Ultrasound-Assisted Extraction of Two Types of Antioxidant Compounds (TPC and TA) from Black Chokeberry (Aronia melanocarpa L.): Optimization of the Individual and Simultaneous Extraction Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Solvents and Reagents
2.3. Ultrasound-Assisted Extraction (UAE)
2.4. Folin-Ciocalteau Procedure
2.5. Identification of Anthocyanins
2.6. Separation and Quantification of Anthocyanins
2.7. Data Statistical Analysis
2.8. Analysis of Sugars
2.9. Analysis of Organic Acids
3. Results and Discussion
3.1. Optimization of TPC Individual Extraction Method
3.2. Optimization of TA Individual Extraction Method
3.3. Discussion on Each Variable That Influences the Extraction
3.4. Optimization of TPC and TA Simultaneous Extraction Method
3.5. Analysis of Sugars and Organic Acids
3.6. Real Sample Implementation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, J.E.; Kim, G.S.; Park, S.; Kim, Y.H.; Kim, M.B.; Lee, W.S.; Jeong, S.W.; Lee, S.J.; Jin, J.S.; Shin, S.C. Determination of chokeberry (Aronia melanocarpa) polyphenol components using liquid chromatography-tandem mass spectrometry: Overall contribution to antioxidant activity. Food Chem. 2014, 146, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A Review on the Characteristic Components and Potential Health Effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Sójka, M.; Kołodziejczyk, K.; Milala, J. Polyphenolic and basic chemical composition of black chokeberry industrial by-products. Ind. Crops Prod. 2013, 51, 77–86. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S. Effect of the Production of Dried Fruits and Juice from Chokeberry (Aronia melanocarpa L.) on the Content and Antioxidative Activity of Bioactive Compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Samoticha, J.; Wojdyło, A.; Lech, K. The influence of different the drying methods on chemical composition and antioxidant activity in chokeberries. LWT Food Sci. Technol. 2016, 66, 484–489. [Google Scholar] [CrossRef]
- Zapolska-Downar, D.; Bryk, D.; Małecki, M.; Hajdukiewicz, K.; Sitkiewicz, D. Aronia melanocarpa fruit extract exhibits anti-inflammatory activity in human aortic endothelial cells. Eur. J. Nutr. 2012, 51, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Gan, R.Y.; Ge, Y.Y.; Zhang, D.; Corke, H. Ultrasonic Treatment Increases Extraction Rate of Common Bean (Phaseolus vulgaris L.) Antioxidants. Antioxidants 2019, 8, 83. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Rhodes, D.; Shen, Y.; Song, W.; Katz, B.; Tomich, J.; Wang, W. Identification and quantification of anthocyanins in transgenic purple tomato. Food Chem. 2016, 202, 184–188. [Google Scholar] [CrossRef]
- Verbeyst, L.; Oey, I.; Van der Plancken, I.; Hendrickx, M.; Van Loey, A. Kinetic study on the thermal and pressure degradation of anthocyanins in strawberries. Food Chem. 2010, 123, 269–274. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Maciel Bindes, M.M.; Miranda Reis, M.H.; Luiz Cardoso, V.; Boffito, D.C. Ultrasound-assisted extraction of bioactive compounds from green tea leaves and clarification with natural coagulants (chitosan and Moringa oleífera seeds). Ultrason. Sonochem. 2019, 51, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, S.Y.; Lin, S.J.; Zhang, J.R.; Gan, R.Y.; Li, H.B. Polyphenolic Profile and Antioxidant Capacity of Extracts from Gordonia axillaris Fruits. Antioxidants 2019, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Hong, J.Y.; Chun, H.S.; Lee, S.K.; Min, H.Y. Ultrasonication-assisted extraction of resveratrol from grapes. J. Food Eng. 2006, 77, 725–730. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Amores-Arrocha, A.; Palma, M.; Barbero, G.F.; Jiménez-Cantizano, A. Alternative Ultrasound-Assisted Method for the Extraction of the Bioactive Compounds Present in Myrtle (Myrtus communis L.). Molecules 2019, 24, 882. [Google Scholar] [CrossRef]
- Watson, M.; Long, H.; Lu, B. Investigation of wrinkling failure mechanics in metal spinning by Box-Behnken design of experiments using finite element method. Int. J. Adv. Manuf. Tech. 2015, 78, 981–995. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Silva Junior, M.M.; Felix, C.S.A.; da Silva, D.L.F.; Santos, A.S.; Santos Neto, J.; de Souza, C.T.; Cruz Junior, R.A.; Souza, A.S. Multivariate optimization techniques in food analysis—A review. Food Chem. 2019, 273, 3–8. [Google Scholar] [CrossRef]
- Vera Candioti, L.; De Zan, M.M.; Cámara, M.S.; Goicoechea, H.C. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Abderrahim, M.; Arribas, S.M.; Condezo-Hoyos, L. A novel high-throughput image based rapid Folin-Ciocalteau assay for assessment of reducing capacity in foods. Talanta 2016, 152, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias-Cárdenas, A.G.; Martínez-Castillo, J.I.; Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; García-Cruz, N.U.; Pacheco, N. Antioxidant Capacity and UPLC-PDA ESI-MS Phenolic Profile of Stevia rebaudiana Dry Powder Extracts Obtained by Ultrasound Assisted Extraction. Agronomy 2018, 8, 170. [Google Scholar] [CrossRef]
- Melvin Samuel, S.; Evy Alice Abigail, M.; Chidambaram, R. Isotherm Modelling, Kinetic Study and Optimization of Batch Parameters Using Response Surface Methodology for Effective Removal of Cr (VI) Using Fungal Biomass. PLoS ONE 2015, 10, e0116884. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Campos-Requena, V.H. Antioxidant Compound Extraction from Maqui (Aristotelia chilensis [Mol] Stuntz) Berries: Optimization by Response Surface Methodology. Antioxidants 2017, 6, 10. [Google Scholar] [CrossRef]
- Candioti, L.V.; Robles, J.C.; Mantovani, E.; Goicoechea, C. Multiple response optimization applied to the development of a capillary electrophoretic method for pharmaceutical analysis. Talanta 2006, 69, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, W.; Saputro, I.E.; Carrera, C.A.; Palma, M.; Barroso, C.G. Multiresponse optimization of a UPLC method for the simultaneous determination of tryptophan and 15 tryptophan-derived compounds using a Box-Behnken design with a desirability function. Food Chem. 2017, 225, 1–9. [Google Scholar] [CrossRef]
- AOAC (Ed.) AOAC Peer Verified Methods Program. In A International, Manual on Policies and Procedures; Maryland: Arlingt, VA, USA, 1998. [Google Scholar]
- Oosthuizen, D.; Goosen, N.J.; Stander, M.A.; Ibrahim, A.D.; Pedavoah, M.M.; Usman, G.O.; Aderinola, T. Solvent Extraction of Polyphenolics from the Indigenous African Fruit Ximenia caffra and Characterization by LC-HRMS. Antioxidants 2018, 7, 103. [Google Scholar] [CrossRef]
- Alderees, F.; Mereddy, R.; Webber, D.; Nirmal, N.; Sultanbawa, Y. Mechanism of Action against Food Spoilage Yeasts and Bioactivity of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum Plant Solvent Extracts. Foods 2018, 7, 179. [Google Scholar] [CrossRef]
- Vajić, U.J.; Grujić-Milanović, J.; Živković, J.; Šavikin, K.; Godevac, D.; Miloradović, Z.; Bugarski, B.; Mihailovic-Stanojevic, N. Optimization of extraction of stinging nettle leaf phenolic compounds using response surface methodology. Ind. Crops Prod. 2015, 74, 912–917. [Google Scholar] [CrossRef]
- Kalt, W.; McDonald, J.E.; Donner, H. Anthocyanins, Phenolics, and Antioxidant Capacity of Processed Lowbush Blueberry Products. Food Chem. Toxicol. 2000, 65, 390–393. [Google Scholar] [CrossRef]
- García-Valcárcel, A.I.; Tadeo, J.L. Fast ultrasound-assisted extraction combined with LC-MS/MS of perfluorinated compounds in manure. J. Sep. Sci. 2013, 36, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barbero, G.F.; Espada-Bellido, E. Assessment of Ultrasound Assisted Extraction as an Alternative Method for the Extraction of Anthocyanins and Total Phenolic Compounds from Maqui Berries (Aristotelia chilensis (Mol.) Stuntz). Agronomy 2019, 9, 148. [Google Scholar] [CrossRef]
- Paini, M.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Binello, A.; Cravotto, G. Influence of ethanol/water ratio in ultrasound and high-pressure/high-temperature phenolic compound extraction from agri-food waste. Int. J. Food Sci. Technol. 2016, 51, 349–358. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT Food Sci. Technol. 2019, 101, 342–350. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.C.; Guiné, R.P.F.; Gonçalves, F.J.A. Evaluation of phenolic compounds, antioxidant activity and bioaccessibility in white crowberry (Corema album). J. Food Meas. Charact. 2017, 11, 1936–1946. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of Microwave-Assisted Extraction for the Recovery of Bioactive Compounds from the Chilean Superfruit (Aristotelia chilensis). Agronomy 2018, 8, 240. [Google Scholar] [CrossRef]
- Bolling, B.W.; Taheri, R.; Pei, R.; Kranz, S.; Yu, M.; Durocher, S.N.; Brand, M.H. Harvest date affects aronia juice polyphenols, sugars, and antioxidant activity, but not anthocyanin stability. Food Chem. 2015, 187, 189–196. [Google Scholar] [CrossRef]
- Hidalgo, G.I.; Almajano, M.P. Red Fruits: Extraction of Antioxidants, Phenolic Content, and radical Scavenging Determination: A Review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Prasad, S. Determination of ascorbic acid and its influence on the bioavailability of iron, zinc and calcium in Fijian food samples. Microchem. J. 2018, 139, 119–124. [Google Scholar] [CrossRef]
- Naude, A.; Nicol, W. Malic acid production through the whole-cell hydration of fumaric acid with immobilised Rhizopus oryzae. Biochem. Eng. J. 2018, 137, 152–161. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Impact of Juice Processing on Blueberry Anthocyanins and Polyphenolics: Comparison of Two Pretreatmentes. Food Chem. Toxicol. 2002, 67, 1660–1667. [Google Scholar] [CrossRef]
- Markkinen, N.; Laaksonen, O.; Nahku, R.; Kuldjarv, R.; Yang, B. Impact of latic acid fermentation on acids, sugars, and phenolic compounds in black chokeberry and sea buckthorn juices. Food Chem. 2019, 286, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Simiqueli, A.A.; Ribeiro Vidigal, M.C.T.; Rodrigues Minim, V.P.; Minim, L.A. Ovalbumin and gum foam and its surface properties as influenced by sucrose and sorbitol. Int. J. Biol. Macromol. 2019, 135, 226–232. [Google Scholar] [CrossRef] [PubMed]
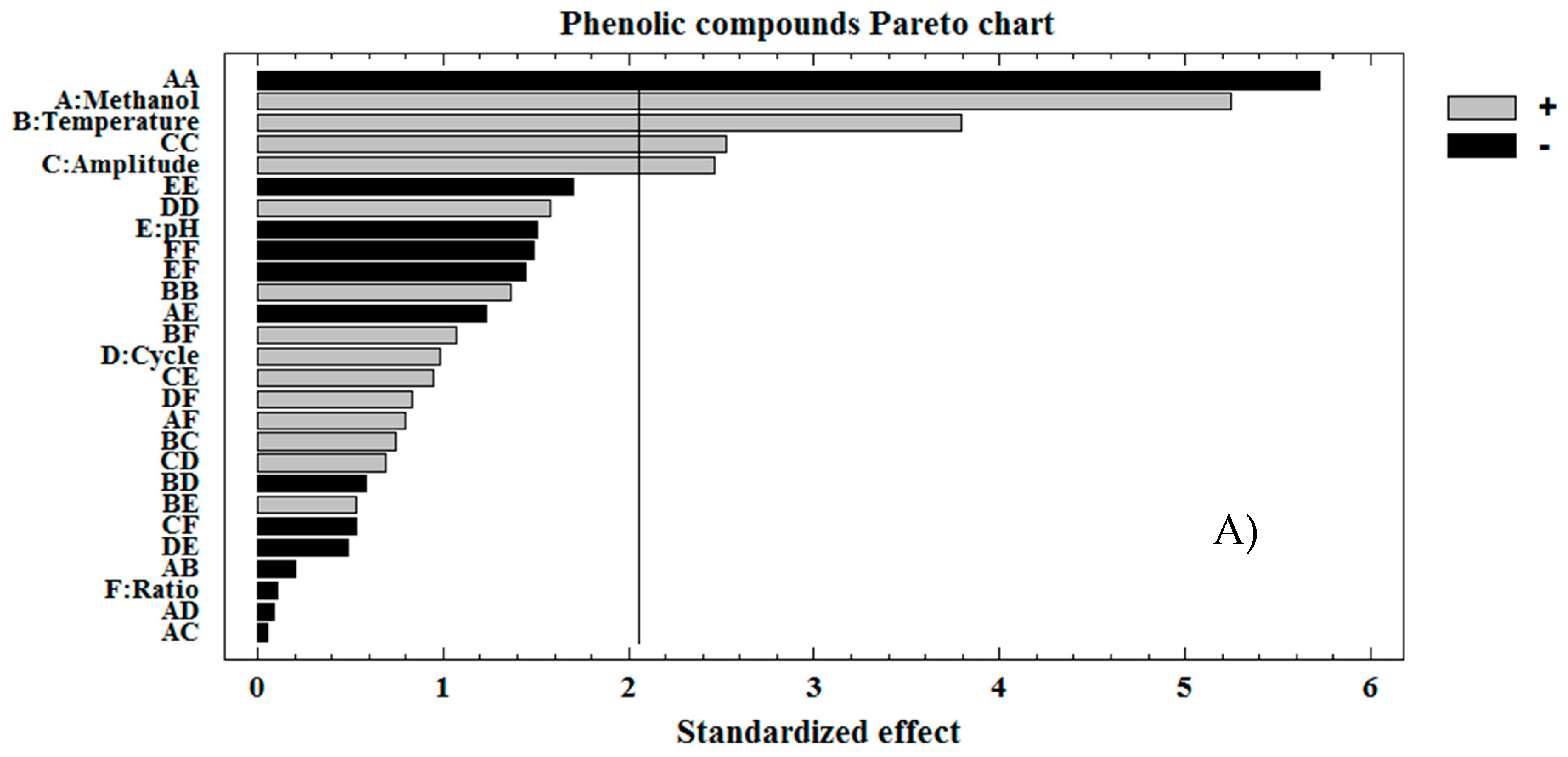
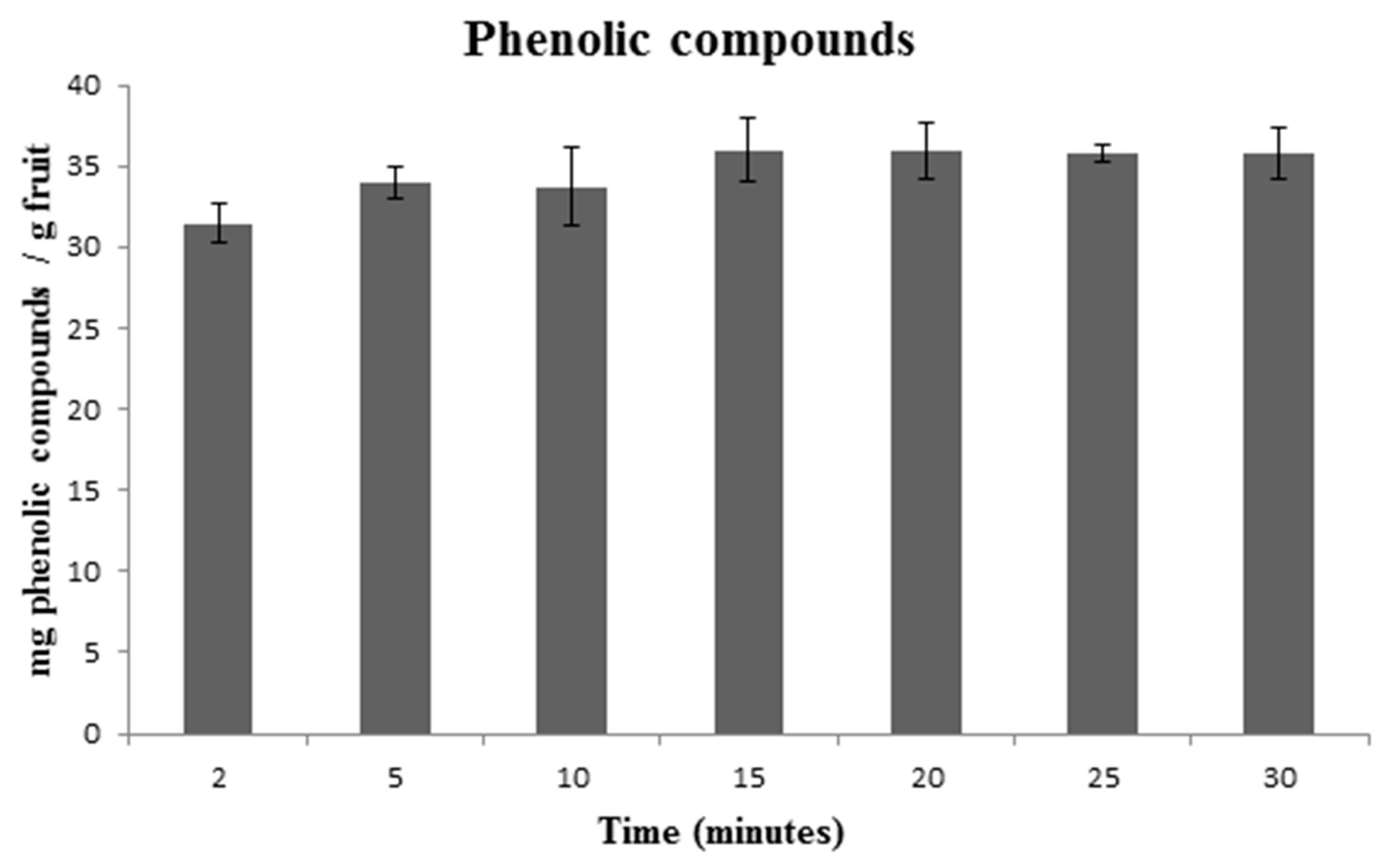
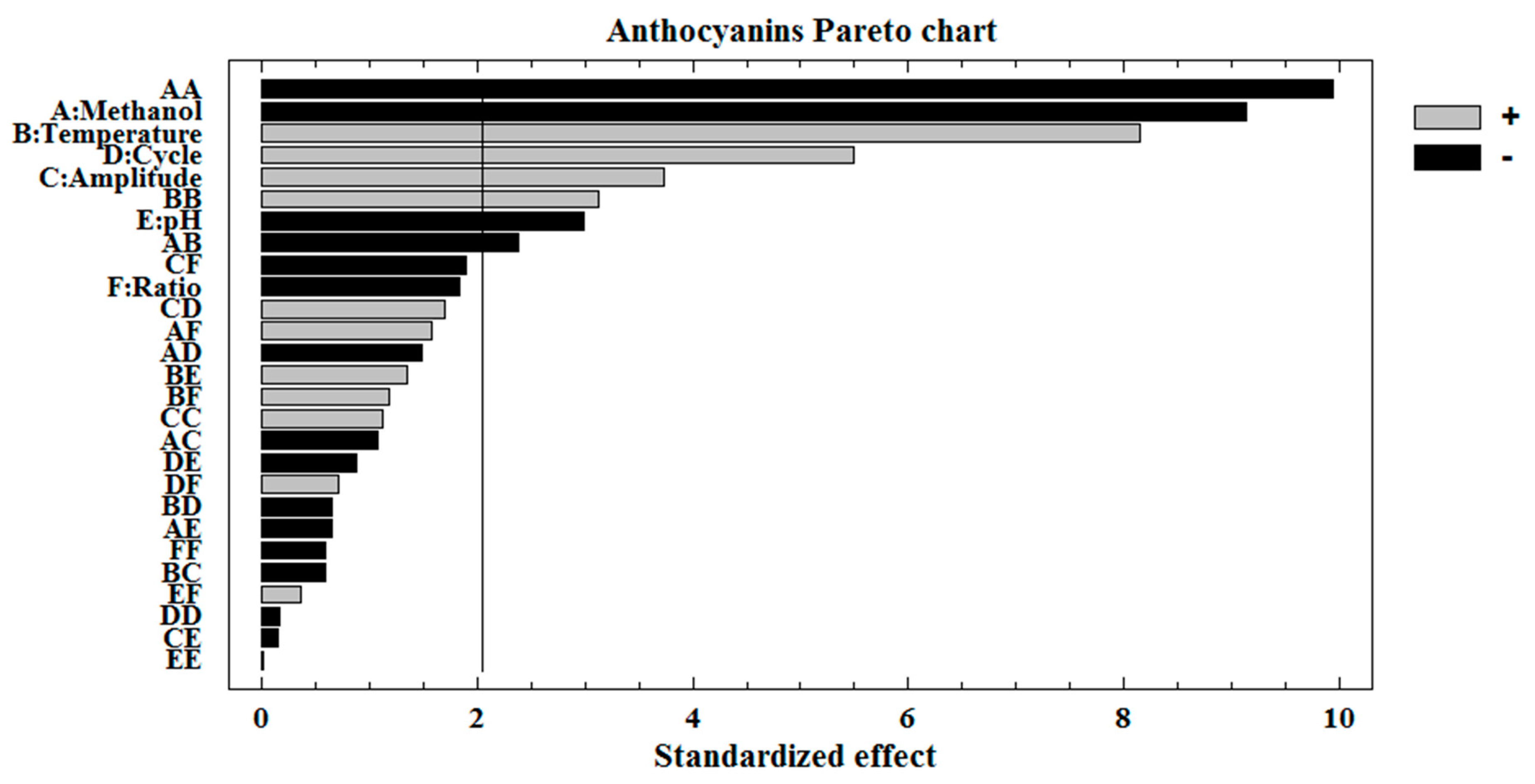
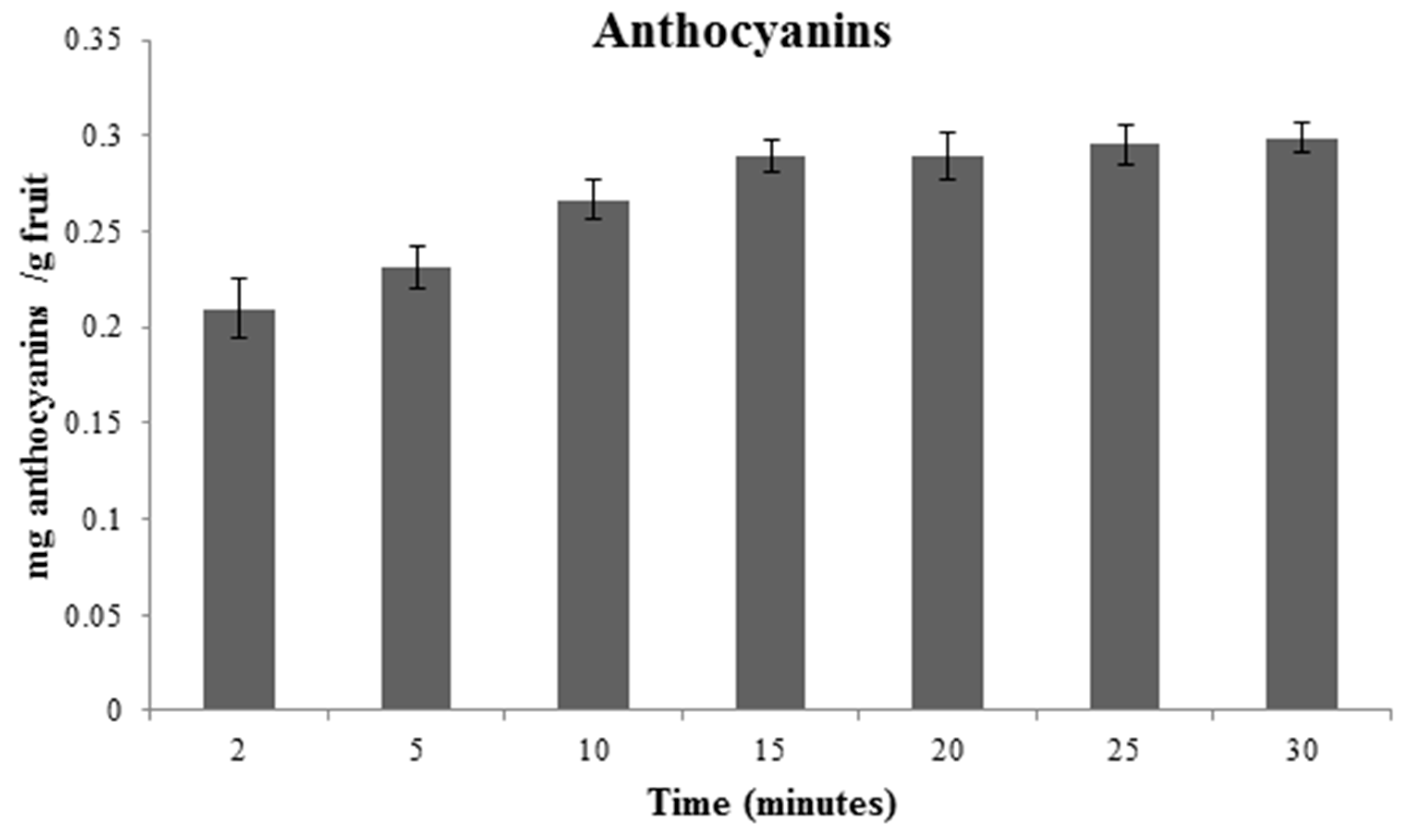
| Source | Sum of Squares | Mean Square | F-Value | p-Value | Coefficient |
|---|---|---|---|---|---|
| A-Solvent | 340.59 | 340.59 | 27.50 | <0.0001 | 3.7671 |
| B-Temperature | 177.83 | 177.83 | 14.36 | 0.0008 | 2.7221 |
| C-Amplitude | 75.23 | 75.23 | 6.07 | 0.0207 | 1.7705 |
| D-Cycle | 12.07 | 12.07 | 0.9741 | 0.3328 | 0.7091 |
| E-pH | 28.20 | 28.20 | 2.28 | 0.1434 | −1.0841 |
| F-Ratio | 0.1430 | 0.1430 | 0.0115 | 0.9153 | −0.0772 |
| AB | 0.5225 | 0.5225 | 0.0422 | 0.8389 | −0.2556 |
| AC | 0.0295 | 0.0295 | 0.0024 | 0.9615 | −0.0607 |
| AD | 0.0879 | 0.0879 | 0.0071 | 0.9335 | −0.0741 |
| AE | 18.86 | 18.86 | 1.52 | 0.2283 | −1.5354 |
| AF | 7.82 | 7.82 | 0.6315 | 0.4340 | 0.9888 |
| BC | 6.89 | 6.89 | 0.5565 | 0.4624 | 0.9282 |
| BD | 4.23 | 4.23 | 0.3418 | 0.5638 | −0.7275 |
| BE | 3.55 | 3.55 | 0.2863 | 0.5972 | 0.4708 |
| BF | 14.24 | 14.24 | 1.15 | 0.2934 | 1.3344 |
| CD | 5.94 | 5.94 | 0.4795 | 0.4948 | 0.8617 |
| CE | 11.21 | 11.21 | 0.9049 | 0.3502 | 1.1837 |
| CF | 3.53 | 3.53 | 0.2849 | 0.5980 | −0.4697 |
| DE | 2.96 | 2.96 | 0.2393 | 0.6288 | −0.6086 |
| DF | 8.60 | 8.60 | 0.6943 | 0.4123 | 1.0368 |
| EF | 25.79 | 25.79 | 2.08 | 0.1610 | −1.7954 |
| A2 | 405.79 | 405.79 | 32.76 | <0.0001 | −6.2810 |
| B2 | 23.08 | 23.08 | 1.86 | 0.1839 | 1.4981 |
| C2 | 78.83 | 78.83 | 6.36 | 0.0181 | 2.7683 |
| D2 | 30.80 | 30.80 | 2.49 | 0.1269 | 1.7305 |
| E2 | 35.86 | 35.86 | 2.90 | 0.1008 | −1.8673 |
| F2 | 27.58 | 27.58 | 2.23 | 0.1477 | −1.6375 |
| Source | Sum of Squares | Mean Square | F-Value | p-Value | Coefficient |
|---|---|---|---|---|---|
| A-Solvent | 0.0331 | 0.0331 | 83.51 | <0.0001 | −0.0371 |
| B-Temperature | 0.0263 | 0.0263 | 66.35 | <0.0001 | 0.0331 |
| C-Amplitude | 0.0055 | 0.0055 | 13.98 | 0.0009 | 0.0152 |
| D-Cycle | 0.0119 | 0.0119 | 30.10 | <0.0001 | 0.0223 |
| E-pH | 0.0035 | 0.0035 | 8.93 | 0.0060 | −0.0121 |
| F-Ratio | 0.0013 | 0.0013 | 3.39 | 0.0771 | −0.0075 |
| AB | 0.0022 | 0.0022 | 5.68 | 0.0248 | −0.0167 |
| AC | 0.0005 | 0.0005 | 1.18 | 0.2882 | −0.0076 |
| AD | 0.0009 | 0.0009 | 2.23 | 0.1475 | −0.0074 |
| AE | 0.0002 | 0.0002 | 0.4254 | 0.5200 | −0.0046 |
| AF | 0.0010 | 0.0010 | 2.50 | 0.1258 | 0.0111 |
| BC | 0.0001 | 0.0001 | 0.3418 | 0.5638 | −0.0041 |
| BD | 0.0002 | 0.0002 | 0.4300 | 0.5177 | −0.0046 |
| BE | 0.0007 | 0.0007 | 1.84 | 0.1864 | 0.0067 |
| BF | 0.0006 | 0.0006 | 1.40 | 0.2466 | 0.0083 |
| CD | 0.0011 | 0.0011 | 2.89 | 0.1013 | 0.0119 |
| CE | 9.031 × 10−6 | 9.031 × 10−6 | 0.0228 | 0.8811 | −0.0010 |
| CF | 0.0014 | 0.0014 | 3.60 | 0.0689 | −0.0094 |
| DE | 0.0003 | 0.0003 | 0.7738 | 0.3871 | −0.0062 |
| DF | 0.0002 | 0.0002 | 0.5154 | 0.4792 | 0.0050 |
| EF | 0.0001 | 0.0001 | 0.1301 | 0.7212 | 0.0025 |
| A2 | 0.0391 | 0.0391 | 98.69 | <0.0001 | −0.0616 |
| B2 | 0.0039 | 0.0039 | 9.81 | 0.0043 | 0.0194 |
| C2 | 0.0005 | 0.0005 | 1.26 | 0.2726 | 0.0070 |
| D2 | 0.0000 | 0.0000 | 0.0261 | 0.8730 | −0.0010 |
| E2 | 6.90 × 10−8 | 6.90 × 10−8 | 0.0002 | 0.9896 | 0.0001 |
| F2 | 0.0001 | 0.0001 | 0.3457 | 0.5616 | −0.0036 |
| Factor | Total Phenolic Compounds | Total Anthocyanins | Total Phenolic Compounds + Total Anthocyanins |
|---|---|---|---|
| % MeOH | 58 | 34 | 54 |
| Temperature (°C) | 70 | 70 | 70 |
| Amplitude (%) | 70 | 70 | 70 |
| Cycle (s) | 0.7 | 0.7 | 0.7 |
| pH | 3.87 | 2 | 2.72 |
| Ratio (g mL−1) | 0.5:17 | 0.5:13.5 | 0.5:18.2 |
| Total average concentration (mg g−1) | 38.0572 | 0.3055 | 37.8231 + 0.2834 |
| Sugars (mg g−1) | Organic Acids (mg g−1) | ||
|---|---|---|---|
| Glucose | 207.9280 | Ascorbic acid | 97.4975 |
| Sorbitol | 118.9995 | Malic acid | 12.6956 |
| Fructose | 92.8570 | Citric acid | 4.8495 |
| Inositol | 0.6837 | Lactic acid | - |
| Maltose | 0.4955 | Total | 115.0427 |
| Glycerol | 0.0983 | ||
| Total | 421.0621 | ||
| Foodstuff Made with Black Aronia | Total Phenolic Compounds (mg g−1) | Total Anthocyanins (mg g−1) | Multiple Response of Both Compounds (mg g−1) TPC/TA |
|---|---|---|---|
| S1: Lyophilized aronia in powder | 34.9415 a | 23.2470 b | 32.6105 a/21.9845 b |
| S2: Raw aronia dehydrated | 13.4554 a | 2.5728 b | 12.0526 a/2.1902 b |
| S3: Aronia berry capsules | 51.1829 a | 26.9411 b | 50.6458 a/24.0754 b |
| S4: Grains of dehydrated raw aronia berries | 37.1157 a | 0.2982 b | 35.4224 a/0.2856 b |
| Foodstuff Made with Black Aronia | S1: Lyophilized Aronia in Powder | S2: Raw Aronia Dehydrated | S3: Aronia Berry Capsules | S4: Grains of Dehydrated Raw Aronia Berries | |
|---|---|---|---|---|---|
| Organic acids (mg g−1) | Ascorbic acid | 59.1270 | 54.0395 | 10.5455 | 67.0127 |
| Malic acid | 11.8760 | 5.4700 | 7.9157 | 13.3520 | |
| Citric acid | 3.8330 | ||||
| Sugars (mg g−1) | Glucose | 344.4775 | 218.3375 | 97.1485 | 179.1065 |
| Sorbitol | 158.0885 | 103.5950 | 62.8810 | 94.7965 | |
| Fructose | 127.4005 | 78.5665 | 42.6710 | 66.5865 | |
| Inositol | 0.6985 | 0.7040 | 0.5430 | 0.4540 | |
| Maltose | 0.4000 | 0.8090 | 0.3480 | 0.3165 | |
| Glycerol | 0.2340 | 0.2595 | 2.0575 | 0.2240 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Espinosa, M.; V. González-de-Peredo, A.; Espada-Bellido, E.; Ferreiro-González, M.; Toledo-Domínguez, J.J.; Carrera, C.; Palma, M.; F. Barbero, G. Ultrasound-Assisted Extraction of Two Types of Antioxidant Compounds (TPC and TA) from Black Chokeberry (Aronia melanocarpa L.): Optimization of the Individual and Simultaneous Extraction Methods. Agronomy 2019, 9, 456. https://doi.org/10.3390/agronomy9080456
Vázquez-Espinosa M, V. González-de-Peredo A, Espada-Bellido E, Ferreiro-González M, Toledo-Domínguez JJ, Carrera C, Palma M, F. Barbero G. Ultrasound-Assisted Extraction of Two Types of Antioxidant Compounds (TPC and TA) from Black Chokeberry (Aronia melanocarpa L.): Optimization of the Individual and Simultaneous Extraction Methods. Agronomy. 2019; 9(8):456. https://doi.org/10.3390/agronomy9080456
Chicago/Turabian StyleVázquez-Espinosa, Mercedes, Ana V. González-de-Peredo, Estrella Espada-Bellido, Marta Ferreiro-González, Juan José Toledo-Domínguez, Ceferino Carrera, Miguel Palma, and Gerardo F. Barbero. 2019. "Ultrasound-Assisted Extraction of Two Types of Antioxidant Compounds (TPC and TA) from Black Chokeberry (Aronia melanocarpa L.): Optimization of the Individual and Simultaneous Extraction Methods" Agronomy 9, no. 8: 456. https://doi.org/10.3390/agronomy9080456
APA StyleVázquez-Espinosa, M., V. González-de-Peredo, A., Espada-Bellido, E., Ferreiro-González, M., Toledo-Domínguez, J. J., Carrera, C., Palma, M., & F. Barbero, G. (2019). Ultrasound-Assisted Extraction of Two Types of Antioxidant Compounds (TPC and TA) from Black Chokeberry (Aronia melanocarpa L.): Optimization of the Individual and Simultaneous Extraction Methods. Agronomy, 9(8), 456. https://doi.org/10.3390/agronomy9080456










