Biostimulants and Microorganisms Boost the Nutritional Composition of Buckwheat (Fagopyrum esculentum Moench) Sprouts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Sampling Preparation and Chemical Analysis
hemicellulose (HCEL) = NDF − ADF
2.3. Statistical Analyses
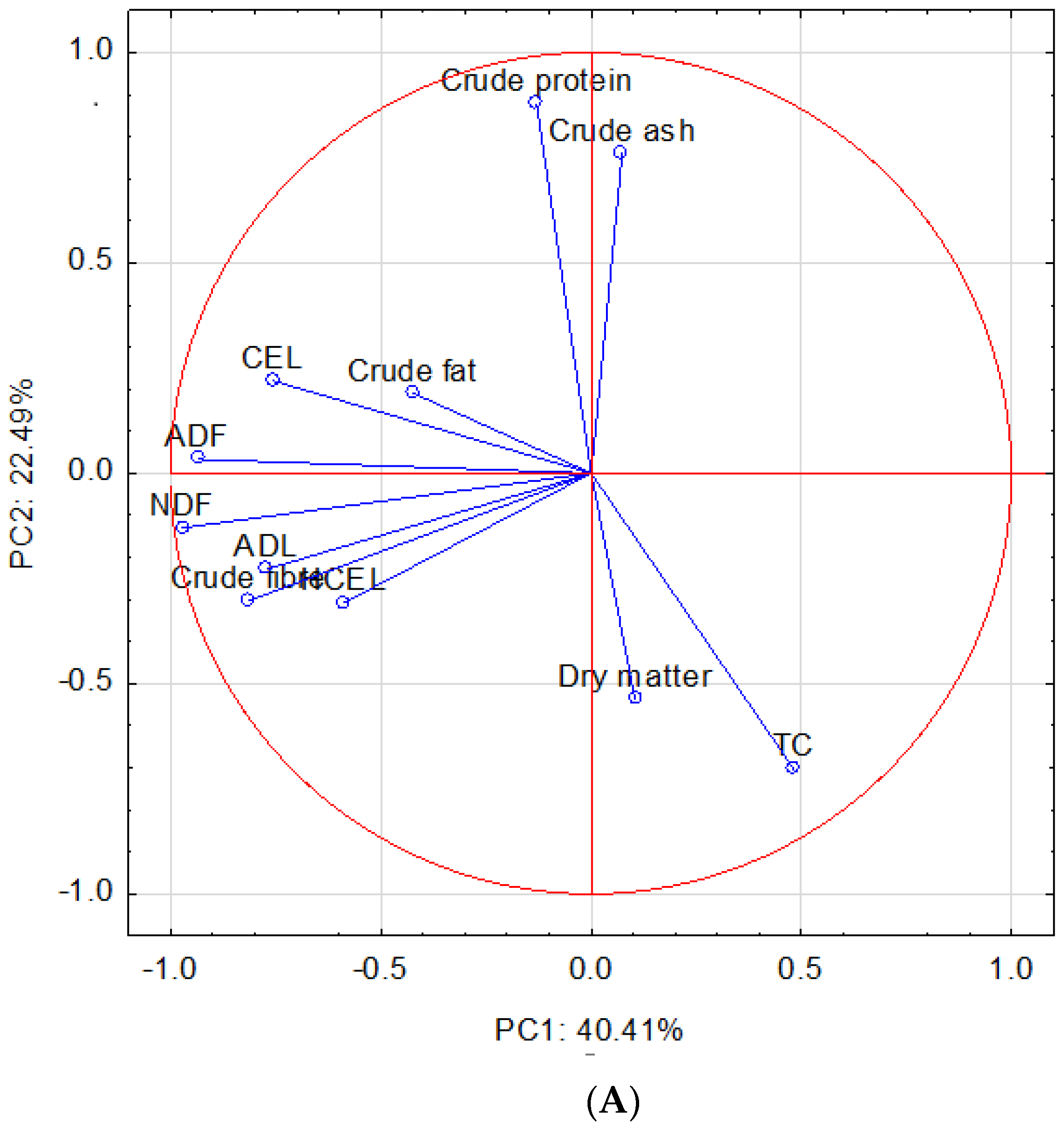
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ebert, A.W. Potential of underutilized traditional vegetables and legume crops to contribute to food and nutritional security, income and more sustainable production systems. Sustainability 2014, 6, 319–335. [Google Scholar] [CrossRef]
- Kahane, R.; Hodgkin, T.; Jaenicke, H.; Hoogendoorn, C.; Hermann, J.; Keatinge, J.; Hughes, J.D.; Looney, N. Agrobiodiversity for food security, health and income. Agron. Sustain. Dev. 2013, 33, 671–693. [Google Scholar] [CrossRef]
- Westhoek, H.; Lesschen, P.J.; Rood, T.; Wagner, S.; De Marco, A.; Murphy-Bokern, D.; Leip, A.; van Grinsven, H.; Sutton, M.A.; Oenema, O. Food choices, health and environment: Effects of cutting Europe’s meat and dairy intake. Global Environ. Chang. 2014, 26, 196–205. [Google Scholar] [CrossRef]
- Ebert, A.W. Sprouts, Microgreens, and Edible Flowers: The Potential for High Value Specialty Produce in Asia. In Proceedings of the SEAVEG Regional Symposium. High Value Vegetables in Southeast Asia: Production, Supply and Demand, Chiang Mai, Thailand, 24–26 January 2012. [Google Scholar]
- Di Gioia, F.; Santamaria, P. The nutritional properties of microgreens. In Microgreens; Di Gioia, F., Santamaria, P., Eds.; Eco-Logica: Bari, Italy, 2015; p. 47. [Google Scholar]
- Kim, S.L.; Kim, S.K.; Park, C.H. Introduction and nutritional evaluation of buckwheat sprout as a new vegetable. Food Res. Int. 2004, 37, 319–327. [Google Scholar] [CrossRef]
- Marton, M.; Mandoki, Z.; Csapo-Kiss, Z.; Csapo, J. The role of sprouts in human nutrition. A review. Acta Univ. Sapientiae Aliment. 2010, 3, 81–117. [Google Scholar]
- Yang, Y.; Meier, F.; Ann Lo, J.; Yuan, W.; Lee Pei Sze, V.; Chung, H.J.; Yuk, H.G. Overview of recent events in the microbiological safety of sprouts and new intervention technologies. Compr. Rev. Food Sci. Food Saf. 2013, 12, 265–280. [Google Scholar] [CrossRef]
- Frederiks, C.; Wesseler, J.H.H. A comparison of EU and US regulatory frameworks for the active substance registration of microbial biological control agents. Pest. Manag. Sci. 2019, 78, 87–103. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Bulgari, R.; Trivellini, A.; Ferrante, A. Effects of two doses of organic extract-based biostimulant on greenhouse lettuce grown under increasing NaCl concentrations. Front. Plant Sci. 2019, 9, 1870. [Google Scholar] [CrossRef]
- Kałużewicz, A.; Gąsecka, M.; Spiżewski, T. Influence of biostimulants on phenolic content in broccoli heads directly after harvest and after storage. Folia Hort. 2017, 29, 221–230. [Google Scholar] [CrossRef]
- Briatia, X.; Jomduang, S.; Park, C.H.; Lumyong, S.; Kanpiengjai, A.; Khanongnuch, C. Enhancing growth of buckwheat sprouts and mircogreens by endophytic bacterium inoculation. Int. J. Agric. Biol. 2017, 19, 374–380. [Google Scholar] [CrossRef]
- Gerbore, J.; Vallance, J.; Yacob, A.; Delmotte, F.; Grizard, D.; Regnault-Roger, C.; Rey, P. Characterization of Phytium oligandrum populations that colonize the rhizosphere of vines form Bordeaux region. FEMS Microbiol. Ecol. 2014, 90, 153–167. [Google Scholar] [CrossRef]
- Singh, A.; Gupta, R.; Pandey, R. Rice seed priming with picomolar rutin enhance rhizospheric Bacillus subtilis CIM colonization and plant growth. PLoS ONE 2016, 11, e01466013. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Hortic. Sci. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Hashem, H.A.; Mansour, H.A.; El-Khawas, S.A.; Hassanein, R.A. The potentiality of marine macro-algae as bio-fertilizers to improve the productivity and salt stress tolerance of canola (Brassica napus L.) plants. Agronomy 2019, 9, 146. [Google Scholar]
- Chanthini, K.M.-P.; Stanley-Raja, V.; Thanigaivel, A.; Karthi, S.; Palanikani, R.; Shyam Sundar, N.; Sivanesh, H.; Soranam, R.; Senthil-Nathan, S. Sustainable agronomic strategies for enhancing the yield and nutritional quality of wild tomato, Solanum Lycopersicum (l) var Cerasiforme Mill. Agronomy 2019, 9, 311. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hort. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Baranowska, A.J. Impact of Growth Biostimulators and Herbicide on Edible Potato Yield. Acta Agroph. 2018, 25, 385–396. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants Research in Some Horticultural Plant Species—A Review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 19th ed.; AOAC: Washington, DC, USA, 2012. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Liu, C.L.; Chen, Y.S.; Yang, J.H.; Chiang, B.H. Antioxidant activity of tartary (Fagopyrum tataricum (L.) Gaertn.) and common (Fagopyrum esculentum Moench) buckwheat sprouts. J. Agric. Food Chem. 2008, 56, 173–178. [Google Scholar] [CrossRef]
- Kim, S.L.; Son, Y.K.; Hwang, J.J.; Kim, S.K.; Hur, H.S.; Park, C.H. Development and utilization of buckwheat sprouts as functional vegetables. Fagopyrum 2001, 18, 49–54. [Google Scholar]
- Paredes-Lopez, O.; Mora-Escobedo, R. Germination of amaranth seeds: Effects on nutrient composition and color. J. Food Sci. 1989, 54, 761–762. [Google Scholar] [CrossRef]
- Gimenez-Bastida, J.A.; Zieliński, H. Buckwheat as a functional food and its effects on health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Piątkowska, E.; Leszczyńska, T.; Pisulewska, E.; Witkowicz, R.; Bystrowska, B.; Francik, R. Identification of polyphenolic compounds and determination of antioxidant in extracts and infusions of buckwheat leaves. Eur. Food Res. Technol. 2017, 244, 333–343. [Google Scholar] [CrossRef]
- Guillon, F.; Champ, M. Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res. Int. 2000, 33, 233–245. [Google Scholar] [CrossRef]
- Linh, N.T.N.; Khoa, D.V.A.; Halas, V. Buckwheat as valuable feed and food resource. NJMBS 2014, 3, 1–8. [Google Scholar]
- Yang, H.J.; Lim, J.H.; Park, K.J.; Kang, S.; Kim, D.S.; Park, S. Methyl jasmolate treated buckwheat sprout powder enhances glucose metabolism by potentiating hepatic insulin signaling in estrogen-deficient rats. Nutrition 2016, 32, 129–137. [Google Scholar] [CrossRef]
- Yiming, Z.; Hong, W.; Linlin, C.; Xiaoli, Z.; Wen, T.; Xinli, S. Evolution of nutrient ingredients in tartary buckwheat seeds during germination. Food Chem. 2015, 186, 244–248. [Google Scholar] [CrossRef]
- Elumalai, L.K.; Rengasamy, R. Synergistic effect of seaweed manure and Bacillus sp. on growth and biochemical constituents of Vigna radiata L. J. Biofertil. Biopes. 2012, 3, 121–128. [Google Scholar] [CrossRef]
- Selvam, G.G.; Sivakumar, K. Influence of seaweed extract as an organic fertilizer on the growth and yield of Arachis hypogea L. and their elemental composition using SEM-energy dispersive spectropic analysis. Asian Pac. J. Reprod. 2014, 3, 18–22. [Google Scholar] [CrossRef]
- Vijayanand, N.; Ramya, S.S.; Rathinavel, S. Potential of liquid extracts of Sargassum wightii on growth, biochemical and yield parameters of cluster bean plant. Asian Pac. J. Reprod. 2014, 3, 150–155. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Critchley, A.T.; Prithiviraj, B. A commercial extract of brown macroalga (Ascophyllum nodosum) affects yield and the nutritional quality of spinach in vitro. Commun. Soil Sci. Plant Anal. 2013, 44, 1873–1884. [Google Scholar] [CrossRef]
- De Gonzalo, G.; Colpa, D.I.; Habib, M.H.M.; Fraaije, M.W. Bacterial enzymes involved in lignin degradation. J. Biotechnol. 2016, 236, 110–119. [Google Scholar] [CrossRef]
- Kellner, H.; Luis, P.; Zimdars, B.; Kiesel, B.; Buscot, F. Diversity of bacterial laccase-like multicopper oxidase genes in forest and grassland Cambisol soil samples. Soil Biol. Biochem. 2008, 40, 638–648. [Google Scholar] [CrossRef]
- Ciepiela, G.A.; Godlewska, A.; Jankowska, J. The effect of seaweed Ecklonia maxima extract and mineral nitrogen on fodder grass chemical composition. Environ. Sci. Pollut. Res. 2016, 23, 2301–2307. [Google Scholar] [CrossRef]

| Treatment | Ingredient (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry Matter | Crude Ash | Crude Protein | Crude Fat | TC | |||||||||||
| Panda | Kora | Mean | Panda | Kora | Mean | Panda | Kora | Mean | Panda | Kora | Mean | Panda | Kora | Mean | |
| Seaweed E. maxima extract | 91.27 d,e | 91.69 f | 91.48 e | 5.37 b,c,d | 5.74 d,e,f | 5.55 b,c | 22.5 f | 22.65 f,g | 22.57 b | 3.82 a,b,c | 3.62 a | 3.72 a,b,c | 38.93 c,d,e | 38.35 c,d,e | 38.64 a |
| Nitrophenols | 90.33 a | 91.60 f | 90.96 b,c | 5.45 c,d,e | 5.47 c,d,e | 5.46 b,c | 23.06 g | 21.99 d,e | 22.52 a,b | 3.79 a,b,c | 3.71 a,b | 3.75 a,b,c | 37.59 b | 38.8 c,d,e | 38.19 a |
| P. oligandrum | 90.48 a | 91.50 e,f | 90.99 b,c | 5.47 c,d,e | 4.8 a | 5.13 a | 23.36 g | 21.09 a,b | 22.22 a | 4.06 c | 3.57 a | 3.82 a,b,c | 36.74 a | 40.99 i | 38.86 a |
| Bacillus subtilis | 90.60 a,b | 91.11 c,d | 90.85 a,b | 5.26 b,c | 5.54 c,d,e | 5.40 b | 22.25 e,f | 22.57 f,g | 22.41 a,b | 3.94 b,c | 3.55 a | 3.75 a,b,c | 41.01 i | 39.66 g,h | 40.33 b |
| Seaweed E. maxima extract + P. oligandrum | 91.06 c,d | 91.05 c,d | 91.05 b,c | 5.48 c,d,e | 5.52 c,d,e | 5.50 b,c | 23.27 g | 21.27 b | 22.27 a,b | 4.05 c | 3.67 a,b | 3.86 c | 38.75 c,d,e | 38.88 c,d,e | 38.81 a |
| Nitrophenols + P. oligandrum | 91.13 c,d | 91.17 c,d | 91.15 c,d | 5.82 e,f | 5.56 c,d,e | 5.68 c | 22.98 g | 23.4 g | 23.19 c | 3.93 b,c | 3.55 a | 3.74 a,b,c | 38.16 c | 38.57 c,d,e | 38.36 a |
| Seaweed E. maxima extract + B. subtilis | 91.68 f | 90.99 c,d | 91.33 d,e | 5.10 a,b | 5.89 f | 5.50 b,c | 22.5 f | 22.5 f | 22.50 a,b | 3.72 a,b | 3.51 a | 3.61 a | 40.14 h | 40.67 i | 40.40 b |
| Nitrophenols + B. subtilis | 90.46 a | 91.05 c,d | 90.76 a | 5.51 c,d,e | 5.07 a,b | 5.29 a,b | 22.67 f,g | 20.81 a | 21.74 a | 4.04 c | 3.62 a | 3.83 b,c | 39.14 d,e,f | 41.74 j | 40.44 b |
| Seaweed E. maxima extract + nitrophenols | 91.61 f | 90.93 c,d | 91.27 d | 4.87 a | 5.95 f | 5.41 b | 21.64 c,d | 22.33 e,f | 21.99 a | 4.05 c | 3.56 a | 3.80 a,bc | 40.01 h | 38.58 c,d,e | 39.29 a |
| Control | 91.26 d,e | 90.8 b,c | 91.03 b,c | 5.24 b,c | 5.55 c,d,e | 5.39 b | 21.65 c,d | 22.18 e,f | 21.92 a | 3.72 a,b | 3.55 a | 3.63 a,b | 39.15 d,e,f,g | 39.58 f,g,h | 39.36 a |
| Mean | 90.98 a | 91.19 b | - | 5.35 a | 5.51 b | - | 22.59 b | 22.08 a | - | 3.91 b | 3.59 a | - | 39.0 a | 39.6 b | - |
| Treatment | Ingredient (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude Fiber | NDF | ADF | |||||||
| Panda | Kora | Mean | Panda | Kora | Mean | Panda | Kora | Mean | |
| Seaweed Ecklonia maxima extract | 20.88 h | 20.16 f | 20.52 e,f | 40.44 i | 35.71 d | 38.07 e | 31.16 g | 27.91 b,c,e | 29.54 c |
| Nitrophenols | 20.27 f | 19.62 d,e | 19.95 c | 38.51 f,g | 35.82 d | 37.16 d | 30.65 f,g | 28.2 c,d,e | 29.42 c |
| Pythium oligandrum | 19.67 d,e | 20.93 h | 20.30 d,e | 35.90 d | 37.56 e,f | 36.73 d | 27.83 b,c,e | 28.66 d,e,f | 28.24 b |
| Bacillus subtilis | 20.21 f | 17.11 a | 18.65 a | 38.25 f | 32.61 b | 35.43 c | 28.47 d,e | 25.00 a | 26.74 a |
| Seaweed Ecklonia maxima extract + Pythium oligandrum | 19.38 c,d | 21.51 i | 20.45 e,f | 37.73 e,f | 36.89 e | 37.31 d | 29.24 d,e,f,g | 28.69 d,e,f | 28.97 b,c |
| Nitrophenols + Pythium oligandrum | 20.26 f | 19.91 e,f | 20.08 c,d | 40.00 h,i | 36.91 e | 38.45 e | 30.53 f,g | 29.73 d,e,f,g | 30.13 c |
| Seaweed Ecklonia maxima extract + Bacillus subtilis | 19.01 c | 18.29 b | 18.6 a | 34.50 c | 31.00 a | 32.75 a | 26.04 a | 25.38 a | 25.71 a |
| Nitrophenols + Bacillus subtilis | 19.29 c,d | 18.99 c | 19.14 b | 35.94 d | 33.24 b | 34.59 b | 26.38 a,b | 26.69 a,b,c | 26.54 a |
| Seaweed Ecklonia maxima extract + nitrophenols | 20.14 f | 20.49 f | 20.31 d,e | 39.19 g,h | 37.15 e | 38.17 e | 29.35 d,e,f,g | 30.13 e,f,g | 29.74 c |
| Control | 21.02 h | 20.37 f | 20.69 f | 39.64 h,i | 37.9 e,f | 38.77 e | 30.15 e,f,g | 29.24 d,e,f,g | 29.69 c |
| Mean | 20.0 b | 19.7 a | - | 38.01 b | 35.48 a | - | 28.98 b | 27.96 a | - |
| Treatment | Ingredient (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ADL | HCEL | CEL | |||||||
| Panda | Kora | Mean | Panda | Kora | Mean | Panda | Kora | Mean | |
| Seaweed Ecklonia maxima extract | 12.10 h | 10.43 e,f | 11.27 e | 9.28 d,e | 7.80 b,c,d,e | 8.54 b | 19.33 c,d | 17.48 a,b,c,d | 18.41 c,d |
| Nitrophenols | 11.44 g | 10.03 c,d,e | 10.74 d | 7.86 b,c,d,e | 7.62 b,c,d,e | 7.74 a,b | 19.21 c,d | 18.17 a,b,c,d | 18.69 c,d |
| Pythium oligandrum | 9.53 a,b,c | 10.74 f | 10.13 c | 8.08 b,c,d,e | 8.91 c,d,e | 8.49 b | 18.3 a,b,c,d | 17.92 a,b,c,d | 18.11 b,c |
| Bacillus subtilis | 9.48 a,b,c | 8.86 a | 9.17 a | 9.79 e | 7.60 b,c,d,e | 8.69 b | 18.99 c,d | 16.15 a | 17.57 b,c |
| Seaweed Ecklonia maxima extract + Pythium oligandrum | 9.68 b,c,d | 10.91 f | 10.29 c | 8.49 b,c,d,e | 8.20 b,c,d,e | 8.34 b | 19.57 d | 17.79 a,b,c,d | 18.68 c,d |
| Nitrophenols + Pythium oligandrum | 10.75 f | 10.27 d,e,f | 10.51 c | 9.48 e | 7.18 a,b,c,d | 8.33 b | 19.78 d | 19.46 d | 19.62 d |
| Seaweed Ecklonia maxima extract + Bacillus subtilis | 9.13 a,b | 9.06 a,b | 9.09 a | 8.46 b,c,d,e | 5.63 a | 7.04 a | 16.92 a,b,c | 16.32 a | 16.62 a |
| Nitrophenols + Bacillus subtilis | 9.94 c,d,e | 9.26 a,b | 9.60 b | 9.56 e | 6.55 a,b | 8.05 a,b | 16.45 a,b | 17.43 a,b,c,d | 16.94 a,b |
| Seaweed Ecklonia maxima extract + nitrophenols | 11.49 g | 11.82 g,h | 11.65 f | 9.84 e | 7.02 a,b,c | 8.43 b | 17.86 a,b,c,d | 18.31 a,b,c,d | 18.08 b,c |
| Control | 12.13 h | 10.4 e,f | 11.26 e | 9.49 e | 8.67 b,c,d,e | 9.08 b | 18.03 a,b,c,d | 18.84 b,c,d | 18.43 c,d |
| Mean | 10.56 b | 10.18 a | - | 9.03 b | 7.52 a | - | 18.44 b | 17.78 a | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witkowicz, R.; Biel, W.; Chłopicka, J.; Galanty, A.; Gleń-Karolczyk, K.; Skrzypek, E.; Krupa, M. Biostimulants and Microorganisms Boost the Nutritional Composition of Buckwheat (Fagopyrum esculentum Moench) Sprouts. Agronomy 2019, 9, 469. https://doi.org/10.3390/agronomy9080469
Witkowicz R, Biel W, Chłopicka J, Galanty A, Gleń-Karolczyk K, Skrzypek E, Krupa M. Biostimulants and Microorganisms Boost the Nutritional Composition of Buckwheat (Fagopyrum esculentum Moench) Sprouts. Agronomy. 2019; 9(8):469. https://doi.org/10.3390/agronomy9080469
Chicago/Turabian StyleWitkowicz, Robert, Wioletta Biel, Joanna Chłopicka, Agnieszka Galanty, Katarzyna Gleń-Karolczyk, Edyta Skrzypek, and Mateusz Krupa. 2019. "Biostimulants and Microorganisms Boost the Nutritional Composition of Buckwheat (Fagopyrum esculentum Moench) Sprouts" Agronomy 9, no. 8: 469. https://doi.org/10.3390/agronomy9080469
APA StyleWitkowicz, R., Biel, W., Chłopicka, J., Galanty, A., Gleń-Karolczyk, K., Skrzypek, E., & Krupa, M. (2019). Biostimulants and Microorganisms Boost the Nutritional Composition of Buckwheat (Fagopyrum esculentum Moench) Sprouts. Agronomy, 9(8), 469. https://doi.org/10.3390/agronomy9080469







