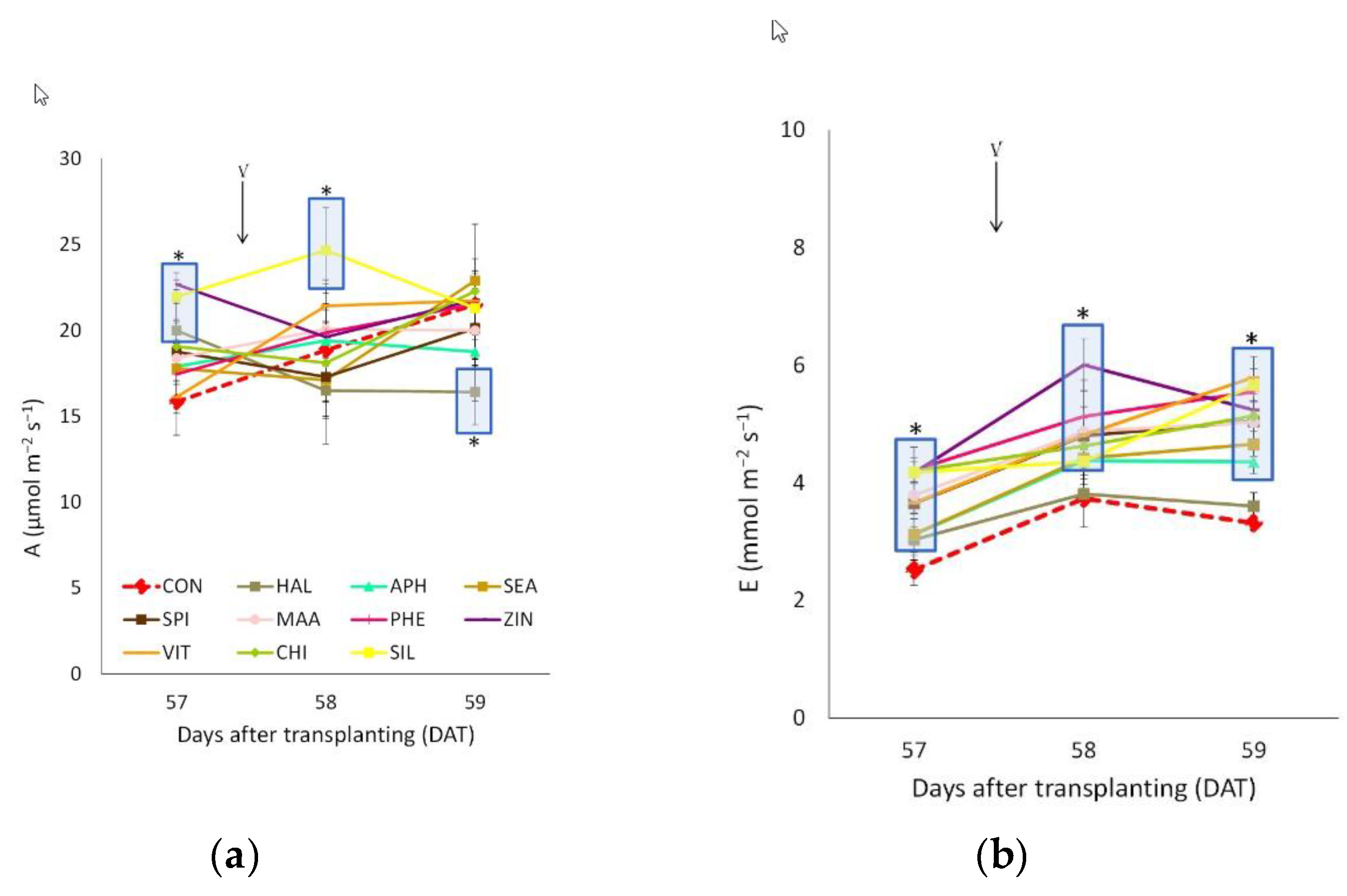
3.3. Leaf Gas Exchanges
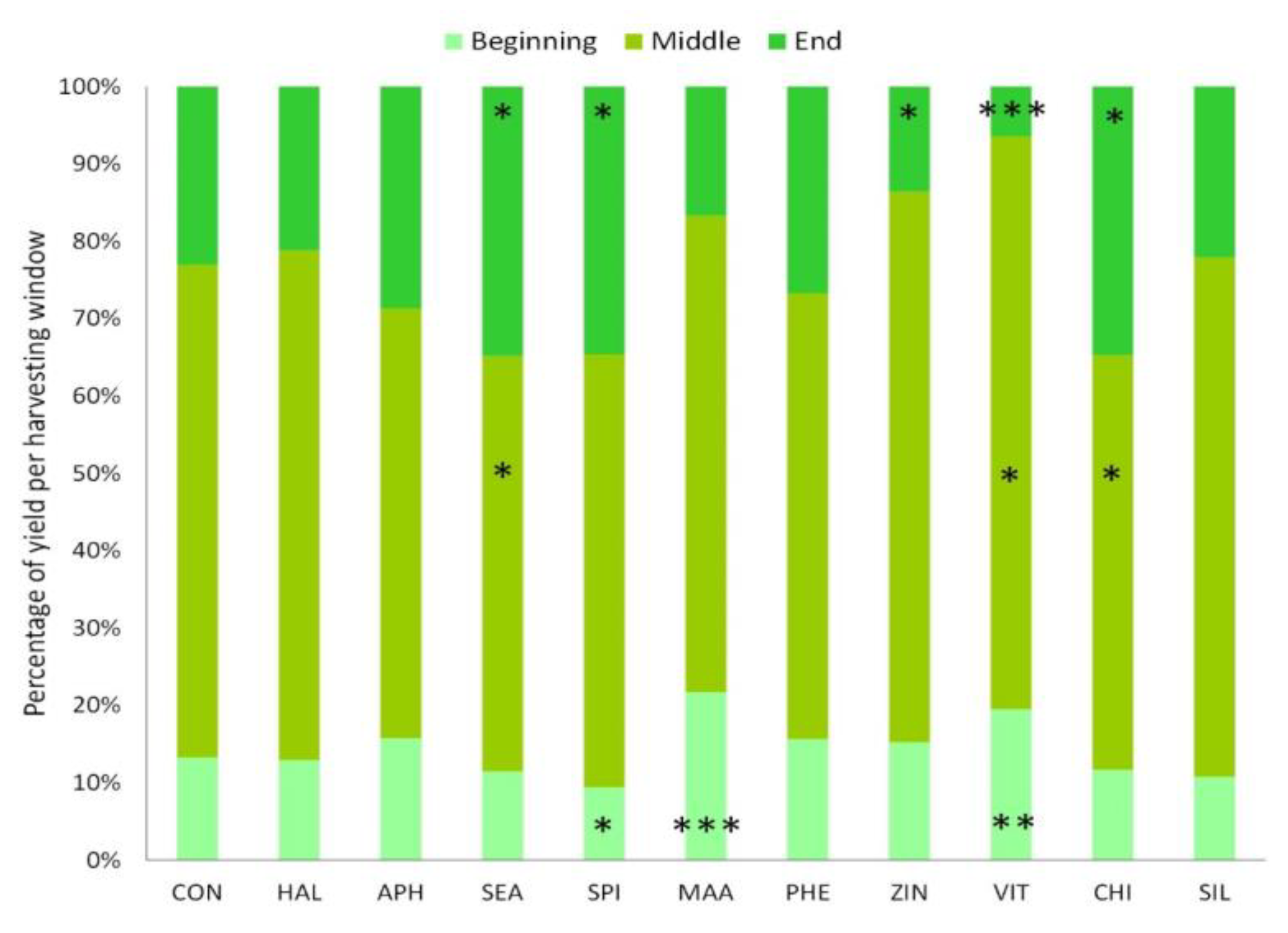
3.4. Yield, Harvest and Fruit Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tagliavini, M.; Baldi, E.; Lucchi, P.; Antonelli, M.; Sorrenti, G.; Baruzzi, G.; Faedi, W. Dynamics of nutrients uptake by strawberry plants (Fragaria × ananassa Dutch.) grown in soil and soilless culture. Eur. J. Agron. 2005, 23, 15–25. [Google Scholar] [CrossRef]
- Albregts, E.E.; Howard, C.M.; Chandler, C.K. Strawberry responses to K rate on a fine sand soil. Hort. Sci. 1991, 26, 135–138. [Google Scholar] [CrossRef]
- Shirko, R.; Nazarideljou, M.J.; Akbar, M.A.; Naser, G. Photosynthetic reaction, mineral uptake, and fruit quality of strawberry affected by different levels of macronutrients. J. Plant Nutr. 2018, 41, 1807–1820. [Google Scholar] [CrossRef]
- Hukkanen, A.T.; Kokko, H.I.; Buchala, A.J.; McDougall, G.J.; Stewart, D.; Kärenlampi, S.O.; Karjalainen, R.O. Benzothiadiazole induces the accumulation of phenolics and improves resistance to powdery mildew in strawberries. J. Agric. Food Chem. 2007, 55, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- de Ponti, T.; Rijk, B.; van Ittersum, M.K. The crop yield gap between organic and conventional agriculture. Agric. Syst. 2012, 108, 1–9. [Google Scholar] [CrossRef]
- van Eysinga, J.R.; van Caem, H.E. Nutrition of glasshouse strawberries with nitrogen, phosphorus and potassium. Sci. Hortic. 1977, 7, 359–368. [Google Scholar] [CrossRef]
- European Council. Available online: https://www.consilium.europa.eu/ (accessed on 11 May 2019).
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, X.; Merewitz, E.; Peng, Y.; Ma, X.; Huang, L.; Yan, Y. Metabolic pathways regulated by chitosan contributing to drought resistance in white clover. J. Proteome Res. 2017, 16, 3039–3052. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Han, W.; Feng, R.; Hu, Y.; Guo, J.; Gong, H. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7, 196. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2018, 82, 277–285. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier Science Publishing Co Inc.: San Diego, CA, USA, 2015; Volume 130, pp. 141–174. ISBN 978-0-12-802137-8. [Google Scholar]
- Mattner, S.W.; Milinkovic, M.; Arioli, T. Increased growth response of strawberry roots to a commercial extract from Durvillaea potatorum and Ascophyllum nodosum. J. Appl. Phycol. 2018, 30, 2943–2951. [Google Scholar] [CrossRef]
- Crouch, I.J.; Beckett, R.P.; Van Staden, J. Effect of seaweed concentrate on the growth and mineral nutrition of nutrient-stressed lettuce. J. Appl. Phycol. 1990, 2, 269–272. [Google Scholar] [CrossRef]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zodape, S.T.; Patolia, J.S. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. South Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Rouphael, Y.; De Micco, V.; Arena, C.; Raimondi, G.; Colla, G.; De Pascale, S. Effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange, and leaf anatomy of zucchini squash grown under saline conditions. J. Appl. Phycol. 2017, 29, 459–470. [Google Scholar] [CrossRef]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. A novel type of seaweed extract as a natural alternative to the use of iron chelates in strawberry production. Sci. Hortic. 2010, 125, 263–269. [Google Scholar] [CrossRef]
- Ghasemi, S.; Khoshgoftarmanesh, A.H.; Afyuni, M.; Hadadzadeh, H. Iron(II)–amino acid chelates alleviate salt-stress induced oxidative damages on tomato grown in nutrient solution culture. Sci. Hortic. 2014, 165, 91–98. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Andreotti, C. Use of biostimulants for organic apple production: Effects on tree growth, yield, and fruit quality at harvest and during storage. Front. Plant Sci. 2018, 9, 1342. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. Hortscience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Mohamed, A.Y.; El-Sehrawy, O.A. Effect of seaweed extract on fruiting of Hindy Bisinnara mango trees. J. Am. Sci. 2013, 9, 537–544. [Google Scholar]
- El-Miniawy, S.; Ragab, M.; Youssef, S.; Metwally, A. Response of strawberry plants to foliar spraying of chitosan. Res. J. Agric. Biol. Sci. 2013, 9, 366–372. [Google Scholar]
- Neri, D.; Lodolini, E.M.; Savini, G.; Sabbatini, P.; Bonanomi, G.; Zucconi, F. Foliar application of humic acids on strawberry (cv Onda). In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS): Leuven, Belgium, November 2002; pp. 297–302. [Google Scholar]
- Roussos, P.A.; Denaxa, N.-K.; Damvakaris, T. Strawberry fruit quality attributes after application of plant growth stimulating compounds. Sci. Hortic. 2009, 119, 138–146. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Zhang, J.; Kirby, C.W.; Ji, X.; Locke, S.J.; Critchley, A.T.; Prithiviraj, B. Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem. 2011, 124, 195–202. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.; Bonini, P.; Colla, G. Plant-and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Cai, Z.; Kastell, A.; Mewis, I.; Knorr, D.; Smetanska, I. Polysaccharide elicitors enhance anthocyanin and phenolic acid accumulation in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Organ Cult. Pctoc 2012, 108, 401–409. [Google Scholar] [CrossRef]
- Portu, J.; López-Alfaro, I.; Gómez-Alonso, S.; López, R.; Garde-Cerdán, T. Changes on grape phenolic composition induced by grapevine foliar applications of phenylalanine and urea. Food Chem. 2015, 180, 171–180. [Google Scholar] [CrossRef]
- El-Sayed, M.E.A. Improving fruit quality and marketing of “Crimson Seedless” grape using some preharvest treatments. J. Hortic. Sci. Ornam. Plants 2013, 5, 218–226. [Google Scholar]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2012, 364, 145–158. [Google Scholar] [CrossRef]
- Landi, L.; Feliziani, E.; Romanazzi, G. Expression of defense genes in strawberry fruits treated with different resistance inducers. J. Agric. Food Chem. 2014, 62, 3047–3056. [Google Scholar] [CrossRef]
- Schiavon, M.; Pizzeghello, D.; Muscolo, A.; Vaccaro, S.; Francioso, O.; Nardi, S. High Molecular Size Humic Substances Enhance Phenylpropanoid Metabolism in Maize (Zea mays L.). J. Chem. Ecol. 2010, 36, 662–669. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef]
- Xie, L.; Wang, Z.H.; Cheng, X.H.; Gao, J.J.; Zhang, Z.P.; Wang, L.J. 5-Aminolevulinic acid promotes anthocyanin accumulation in Fuji apples. Plant Growth Regul. 2013, 69, 295–303. [Google Scholar] [CrossRef]
- Youssef, K.; Sanzani, S.M.; Ligorio, A.; Ippolito, A.; Terry, L.A. Sodium carbonate and bicarbonate treatments induce resistance to postharvest green mould on citrus fruit. Postharvest Biol. Technol. 2014, 87, 61–69. [Google Scholar] [CrossRef]
- Saa, S.; Olivos-Del Rio, A.; Castro, S.; Brown, P.H. Foliar application of microbial and plant based biostimulants increases growth and potassium uptake in almond (Prunus dulcis [Mill.] D. A. Webb). Front. Plant Sci 2015, 6. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Sacks, E.J.; Shaw, D.V. Color change in fresh strawberry fruit of seven genotypes stored at 0C. HortScience 1993, 28, 209–210. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. Aoac Int. 2005, 88, 1269–1278. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bassi, M.; Lubes, G.; Bianchi, F.; Agnolet, S.; Ciesa, F.; Brunner, K.; Guerra, W.; Robatscher, P.; Oberhuber, M. Ascorbic acid content in apple pulp, peel, and monovarietal cloudy juices of 64 different cultivars. Int. J. Food Prop. 2018, 1–9. [Google Scholar] [CrossRef]
- AOAC Vitamin C in juices and vitamin preparations. Official Method 967.21; AOAC Official Methods of Analysis, 18th; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005; p. 45.1.14.
- ISO 16634-1:2008. Food Products—Determination of the Total Nitrogen Content by Combustion According to the Dumas Principle and Calculation of the Crude Protein Content—Part 1: Oilseeds and Animal Feeding Stuffs; International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- EPA 3052. Microwave assisted acid digestion of siliceous and organically based matrics; U.S. Environmental Protection Agency: Washington, DC, USA, 1996.
- EPA 6010D. Inductively coupled plasma–Optical emission spectrometry; U.S. Environmental Protection Agency: Washington, DC, USA, 2018.
- Dunnett, C.W. Multiple comparisons between several treatments and a specified treatment. In Proceedings of the Linear Statistical Inference; Caliński, T., Klonecki, W., Eds.; Springer: New York, NY, USA, 1985; pp. 39–47. [Google Scholar]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. Bmc Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef]
- Jarosz, Z.; Konopinska, J. Effect of substrate type and nitrogen fertilization upon yielding and chemical composition of ‘Elsanta’ strawberry cultivar grown in unheated foil tunnel. Acta Sci. Pol. Hortorum Cultus 2010, 9, 87–96. [Google Scholar]
- Saied, A.S.; Keutgen, A.J.; Noga, G. The influence of NaCl salinity on growth, yield and fruit quality of strawberry cvs. ‘Elsanta’ and ‘Korona’. Sci. Hortic. 2005, 103, 289–303. [Google Scholar] [CrossRef]
- Casierra-Posada, F.; Torres, I.D.; Blanke, M.M. Fruit quality and yield in partially defoliated strawberry plants in the tropical highlands. Gesunde Pflanz. 2013, 65, 107–112. [Google Scholar] [CrossRef]
- Almaliotis, D.; Velemis, D.; Bladenopoulou, S.; Karapetsas, N. Leaf nutrient levels of strawberries (cv Tudla) in relation to crop yield. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2002; pp. 447–450. [Google Scholar]
- Campbell, C.R.; Miner, G.S. Strawberry, annual hill culture. In Reference sufficiency ranges for plant analysis in the southern region of the United States; Southern Cooperative Series Bulletin 394; North Carolina Department of Agriculture & Consumer Services: Raleigh, NC, USA, 2000; ISBN 1-58161-394-6. [Google Scholar]
- Jamali, B.; Eshghi, S.; Tafazoli, E. Mineral composition of ‘Selva’ strawberry as affected by time of application of nitric oxide under saline conditions. Hortic. Env. Biotechnol. 2015, 56, 273–279. [Google Scholar] [CrossRef]
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Górecki, H. Effect of the new plant growth biostimulants based on amino acids on yield and grain quality of winter wheat. Molecules 2018, 23, 470. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, A.L.; Guneri, M.; Ashraf, M. Mitigation effects of silicon on tomato plants bearing fruit grown at high boron levels. J. Plant Nutr. 2011, 34, 1985–1994. [Google Scholar] [CrossRef]
- Tsadilas, C.D.; Kassioti, T.; Mitsios, I.K. Influence of liming and nitrogen forms on boron uptake by tobacco. Commun. Soil Sci. Plant Anal. 2005, 36, 701–708. [Google Scholar] [CrossRef]
- Hellequin, E.; Monard, C.; Quaiser, A.; Henriot, M.; Klarzynski, O.; Binet, F. Specific recruitment of soil bacteria and fungi decomposers following a biostimulant application increased crop residues mineralization. PLoS ONE 2019, 13, e0209089. [Google Scholar] [CrossRef]
- Ertani, A.; Sambo, P.; Nicoletto, C.; Santagata, S.; Schiavon, M.; Nardi, S. The use of organic biostimulants in hot pepper plants to help low input sustainable agriculture. Chem. Biol. Technol. Agric. 2015, 2, 11. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Goyer, A. Thiamine in plants: Aspects of its metabolism and functions. Phytochemistry 2010, 71, 1615–1624. [Google Scholar] [CrossRef]
- Rapala-Kozik, M. Vitamin B1 (thiamine): A cofactor for enzymes involved in the main metabolic pathways and an environmental stress protectant. In Advances in Botanical Research; Rébeillé, F., Douce, R., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 58, pp. 37–91. ISBN 0065-2296. [Google Scholar]
- Mukta, J.; Rahman, M.; As Sabir, A.; Gupta, D.R.; Surovy, M.Z.; Rahman, M.; Islam, M.T. Chitosan and plant probiotics application enhance growth and yield of strawberry. Biocatal. Agric. Biotechnol. 2017, 11, 9–18. [Google Scholar] [CrossRef]
- Hajiboland, R.; Moradtalab, N.; Eshaghi, Z.; Feizy, J. Effect of silicon supplementation on growth and metabolism of strawberry plants at three developmental stages. N. Z. J. Crop Hortic. Sci. 2018, 46, 144–161. [Google Scholar] [CrossRef]
- Agostinho, F.; Tubana, B.; Martins, M.; Datnoff, L. Effect of different silicon sources on yield and silicon uptake of rice grown under varying phosphorus rates. Plants 2017, 6, 35. [Google Scholar] [CrossRef]
- Artyszak, A. Effect of silicon fertilization on Crop yield quantity and quality: A literature review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef]
- DiMario, R.J.; Clayton, H.; Mukherjee, A.; Ludwig, M.; Moroney, J.V. Plant carbonic anhydrases: Structures, locations, evolution, and physiological roles. Mol. Plant 2017, 10, 30–46. [Google Scholar] [CrossRef]
- Sturikova, H.; Krystofova, O.; Huska, D.; Adam, V. Zinc, zinc nanoparticles and plants. J. Hazard. Mater. 2018, 349, 101–110. [Google Scholar] [CrossRef]
- Mattiello, E.M.; Ruiz, H.A.; Neves, J.C.L.; Ventrella, M.C.; Araújo, W.L. Zinc deficiency affects physiological and anatomical characteristics in maize leaves. J. Plant Physiol. 2015, 183, 138–143. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Bhaskara Reddy, M.; Belkacemi, K.; Corcuff, R.; Castaigne, F.; Arul, J. Effect of pre-harvest chitosan sprays on post-harvest infection by Botrytis cinerea and quality of strawberry fruit. Postharvest Biol. Technol. 2000, 20, 39–51. [Google Scholar] [CrossRef]
- Hernández-Muñoz, P.; Almenar, E.; Valle, V.D.; Velez, D.; Gavara, R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria × ananassa) quality during refrigerated storage. Food Chem. 2008, 110, 428–435. [Google Scholar] [CrossRef]
- Khan, A.S.; Ahmad, B.; Jaskani, M.J.; Ahmad, R.; Malik, A.U. Foliar application of mixture of amino acids and seaweed (Ascophyllum nodosum) extract improve growth and physicochemical properties of grapes. Int. J. Agric. Biol. 2012, 14, 383–388. [Google Scholar]
- Cluzet, S.; Torregrosa, C.; Jacquet, C.; Lafitte, C.; Fournier, J.; Mercier, L.; Salamagne, S.; Briand, X.; Esquerre’-Tugaye’, M.-T.; Dumas, B. Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp. Plant Cell Env. 2004, 27, 917–928. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant. Sci. 2014, 5. [Google Scholar] [CrossRef]
- Olkhovych, O.; Volkogon, M.; Taran, N.; Batsmanova, L.; Kravchenko, I. The Effect of copper and zinc nanoparticles on the growth parameters, contents of ascorbic acid, and qualitative composition of amino acids and acylcarnitines in Pistia stratiotes L. (Araceae). Nanoscale Res. Lett. 2016, 11. [Google Scholar] [CrossRef]
| Physico-Chemical Composition of Substrate | Composition of the Nutrient Solution Used at Transplant | ||
|---|---|---|---|
| pH | 5.1 | N (mg L−1) | 240 |
| Dry matter (%) | 42.6 | P2O5 (mg L−1) | 100 |
| Humidity (%) | 57.4 | K2O (mg L−1) | 100 |
| Soluble salts (g L−1) | 2.0 | B (mg L−1) | 0.2 |
| NO3 (mg L−1) | 3.3 | Cu (mg L−1) | 0.1 |
| NH4 (mg L−1) | 462.7 | Fe (mg L−1) | 1.2 |
| P2O5 (mg L−1) | 37.0 | Mn (mg L−1) | 0.5 |
| K2O (mg L−1) | 245.0 | Mo (mg L−1) | 0.1 |
| Mg (mg L−1) | 121.0 | Zn (mg L−1) | 0.3 |
| Na (mg L−1) | 13.0 | ||
| B (mg L−1) | 0.26 | ||
| Fe (mg L−1) | 63.0 | ||
| Mn (mg L−1) | 10.2 | ||
| Cu (mg L−1) | 1.2 | ||
| Zn (mg L−1) | 2.1 | ||
| Dry Weight of Plant Organs (g DW a plant−1) | Mineral Element Concentration | Leaves | Roots | |
|---|---|---|---|---|
| Leaves | 2.07 ± 0.31 | N (%) | 3.90 ± 0.28 | 2.65 ± 0.19 |
| Crown | 0.71 ± 0.23 | P (%) | 0.55 ± 0.10 | 0.43 ± 0.07 |
| Roots | 2.17 ± 0.58 | K (%) | 3.14 ± 0.20 | 0.76 ± 0.16 |
| Total weight | 4.95 ± 0.97 | Ca (%) | 1.39 ± 0.20 | 1.33 ± 0.34 |
| Mg (%) | 0.42 ± 0.03 | 0.32 ± 0.06 | ||
| S (%) | 0.52 ± 0.26 | 0.42 ± 0.13 | ||
| B (mg kg−1) | 35.58 ± 13.20 | 22.93 ± 1.66 | ||
| Cu (mg kg−1) | 19.98 ± 9.67 | 115.13 ± 65.82 | ||
| Fe (mg kg−1) | 74.68 ± 32.96 | 149.83 ± 40.22 | ||
| Mn (mg kg−1) | 168.35 ± 5.84 | 61.80 ± 23.66 | ||
| Zn (mg kg−1) | 33.35 ± 5.32 | 85.68 ± 40.17 | ||
| Na (mg kg−1) | 163.59 ± 91.87 | 299.73 ± 100.71 | ||
| Si (mg kg−1) | 382.35 ± 61.93 | 181.83 ± 32.92 | ||
| Treatment | Active Ingredients | Moisture (%) | Ash (%) | Density (kg dm−3) | Organic Matter (%) | pH | Electrical Conductivity (dS m−1) | Total Organic C (% w w−1) | Total Organic N (% w w−1) | Free Amino Acids (% w w−1) | Total Amino Acids (% w w−1) | Other Characteristics | Concentration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | Water | ||||||||||||
| HAL | Humic acids | - | - | 1.1 | - | 9.2 | 1.2 | 7.5 | 0.1 | - | - | - | 1.0 g L−1 |
| APH | Alfalfa protein hydrolysate | 70.0 | 7.0 | 1.2 | 23.0 | 5.5 | 1.6 | - | - | 1.5 | 5.1 | - | 3.0 g L−1 |
| SEA | Macroseaweed extract | 84.0 | 1.5 | 1.0 | 14.5 | 4.5 | 0.4 | 3 | ≤0.1 | - | - | From A. nodosum | 4.0 g L−1 |
| SPI | Microalga hydrolysate | - | - | 1.2 | - | 5.5 | 1.5 | 16.8 | 3.9 | 6.5 | - | From Spirulina spp (37% hydrol. degree) | 4.0 g L−1 |
| MAA | Mix of amino acids | 45.0 | 5.0 | 1.2 | 50.0 | 5.5 | 0.8 | 24.5 | 9 | 1.5 | 55.0 | - | 3.0 g L−1 |
| PHE | MAA combined with pure phenylalanine | 55.0 | 5.0 | 1.2 | 40.0 | 5.5 | - | 19.6 | 7.2 | 1.8 | 45.0 | Phenylalanine (1%) | 3.0 g L−1 |
| ZIN | MAA combined with zinc | 55.0 | 7.0 | 1.2 | 38.0 | 5.5 | - | 19.6 | 7.2 | 0.8 | 44.0 | Zn (2%) | 3.0 g L−1 |
| VIT | B-group vitamins (Sigma-Aldrich, USA) | - | - | - | - | - | - | - | - | - | - | B1-thiamine (33.3%), B2-riboflavin (33.3%), B6-pyridoxine (33.3%) | 1.0 g L−1 |
| CHI | Chitosan—ChitoPlant Solution® (Agritalia, Italy) | 98.3 | 0.01 | - | 1.7 | 5.2 | - | - | - | - | - | - | 10 mL L−1 |
| SIL | Siliforce® (ILSA S.p.A., Italy) | - | - | 1.2 | - | 2.0 | - | - | - | - | - | Si (8 g kg−1), Zn (1.8%), Mo (0.2%) | 0.3 mL L−1 |
| Treatment | Leaves | Shoots and Stolons | Crowns | Roots | Fruits |
|---|---|---|---|---|---|
| CON | 3.35 ± 1.32 1 | 0.87 ± 0.38 | 0.04 ± 0.25 | 0.59 ± 0.87 | 4.01 ± 0.19 |
| HAL | 3.17 ± 2.36 | 0.47 ± 0.20 | 0.07 ± 0.16 | 2.26 ± 1.72 | 3.96 ± 0.15 |
| APH | 3.47 ± 2.08 | 0.85 ± 0.30 | 0.13 ± 0.21 | 4.19 ± 0.34 *** | 4.31 ± 0.15 |
| SEA | 2.05 ± 1.16 | 0.59 ± 0.22 | 0.08 ± 0.13 | 3.06 ± 1.49 * | 4.26 ± 0.33 |
| SPI | 4.85 ± 1.81 | 0.83 ± 0.21 | 0.25 ± 0.25 | 2.91 ± 1.19 * | 3.76 ± 0.20 |
| MAA | 0.87 ± 0.78 * | 0.56 ± 0.39 | 0.10 ± 0.22 | 0.79 ± 0.97 | 1.98 ± 0.58 *** |
| PHE | 1.70 ± 1.47 | 0.45 ± 0.37 | 0.00 ± 0.17 | 2.00 ± 1.70 | 3.01 ± 0.16 *** |
| ZIN | 2.40 ± 1.00 | 0.65 ± 0.26 | 0.01 ± 0.22 | 1.56 ± 0.64 | 3.79 ± 0.08 |
| VIT | 5.30 ± 1.17 | 1.16 ± 0.21 | 0.33 ± 0.39 | 4.13 ± 2.29 ** | 4.32 ± 0.12 |
| CHI | 4.81 ± 0.85 | 0.86 ± 0.42 | 0.58 ± 0.37 | 3.75 ± 1.85 ** | 5.05 ± 0.39 *** |
| SIL | 3.14 ± 1.42 | 0.69 ± 0.44 | 0.12 ± 0.31 | 1.49 ± 1.19 | 4.64 ± 0.57 * |
| Treatment | N (% DW) | P (% DW) | K (% DW) | Ca (% DW) | Mg (% DW) | S (% DW) |
|---|---|---|---|---|---|---|
| CON | 2.59 ± 0.25 1 | 0.51 ± 0.04 | 2.52 ± 0.18 | 2.37 ± 0.15 | 0.38 ± 0.02 | 0.22 ± 0.03 |
| HAL | 2.46 ± 0.04 | 0.52 ± 0.05 | 2.72 ± 0.42 | 2.27 ± 0.27 | 0.39 ± 0.02 | 0.22 ± 0.03 |
| APH | 2.60 ± 0.27 | 0.58 ± 0.08 | 2.64 ± 0.12 | 2.26 ± 0.22 | 0.38 ± 0.01 | 0.22 ± 0.03 |
| SEA | 2.56 ± 0.17 | 0.55 ± 0.05 | 2.81 ± 0.38 | 2.25 ± 0.20 | 0.39 ± 0.03 | 0.24 ± 0.05 |
| SPI | 2.49 ± 0.35 | 0.56 ± 0.07 | 2.58 ± 0.23 | 2.42 ± 0.16 | 0.42 ± 0.04 | 0.24 ± 0.07 |
| MAA | 2.56 ± 0.09 | 0.62 ± 0.04 | 2.59 ± 0.12 | 2.14 ± 0.13 | 0.38 ± 0.03 | 0.20 ± 0.01 |
| PHE | 2.74 ± 0.39 | 0.52 ± 0.11 | 2.77 ± 0.08 | 2.42 ± 0.32 | 0.40 ± 0.03 | 0.24 ± 0.05 |
| ZIN | 2.47 ± 0.16 | 0.52 ± 0.09 | 2.75 ± 0.08 | 2.16 ± 0.24 | 0.37 ± 0.03 | 0.21 ± 0.05 |
| VIT | 2.38 ± 0.29 | 0.62 ± 0.02 | 2.79 ± 0.32 | 2.24 ± 0.18 | 0.42 ± 0.02 | 0.19 ± 0.03 |
| CHI | 2.36 ± 0.17 | 0.54 ± 0.05 | 2.69 ± 0.15 | 2.10 ± 0.15 | 0.39 ± 0.01 | 0.20 ± 0.01 |
| SIL | 2.38 ± 0.21 | 0.51 ± 0.06 | 2.74 ± 0.25 | 2.16 ± 0.15 | 0.37 ± 0.03 | 0.18 ± 0.03 |
| Treatment | B (mg kg−1 DW) | Fe (mg kg−1 DW) | Mn (mg kg−1 DW) | Cu (mg kg−1 DW) | Zn (mg kg−1 DW) | Na (mg kg−1 DW) | Si (mg kg−1 DW) |
|---|---|---|---|---|---|---|---|
| CON | 66.58 ± 10.93 | 81.65 ± 4.89 | 381.88 ± 107.48 | 7.00 ± 0.00 | 28.93 ± 3.01 | 240.12 ± 84.80 | 416.88 ± 61.75 |
| HAL | 58.75 ± 3.78 | 111.15 ± 28.87 | 331.58 ± 47.57 | 10.10 ± 3.59 | 34.53 ± 10.27 | 319.56 ± 45.58 | 538.53 ± 158.67 |
| APH | 60.55 ± 7.41 | 102.68 ± 32.96 | 355.08 ± 60.70 | 7.35 ± 0.64 | 25.98 ± 1.79 | 251.92 ± 126.22 | 782.65 ± 68.74 *** |
| SEA | 55.93 ± 3.32 | 197.75 ± 143.89 | 300.90 ± 101.74 | 7.15 ± 0.30 | 28.73 ± 6.49 | 363.06 ± 74.44 | 424.30 ± 18.28 |
| SPI | 62.08 ± 16.99 | 114.60 ± 13.64 | 378.10 ± 104.30 | 7.00 ± 0.00 | 31.75 ± 2.32 | 327.93 ± 127.08 | 471.50 ± 33.98 |
| MAA | 61.18 ± 5.57 | 800.55 ± 1348.74 | 367.60 ± 57.87 | 9.53 ± 3.18 | 34.88 ± 7.92 | 243.16 ± 66.33 | 457.83 ± 113.69 |
| PHE | 61.75 ± 13.01 | 94.93 ± 16.11 | 375.75 ± 117.59 | 7.38 ± 0.75 | 31.38 ± 4.81 | 440.30± 262.85 | 480.68 ± 146.04 |
| ZIN | 59.43 ± 6.13 | 310.85 ± 332.71 | 285.73 ± 83.13 | 7.65 ± 1.23 | 127.43 ± 13.95 *** | 270.27 ± 68.68 | 624.68 ± 98.52 ** |
| VIT | 62.40 ± 5.67 | 91.75 ± 13.21 | 347.75 ± 59.23 | 7.58 ± 0.67 | 36.15 ± 4.11 | 234.82 ± 79.06 | 398.65 ± 71.72 |
| CHI | 59.50 ± 4.46 | 115.40 ± 43.02 | 393.08 ± 38.55 | 7.13 ± 0.25 | 30.90 ± 0.85 | 218.92 ± 20.99 | 487.33 ± 110.15 |
| SIL | 60.40 ± 4.43 | 93.48 ± 23.08 | 366.53 ± 87.71 | 7.23 ± 0.45 | 83.80 ± 13.61 *** | 254.18 ± 126.09 | 689.13 ± 41.81 *** |
| Treatment | N (% DW) | P (% DW) | K (% DW) | Ca (% DW) | Mg (% DW) | S (% DW) |
|---|---|---|---|---|---|---|
| CON | 1.44 ± 0.12 1 | 0.15 ± 0.03 | 0.49 ± 0.18 | 1.60 ± 0.22 | 0.21 ± 0.02 | 0.35 ± 0.03 |
| HAL | 1.28 ± 0.17 | 0.13 ± 0.02 | 0.46 ± 0.16 | 1.74 ± 0.17 | 0.22 ± 0.01 | 0.37 ± 0.08 |
| APH | 1.45 ± 0.14 | 0.14 ± 0.05 | 0.44 ± 0.22 | 1.85 ± 0.29 | 0.23 ± 0.05 | 0.45 ± 0.09 |
| SEA | 1.41 ± 0.15 | 0.14 ± 0.03 | 0.40 ± 0.08 | 1.97 ± 0.16 | 0.23 ± 0.02 | 0.50 ± 0.08 |
| SPI | 1.35 ± 0.17 | 0.13 ± 0.02 | 0.43 ± 0.09 | 1.77 ± 0.16 | 0.22 ± 0.03 | 0.40 ± 0.07 |
| MAA | 1.65 ± 0.18 | 0.18 ± 0.03 | 0.49 ± 0.07 | 1.68 ± 0.21 | 0.22 ± 0.03 | 0.46 ± 0.13 |
| PHE | 1.39 ± 0.17 | 0.14 ± 0.02 | 0.48 ± 0.18 | 1.86 ± 0.23 | 0.20 ± 0.02 | 0.45 ± 0.13 |
| ZIN | 1.45 ± 0.08 | 0.15 ± 0.02 | 0.50 ± 0.14 | 1.82 ± 0.17 | 0.24 ± 0.04 | 0.43 ± 0.07 |
| VIT | 1.44 ± 0.08 | 0.14 ± 0.02 | 0.45 ± 0.09 | 1.91 ± 0.35 | 0.26 ± 0.01 | 0.57 ± 0.28 |
| CHI | 1.26 ± 0.19 | 0.13 ± 0.03 | 0.39 ± 0.03 | 1.93 ± 0.17 | 0.23 ± 0.01 | 0.46 ± 0.12 |
| SIL | 1.40 ± 0.07 | 0.17 ± 0.03 | 0.59 ± 0.18 | 1.69 ± 0.25 | 0.23 ± 0.02 | 0.41 ± 0.13 |
| Treatment | B (mg kg−1 DW) | Fe (mg kg−1 DW) | Mn (mg kg−1 DW) | Cu (mg kg−1 DW) | Zn (mg kg−1 DW) | Na (mg kg−1 DW) | Si (mg kg−1 DW) |
|---|---|---|---|---|---|---|---|
| CON | 17.28 ± 2.29 | 484.63 ± 94.84 | 56,25 ± 23,07 | 80.08 ± 53.24 | 77.08 ± 43.06 | 702.14 ± 81.07 | 350.25 ± 78.52 |
| HAL | 13.10 ± 3.45 * | 1227.43 ± 538.00 | 51,43 ± 22,80 | 98.13 ± 73.45 | 67.48 ± 56.12 | 662.19 ± 118.41 | 528.63 ± 97.22 * |
| APH | 11.43 ± 2.66 ** | 620.83 ± 215.52 | 81,40 ± 12,66 | 52.65 ± 19.62 | 44.98 ± 30.09 | 624.75 ± 77.51 | 444.93 ± 92.27 |
| SEA | 10.90 ± 0.98 *** | 724.53 ± 214.65 | 55,35 ± 38,78 | 146.40 ± 84.29 | 42.95 ± 15.41 | 681.68 ± 81.97 | 538.88 ± 105.99 * |
| SPI | 12.35 ± 2.64 ** | 1807.48 ± 1745.77 ** | 75,45 ± 31,44 | 68.90 ± 52.69 | 57.78 ± 38.74 | 624.80 ± 141.86 | 683.63 ± 182.23 *** |
| MAA | 13.73 ± 3.36 | 648.95 ± 150.92 | 70,58 ± 19,96 | 207.95 ± 222.50 | 127.05 ± 94.10 | 579.94 ± 100.10 | 515.35 ± 127.19 |
| PHE | 12.98 ± 1.78 * | 691.78 ± 309.30 | 97,50 ± 88,66 | 83.70 ± 113.54 | 40.78 ± 15.81 | 586.40 ± 61.30 | 461.80 ± 117.93 |
| ZIN | 12.33 ± 2.46 ** | 808.08 ± 157.22 | 70,25 ± 38,06 | 69.63 ± 52.43 | 139.78 ± 32.20 * | 643.41 ± 191.04 | 689.03 ± 93.68 *** |
| VIT | 8.53 ± 1.98 *** | 1461.73 ± 956.78 * | 100,13 ± 73,10 | 228.33 ± 63.72 | 95.98 ± 35.03 | 600.22 ± 90.50 | 631.18 ± 99.86 ** |
| CHI | 10.33 ± 2.76 *** | 1079.63 ± 661.56 | 133,63 ± 39,71 | 80.23 ± 41.43 | 38.28 ± 8.27 | 655.94 ± 19.66 | 623.28 ± 187.63 ** |
| SIL | 12.68 ± 1.91 * | 519.48 ± 183.10 | 55,30 ± 29,18 | 69.08 ± 50.39 | 67.98 ± 22.73 | 565.72 ± 98.53 | 590.98 ± 57.86 ** |
| Treatment | Total Yield (g plant−1 FW a) | Number Fruits Plant−1 (N°) | Mean Fruit Weight (g FW) |
|---|---|---|---|
| CON | 50.84 ± 3.57 1 | 7.75 ± 0.25 | 6.55 ± 0.24 |
| HAL | 50.95 ± 1.95 | 7.42 ± 0.72 | 6.97 ± 0.42 |
| APH | 51.67 ± 2.90 | 7.00 ± 0.25 | 7.43 ± 0.46 * |
| SEA | 53.57 ± 1.17 | 8.25 ± 0.87 | 6.52 ± 0.40 |
| SPI | 51.05 ± 7.71 | 7.17 ± 0.38 | 7.16 ± 0.07 |
| MAA | 25.95 ± 4.69 *** | 4.50 ± 0.66 *** | 5.74 ± 0.76 * |
| PHE | 39.59 ± 2.73 ** | 6.58 ± 0.38 * | 6.08 ± 0.43 |
| ZIN | 47.51 ± 1.89 | 7.17 ± 0.58 | 6.82 ± 0.84 |
| VIT | 54.58 ± 2.94 | 8.25 ± 0.25 | 6.60 ± 0.09 |
| CHI | 64.88 ± 3.02 *** | 8.42 ± 0.80 | 7.72 ± 0.22 ** |
| SIL | 59.57 ± 6.29 * | 7.92 ± 0.88 | 7.54 ± 0.11 * |
| Treatment | FF (kg cm−2) | TSS (° Brix) | TA (g L−1) | C * | h° |
|---|---|---|---|---|---|
| CON | 0.71 ± 0.02 1 | 7.07 ± 0.06 | 9.11 ± 0.09 | 27.41 ± 1.36 | 18.31 ± 3.46 |
| HAL | 0.69 ± 0.02 | 7.73 ± 0.61 | 9.32 ± 0.08 | 31.58 ± 2.71 * | 17.72 ± 3.90 |
| APH | 0.73 ± 0.02 | 7.07 ± 0.51 | 9.48 ± 0.18 | 34.51 ± 2.54 *** | 17.28 ± 3.70 |
| SEA | 0.72 ± 0.01 | 7.73 ± 0.50 | 9.74 ± 0.19 | 34.72 ± 3.74 *** | 18.68 ± 2.01 |
| SPI | 0.58 ± 0.09 ** | 7.03 ± 0.15 | 10.19 ± 0.99 * | 29.89 ± 1.07 | 16.13 ± 2.84 |
| MAA | 0.73 ± 0.04 | 8.70 ± 0.70 *** | 8.66 ± 0.40 | 30.06 ± 0.90 | 16.98 ± 0.63 |
| PHE | 0.72 ± 0.03 | 7.23 ± 0.25 | 9.60 ± 0.29 | 28.69 ± 3.23 | 16.23 ± 0.80 |
| ZIN | 0.71 ± 0.03 | 7.97 ± 0.2 * | 9.44 ± 0.11 | 32.86 ± 2.37 ** | 17.03 ± 4.61 |
| VIT | 0.74 ± 0.05 | 7.80 ± 0.44 * | 8.79 ± 0.55 | 30.25 ± 1.99 | 14.38 ± 3.18 |
| CHI | 0.84 ± 0.01 ** | 6.97 ± 0.15 | 8.72 ± 1.00 | 29.05 ± 1.81 | 16.94 ± 2.84 |
| SIL | 0.73 ± 0.11 | 7.37 ± 0.15 | 9.34 ± 0.27 | 29.75 ± 1.04 | 17.08 ± 1.55 |
| Treatment | TPC (mg GAE 100 g−1 DW) | TAC (mg CGE 100 g−1 DW) | ABTS (mg Trolox 100 g−1 DW) | AA (mg AA 100 g−1 DW) |
|---|---|---|---|---|
| CON | 2303.95 ± 116.11 1 | 354.89 ± 55.57 | 2886.04 ± 110.02 | 556.69 ± 37.18 |
| HAL | 2455.53 ± 406.32 | 435.01 ± 85.90 | 2865.18 ± 402.51 | 617.55 ± 102.80 |
| APH | 2803.17 ± 488.83 * | 461.48 ± 56.26 | 3420.95 ± 252.11 | 572.33 ± 12.77 |
| SEA | 2734.73 ± 261.74 * | 478.59 ± 126.19 | 3178.61 ± 296.83 | 548.29 ± 29.01 |
| SPI | 2211.79 ± 105.48 | 408.96 ± 13.62 | 2799.13 ± 44.49 | 582.32 ± 53.40 |
| MAA | 2327.76 ± 122.79 | 369.94 ± 139.69 | 3318.91 ± 463.59 | 587.54 ± 42.27 |
| PHE | 2506.83 ± 240.52 | 475.10 ± 83.04 | 2990.51 ± 316.85 | 539.14 ± 51.51 |
| ZIN | 2098.88 ± 152.98 | 404.44 ± 18.33 | 3011.75 ± 599.56 | 422.90 ± 56.38 * |
| VIT | 2114.04 ± 270.33 | 396.17 ± 100.75 | 2831.57 ± 55.35 | 469.69 ± 10.50 |
| CHI | 2400.41 ± 40.91 | 417.22 ± 31.63 | 3047.21 ± 53.23 | 599.14 ± 84.73 |
| SIL | 2687.08 ± 197.94 | 307.97 ± 37.55 | 3220.11 ± 91.69 | 452.43 ± 94.26 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar Applications of Biostimulants Promote Growth, Yield and Fruit Quality of Strawberry Plants Grown under Nutrient Limitation. Agronomy 2019, 9, 483. https://doi.org/10.3390/agronomy9090483
Soppelsa S, Kelderer M, Casera C, Bassi M, Robatscher P, Matteazzi A, Andreotti C. Foliar Applications of Biostimulants Promote Growth, Yield and Fruit Quality of Strawberry Plants Grown under Nutrient Limitation. Agronomy. 2019; 9(9):483. https://doi.org/10.3390/agronomy9090483
Chicago/Turabian StyleSoppelsa, Sebastian, Markus Kelderer, Claudio Casera, Michele Bassi, Peter Robatscher, Aldo Matteazzi, and Carlo Andreotti. 2019. "Foliar Applications of Biostimulants Promote Growth, Yield and Fruit Quality of Strawberry Plants Grown under Nutrient Limitation" Agronomy 9, no. 9: 483. https://doi.org/10.3390/agronomy9090483
APA StyleSoppelsa, S., Kelderer, M., Casera, C., Bassi, M., Robatscher, P., Matteazzi, A., & Andreotti, C. (2019). Foliar Applications of Biostimulants Promote Growth, Yield and Fruit Quality of Strawberry Plants Grown under Nutrient Limitation. Agronomy, 9(9), 483. https://doi.org/10.3390/agronomy9090483






