Biostimulant Application Enhances Fruit Setting in Eggplant—An Insight into the Biology of Flowering
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Arrangement
2.2. Cultivation Procedures
2.3. Weather Conditions
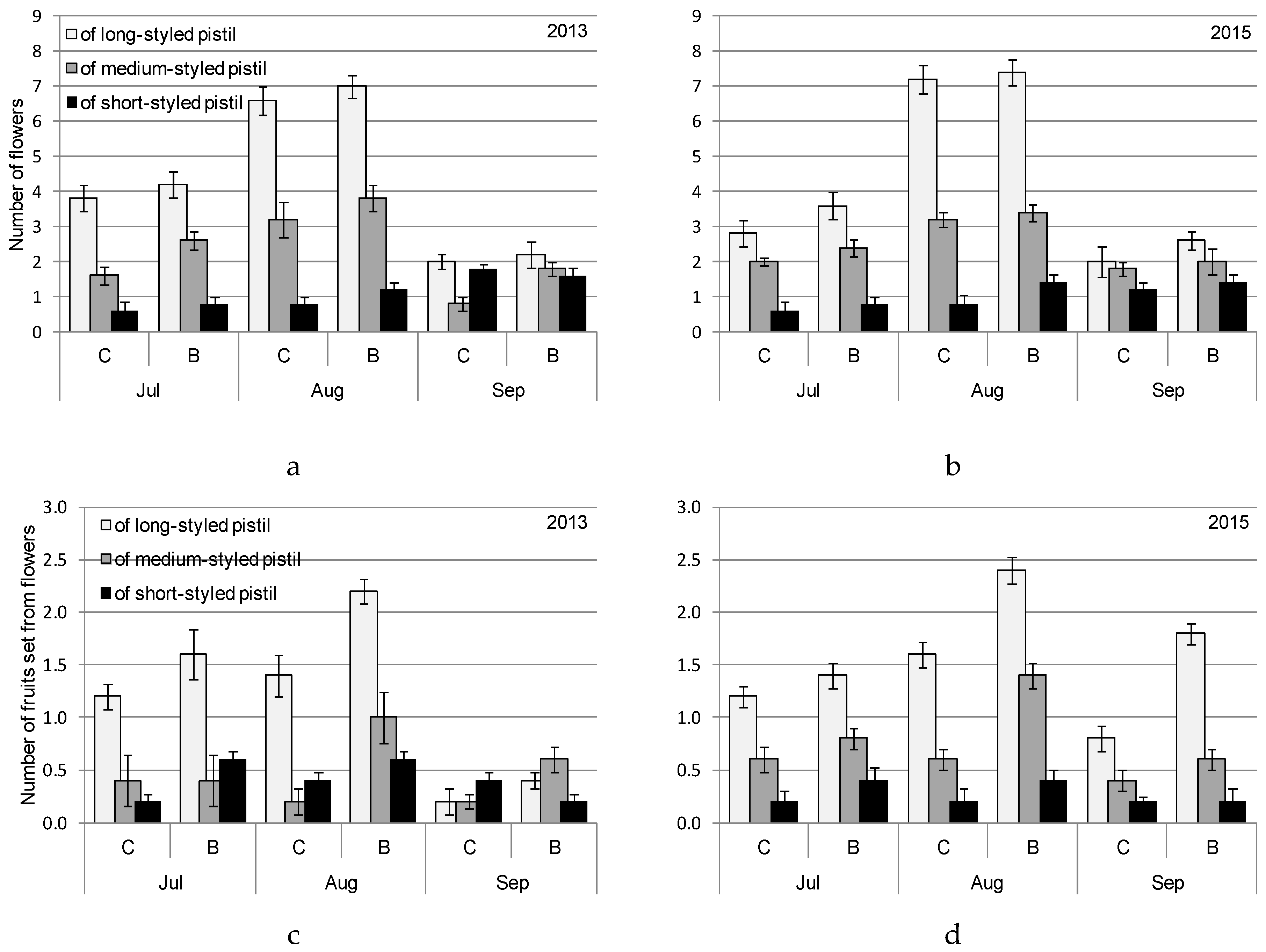
2.4. Procedures for Flowering and Fruit Setting Observations
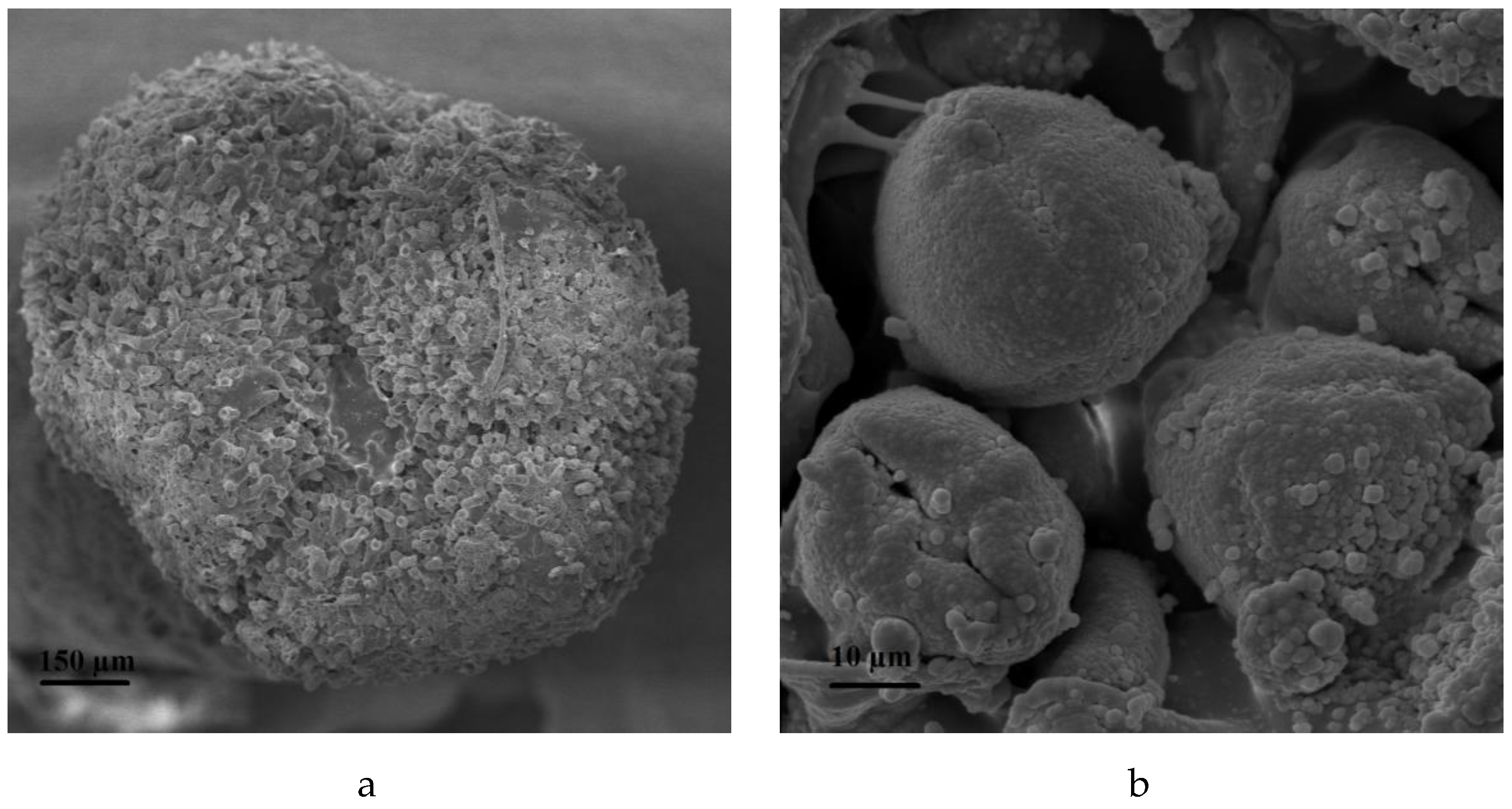
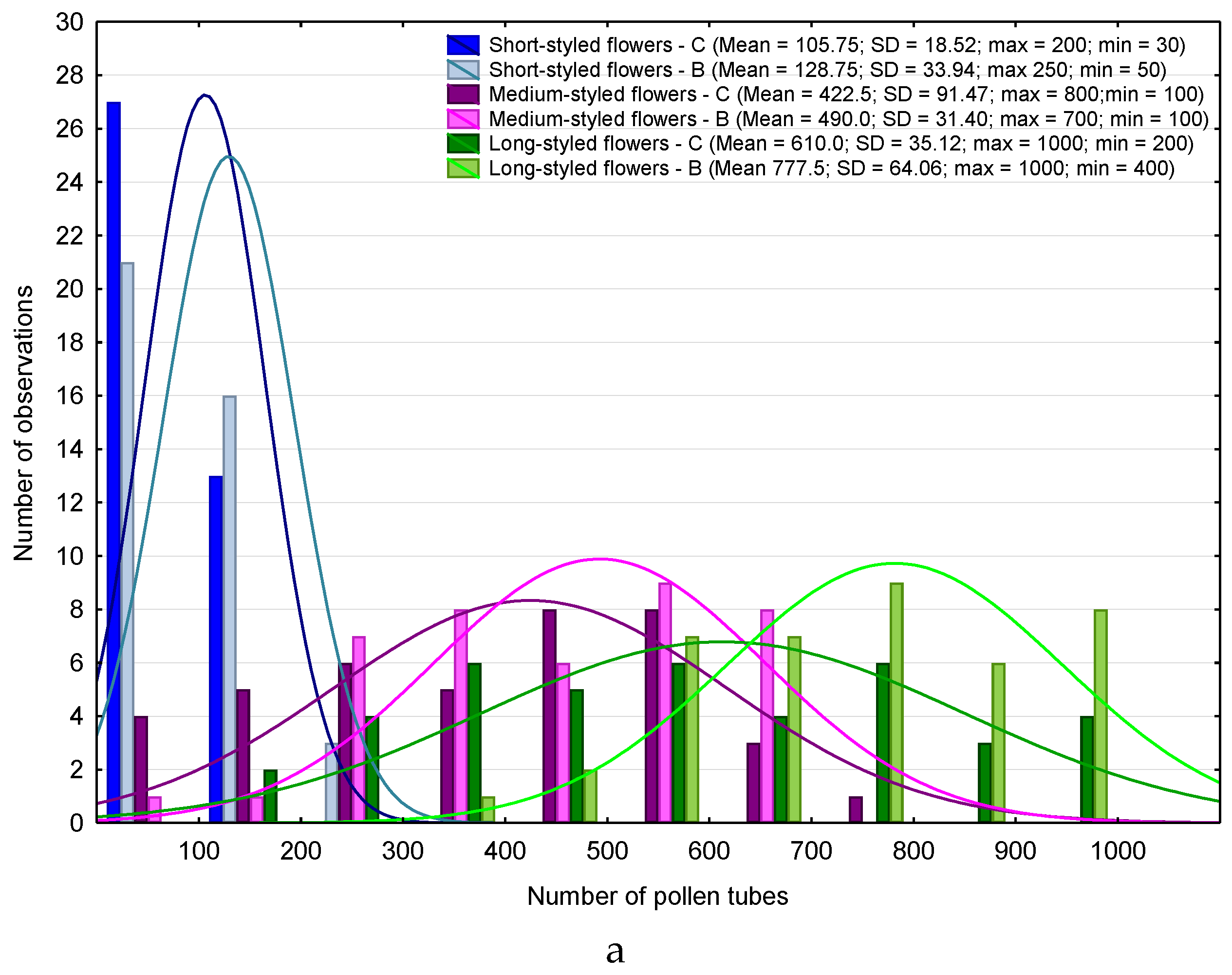
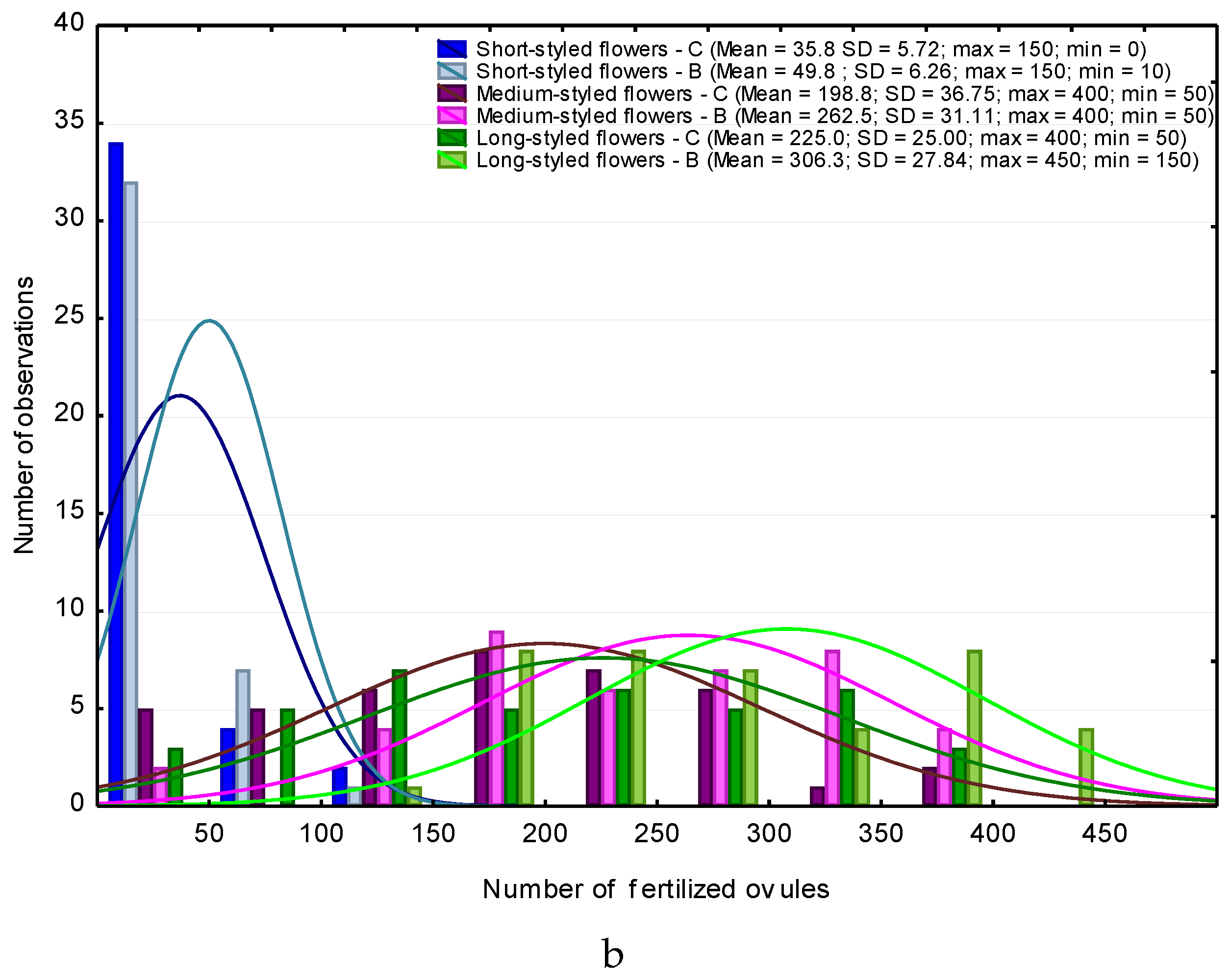
2.5. Procedures for Microscopic Observations
2.6. Statistical Analyses
3. Results
4. Discussion
4.1. Heterostyly Expression in Eggplant as Affected by Biostimulant Treatment and Cultivar
4.2. Biostimulant-Affected Flower and Fruit Set Effectiveness
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pandit, M.K.; Thapa, H.; Akhtar, S.; Hazra, P. Evaluation of brinjal genotypes for growth and reproductive characters with seasonal variation. J. Crop. Weed 2010, 6, 31–34. [Google Scholar]
- Jagatheeswari, D. Morphological studies on flowering plants (Solanaceae). Int. Lett. Nat. Sci. 2014, 10, 36–43. [Google Scholar] [CrossRef]
- Keller, B.; Thomson, J.D.; Conti, E. Heterostyly promotes disassortative pollination and reduces sexual interference in Darwin’s primroses: Evidence from experimental studies. Funct. Ecol. 2014, 28, 1413–1425. [Google Scholar] [CrossRef]
- Glover, B.J.; Bunnewell, S.; Martin, C. Convergent evolution within the genus Solanum: The specialised anther cone develops through alternative pathways. Gene 2004, 331, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Karapanos, I.C.; Mahmood, S.; Thanopoulos, C. Fruit set in solanaceous vegetable crops as affected by floral and environmental factors. Eur. J. Plant Sci. Biotechnol. 2008, 2, 88–105. [Google Scholar]
- Sękara, A.; Bieniasz, M. Pollination, fertilization and fruit formation in eggplant (Solanum melongena L.). Acta Agrobot. 2008, 61, 107–113. [Google Scholar]
- Kowalska, G. The influence of heterostyly, pollination method and harmonization on eggplant’s (Solanum melongena L.) flowering and fruiting. Acta Agrobot. 2003, 56, 61–76. [Google Scholar] [CrossRef]
- Kowalska, G. Eggplant (Solanum melongena L.) flowering and fruiting dynamics depending on pistil type as well as way of pollination and flower harmonization. Folia Hortic. 2006, 18, 17–29. [Google Scholar]
- Araméndiz Tatis, H.; Cardona Ayala, C.; Espitia Camacho, M. Floral morphology characterization of two cultivars of eggplant (Solanum melongena L.) (Solanaceae). Rev. Fac. Nac. Agron. Medellin 2009, 62, 5125–5134. [Google Scholar]
- Rylski, I.; Nothmann, J.; Arcan, L. Differential fertility in short-styled eggplant flowers. Sci. Hortic. 1984, 22, 39–46. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, A.; Li, W.; Jiang, Y.; Song, S.; Li, Y.; Chen, R. Comparative proteomic analysis of eggplant (Solanum melongena L.) heterostylous pistil development. PLoS ONE 2017, 12, e0179018. [Google Scholar] [CrossRef] [PubMed]
- Gemmill-Herren, B.; Ochieng, A.O. Role of native bees and natural habitats in eggplant (Solanum melongena) pollination in Kenya. Agric. Ecosyst. Environ. 2008, 127, 31–36. [Google Scholar] [CrossRef]
- Kikuchi, K.; Honda, I.; Matsuo, S.; Fukuda, M.; Saito, T. Stability of fruit set of newly selected parthenocarpic eggplant lines. Sci. Hortic. 2008, 115, 111–116. [Google Scholar] [CrossRef]
- Makrogianni, D.I.; Karapanos, I.C.; Passam, H.C. Seasonal fluctuations in pollen production and viability in eggplant and the quality of seed-containing and seedless (auxin-set) fruits. J. Plant Growth Regul. 2018, 37, 937–946. [Google Scholar] [CrossRef]
- Sharma, M.D. Effect of plant growth regulators on growth and yield of brinjal at Khajura, Banke. J. Inst. Agric. Anim. Sci. 2006, 27, 153–156. [Google Scholar] [CrossRef][Green Version]
- Netam, J.L.; Sharma, R. Efficacy of plant growth regulators on growth characters and yield attributes in brinjal (Solanum melongena L.) cv. Brinjal. 3112. J. Agric. Veterin. Sci. 2014, 7, 27–30. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; D’Anna, F.; Palazzolo, E.; Mennella, G.; Rotino, G.L. Hybrids and allied species as potential rootstocks for eggplant: Effect of grafting on vigour, yield and overall fruit quality traits. Sci. Hort. 2018, 228, 81–90. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; Rotino, G.L.; Palazzolo, E.; Mennella, G.; D’Anna, F. Solanum aethiopicum gr. gilo and its interspecific hybrid with S. melongena as alternative rootstocks for eggplant: Effects on vigor, yield, and fruit physicochemical properties of cultivar ‘Scarlatti’. Agronomy 2019, 9, 223. [Google Scholar] [CrossRef]
- Caruso, G.; Pokluda, R.; Sękara, A.; Kalisz, A.; Jezdinský, A.; Kopta, T.; Grabowska, A. Agricultural practices, biology and quality of eggplant cultivated in Central Europe. A Review. Hort. Sci. 2017, 44, 21–42. [Google Scholar] [CrossRef]
- Grabowska, A.; Kunicki, E.; Sękara, A.; Kalisz, A.; Jezdinský, A.; Gintrowicz, K. The effect of biostimulants on the quality parameters of tomato grown for the processing industry. Agrochimica 2015, 59, 203–217. [Google Scholar]
- Kopta, T.; Pavlikova, M.; Sękara, A.; Pokluda, R.; Marsalek, B. Effect of bacterial-algal biostimulant on the yield and internal quality of lettuce (Lactuca sativa L.) produced for spring and summer crop. Not. Bot. Horti. Agrobot. Cluj-Napoca 2018, 46, 615–621. [Google Scholar] [CrossRef]
- DuJardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; Dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 1–12. [Google Scholar] [CrossRef]
- Sharma, H.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front Plant Sci. 2017, 7, 2049–2080. [Google Scholar] [CrossRef] [PubMed]
- Pohl, A.; Grabowska, A.; Kalisz, A.; Sękara, A. Preliminary screening of biostimulative effects of Göemar BM-86 on eggplant cultivars grown under field conditions in Poland. Acta Agrobot. 2018, 71, 1752. [Google Scholar] [CrossRef]
- Pohl, A.; Grabowska, A.; Kalisz, A.; Sękara, A. The eggplant yield and fruit composition as affected by genetic factor and biostimulant application. Not. Bot. Horti. Agrobot. Cluj-Napoca 2019, 47. [Google Scholar] [CrossRef]
- Sękara, A.; Bączek-Kwinta, R.; Gawęda, M.; Kalisz, A.; Pokluda, R.; Jezdinský, A. Sequential abiotic stress applied to juvenile eggplant modifies the seedlings parameters, plant ontogeny and yield. Hort. Sci. 2016, 43, 149–157. [Google Scholar] [CrossRef]
- UPL Limited, Arysta Lifescience. Available online: https://www.arystalifescience.com/ (accessed on 23 January 2019).
- Sękara, A. Biology of the Vegetative and Generative Development of Eggplant (Solanum melongena L.) in the Field Production. Chosen Aspects; Zesz Nauk UR Wydawnictwo Uniwersytetu Rolniczego: Krakow, Poland, 2010; Volume 459, pp. 19–24. [Google Scholar]
- Martin, F.W. Staining and observing pollen tubes in the style by means of fluorescence. Stain. Technol. 1959, 34, 125–128. [Google Scholar] [CrossRef]
- Srinivas, G.; Jayappa, A.H.; Patel, A.I. Heterostyly: A threat to potential fruit yield in brinjal (Solanum melongena L.). Adv. Life Sci. 2016, 5, 1211–1215. [Google Scholar]
- Hazra, P.; Mandal, J.; Mukhopadhyay, T.P. Pollination behaviour and natural hybridization in Solanum melongena L., and utilization of functional male sterile line in hybrid seed production. Caps. Eggpl. Newsl. 2003, 22, 143–146. [Google Scholar]
- Arthur, G.D.; Stirk, W.A.; Van Staden, J. Effect of a seaweed concentrate on the growth and yield of three varieties of Capsicum annuum. S. Afr. J. Bot. 2003, 69, 207–211. [Google Scholar] [CrossRef]
- Helaly, M.N.; Arafa, A.A.; Ibrahim, H.M.; Ghoniem, K.H. Improving growth and productivity of tomato by some biostimulants and micronutrients with or without mulching. J. Phytol. 2018, 10, 15–23. [Google Scholar]
- Khah, E.M.; Antonopoulos, A.; Passam, H.C. Floral behaviour and fruit set in four cultivars of aubergine. Acta Hortic. 2002, 579, 259–264. [Google Scholar] [CrossRef]
- Fornes, F.; Sánchez-Perales, M.; Guardiola, J.L. Effect of a seaweed extract on the productivity of ‘de Nules’ Clementine mandarin and Navelina orange. Bot. Mar. 2002, 45, 486–489. [Google Scholar] [CrossRef]
- Basak, A. Effect of preharvest treatment with seaweed products, Kelpak® and Goëmar BM 86®, on fruit quality in apple. Int. J. Fruit Sci. 2008, 8, 1–14. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Naira, A.; Moieza, A. Effect of plant biostimulants on flowering, fruit drop, yield and return bloom of pomegranate cv. Kandhari Kabuli. Asian J. Hortic. 2012, 7, 473–477. [Google Scholar]
- Morales-Payan, J.P.; Candelas, C.D. Increasing organic avocado fruit yield using a Ascophyllum nodosum biostimulant and fertilization. Acta Hortic. 2014, 1042, 121–124. [Google Scholar] [CrossRef]
- Chouliaras, V.; Tasioula, M.; Chatzissavvidis, C.; Therios, I.; Tsabolatidou, E. The effects of a seaweed extract in addition to nitrogen and boron fertilization on productivity, fruit maturation, leaf nutritional status and oil quality of the olive (Olea europaea L.) cultivar Koroneiki. J. Sci. Food Agric. 2009, 89, 984–988. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Elings, A.; Bakker, M.J.; Brajeul, E.; Dieleman, J.A.; de Visser, P.H.B.; Heuvelink, E. Modelling dry matter production and partitioning in sweet pepper. Acta Hortic. 2006, 718, 121–128. [Google Scholar] [CrossRef]
- Abd El-Gawad, H.G.; Osman, H.S. Effect of exogenous application of boricacid and seaweed extract on growth, biochemical content and yield of eggplant. J. Hortic. Sci. Ornam. Plants 2014, 6, 133–143. [Google Scholar]
- Gómez-Cadenas, A.; Pérez-Santamarina, R.; Ghorbel, R. Effect of a biostimulant product containing macronutrients and a carboxylic acid (AMEC®) on citrus fruitlet abscission. Acta Hort. 2012, 189–193. [Google Scholar]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. Perspectives on the use of a seaweed extract to moderate the negative effects of alternate bearing in apple trees. J. Hort. Sci. Biotechnol. 2009, 84, 131–137. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]


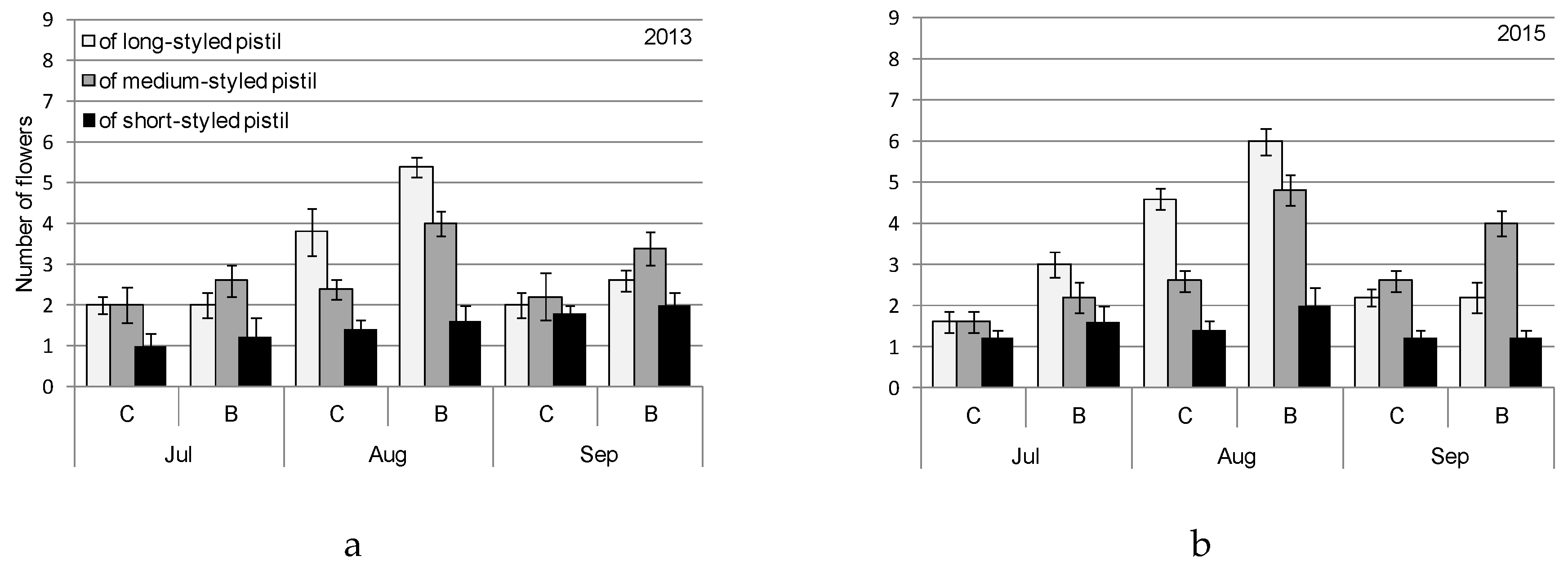
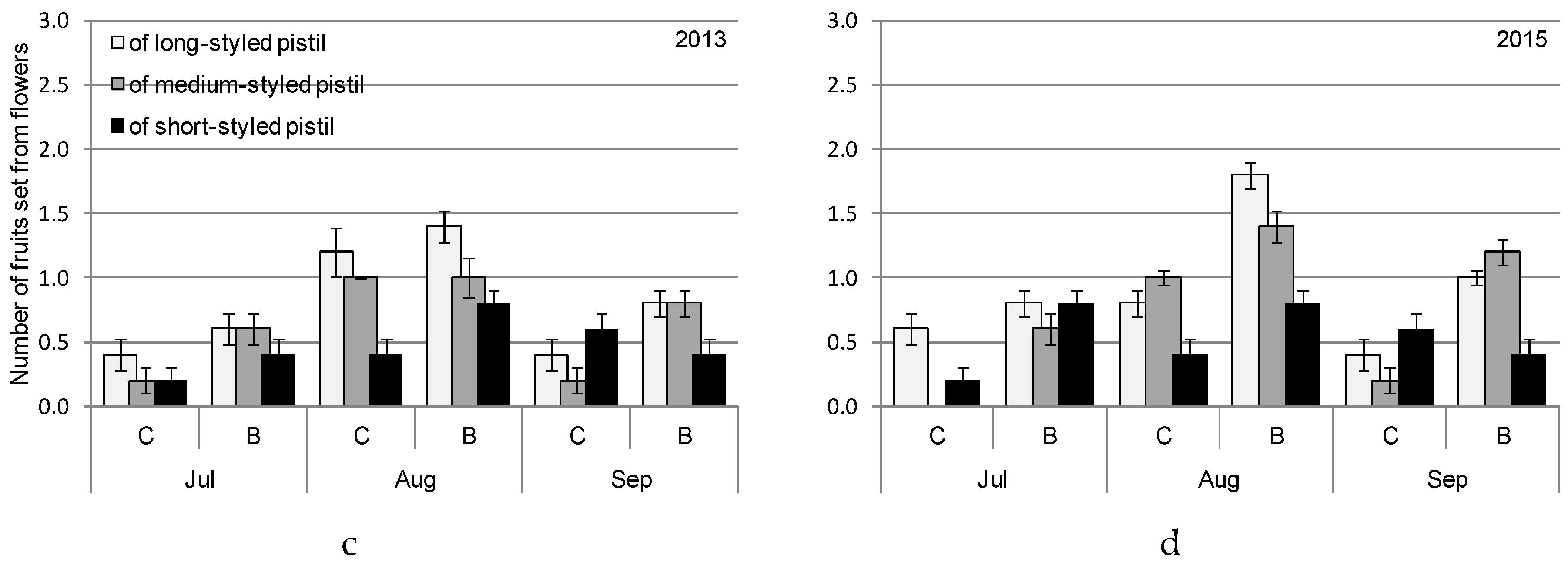

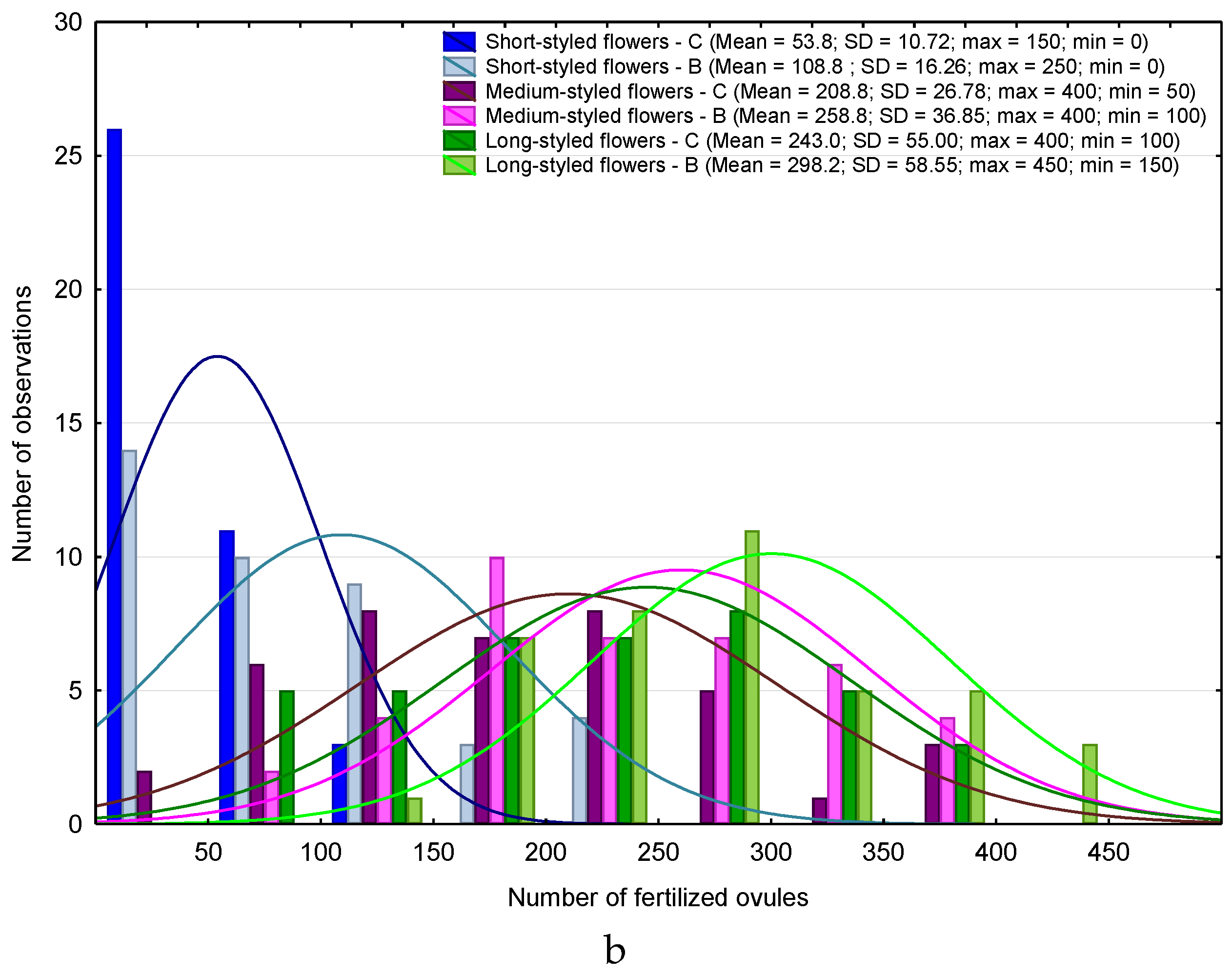
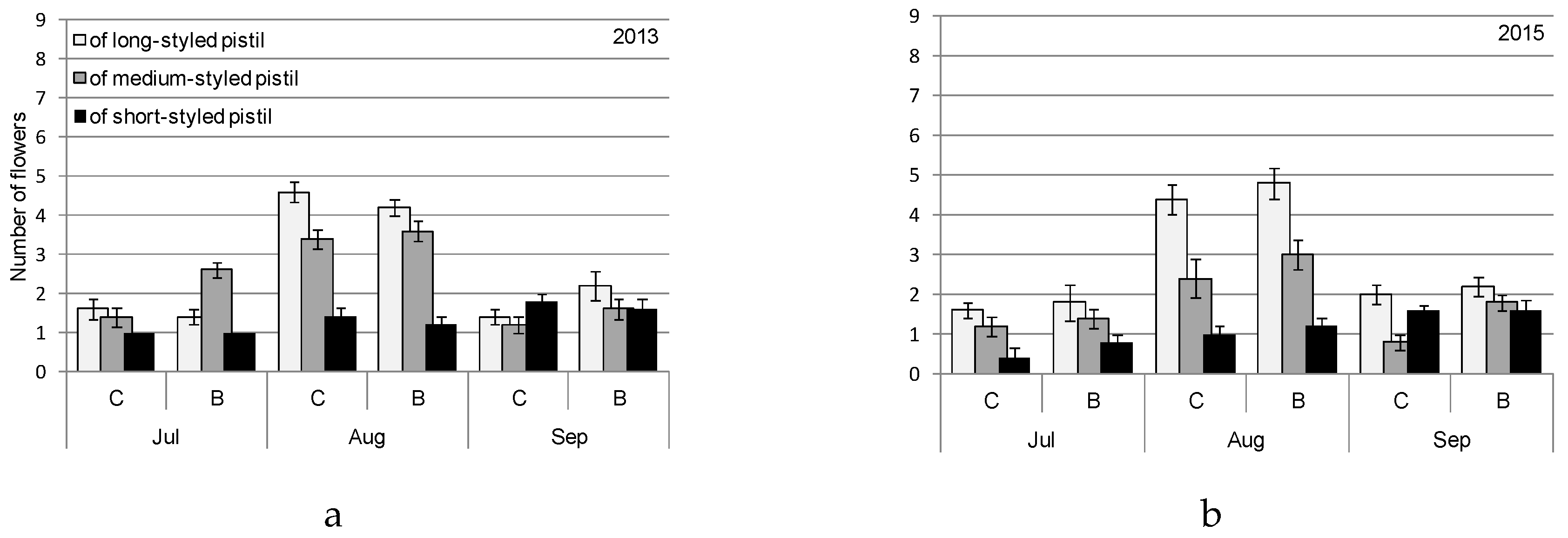

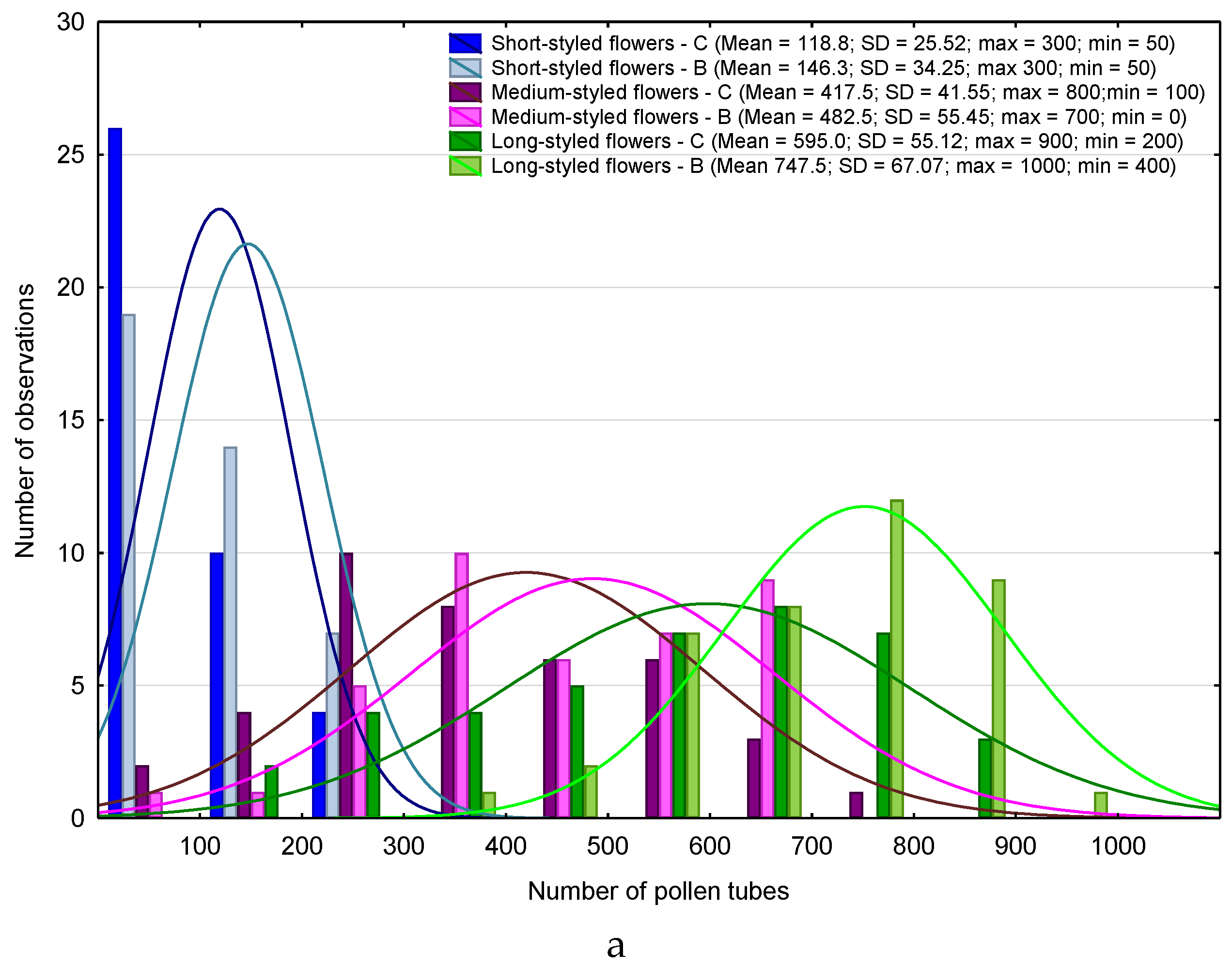
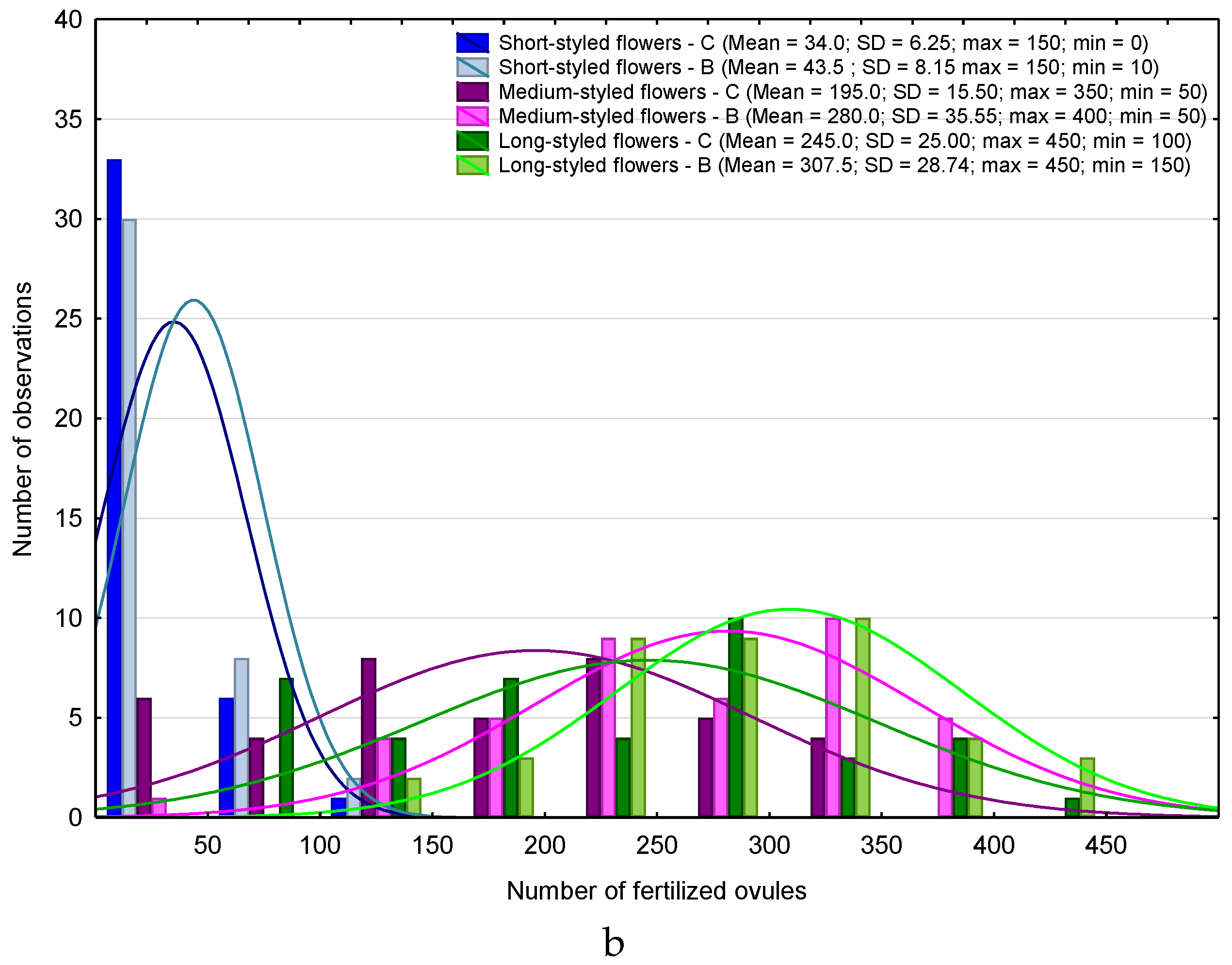
| Month | 2013 | 2015 | ||||
|---|---|---|---|---|---|---|
| Temperature (°C) | PAR (µmol m−2 s−1) | Sum of Rainfall (mm) | Temperature (°C) | PAR (µmol m−2 s−1) | Sum of Rainfall (mm) | |
| May | 14.3 | 345 | 83 | 13.1 | 357 | 93 |
| June | 17.6 | 392 | 188 | 17.5 | 401 | 40 |
| July | 19.4 | 477 | 28 | 20.4 | 489 | 39 |
| August | 18.8 | 396 | 51 | 21.2 | 357 | 58 |
| September | 12.1 | 256 | 63 | 14.9 | 283 | 60 |
| Parameter | Year | Type of Flower | |||||
|---|---|---|---|---|---|---|---|
| With Long-Styled Pistil | With Medium-Styled Pistil | With Short-Styled Pistil | |||||
| C * | B | C | B | C | B | ||
| “Epic” F1 | |||||||
| Number of particular types of flowers per plant | 2013 | 12.2 d ** | 13.4 d | 5.6 b | 8.2 c | 3.4 a | 3.5 ab |
| 2015 | 12.1 c | 13.5 c | 6.4 b | 7.6 b | 3.5 a | 3.6 a | |
| Number of fruits per plant set from particular types of flowers | 2013 | 2.4 b | 4.2 d | 0.8 a | 2.0 b | 1.0 a | 1.4 ab |
| 2015 | 3.2 b | 5.6 c | 1.0 a | 3.2 b | 1.0 a | 1.2 a | |
| Effectiveness of fruit setting as depended on type of flower (%) | 2013 | 20.7 | 27.6 | 15.6 | 24.7 | 26.7 | 40.0 |
| 2015 | 33.7 | 48.8 | 18.9 | 46.7 | 26.6 | 36.7 | |
| Number of seeds per fruit | 2013 | 332 d | 365 e | 245 b | 289 c | 102 a | 125 a |
| 2015 | 358 e | 372 f | 255 c | 312 d | 95 a | 138 b | |
| “Flavine” F1 | |||||||
| Number of particular types of flowers per plant | 2013 | 7.8 cd | 10.0 d | 6.6 bc | 10.0 d | 4.2 a | 4.8 ab |
| 2015 | 8.4 b | 11.2 c | 6.8 b | 11.0 c | 3.8 a | 4.8 a | |
| Number of fruits per plant set from particular types of flowers | 2013 | 2.0 abc | 2.8 b | 1.4 ab | 2.4 bc | 1.2 a | 1.6 ab |
| 2015 | 1.8 a | 3.6 b | 1.2 a | 3.2 b | 1.2 a | 2.0 a | |
| Effectiveness of fruit setting as depended on type of flower (%) | 2013 | 26.9 | 28.7 | 22.8 | 26.0 | 20.0 | 33.3 |
| 2015 | 26.0 | 36.2 | 16.7 | 27.2 | 30.0 | 41.1 | |
| Number of seeds per fruit | 2013 | 312 c | 328 c | 258 b | 325 c | 142 a | 155 a |
| 2015 | 322 c | 333 d | 289 b | 316 c | 134 a | 143 a | |
| “Gascona” F1 | |||||||
| Number of particular types of flowers per plant | 2013 | 7.6 c | 8.0 c | 5.6 b | 8.2 c | 3.4 a | 3.6 a |
| 2015 | 7.6 bc | 7.8 c | 6.0 b | 7.8 c | 4.2 a | 3.8 a | |
| Number of fruits per plant set from particular types of flowers | 2013 | 2.0 abc | 3.2 c | 1.6 ab | 2.4 bc | 1.0 a | 0.8 a |
| 2015 | 2.4 ab | 3.0 b | 1.6 a | 2.2 ab | 1.4 a | 1.4 a | |
| Effectiveness of fruit setting as depended on type of flower (%) | 2013 | 26.2 | 41.5 | 22.6 | 29.1 | 23.3 | 20.0 |
| 2015 | 38.7 | 37.1 | 11.7 | 30.6 | 36.7 | 33.3 | |
| Number of seeds per fruit | 2013 | 289 d | 322 e | 257 c | 285 d | 78 a | 127 b |
| 2015 | 321 e | 356 f | 269 c | 297 d | 98 a | 159 b | |
| ANOVA Source of Variation | “Epic” F1 | |||
|---|---|---|---|---|
| No of Flowers 2013 | No of Fruits 2013 | No of Flowers 2015 | No of Fruits 2015 | |
| Type of flower (F) | *** | *** | *** | *** |
| Biostimulant (B) | ** | * | *** | *** |
| Month (M) | *** | *** | * | ** |
| F × B | ns | * | ns | * |
| F × M | *** | *** | *** | ns |
| B × M | ns | ns | ns | * |
| ANOVASource of Variation | “Flavine” F1 | |||
|---|---|---|---|---|
| No of Flowers 2013 | No of Fruits 2013 | No of Flowers 2015 | No of Fruits 2015 | |
| Type of flower (F) | *** | *** | *** | ** |
| Biostimulant (B) | ** | *** | *** | *** |
| Month (M) | *** | *** | *** | *** |
| F × B | ns | * | * | ns |
| F × M | *** | *** | *** | ns |
| B × M | ns | * | * | ns |
| ANOVASource of Variation | “Gascona” F1 | |||
|---|---|---|---|---|
| No of Flowers 2013 | No of Fruits 2013 | No of Flowers 2015 | No of Fruits 2015 | |
| Type of flower (F) | *** | *** | *** | * |
| Biostimulant (B) | * | *** | ns | ns |
| Month (M) | *** | ns | *** | *** |
| F × B | * | ns | * | ns |
| F × M | *** | ns | *** | * |
| B × M | ns | ns | ns | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pohl, A.; Grabowska, A.; Kalisz, A.; Sękara, A. Biostimulant Application Enhances Fruit Setting in Eggplant—An Insight into the Biology of Flowering. Agronomy 2019, 9, 482. https://doi.org/10.3390/agronomy9090482
Pohl A, Grabowska A, Kalisz A, Sękara A. Biostimulant Application Enhances Fruit Setting in Eggplant—An Insight into the Biology of Flowering. Agronomy. 2019; 9(9):482. https://doi.org/10.3390/agronomy9090482
Chicago/Turabian StylePohl, Alicja, Aneta Grabowska, Andrzej Kalisz, and Agnieszka Sękara. 2019. "Biostimulant Application Enhances Fruit Setting in Eggplant—An Insight into the Biology of Flowering" Agronomy 9, no. 9: 482. https://doi.org/10.3390/agronomy9090482
APA StylePohl, A., Grabowska, A., Kalisz, A., & Sękara, A. (2019). Biostimulant Application Enhances Fruit Setting in Eggplant—An Insight into the Biology of Flowering. Agronomy, 9(9), 482. https://doi.org/10.3390/agronomy9090482






