Temporal Interactions between Root-Lesion Nematodes and the Fungus Rhizoctonia Solani Lead to Reduced Potato Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungus
2.2. Nematodes
2.3. Experimental Setup
2.4. Harvest
2.5. Nematode Extraction
2.6. Statistical Analyses
3. Results
3.1. Impact on Stems
3.2. Impact on Stolons
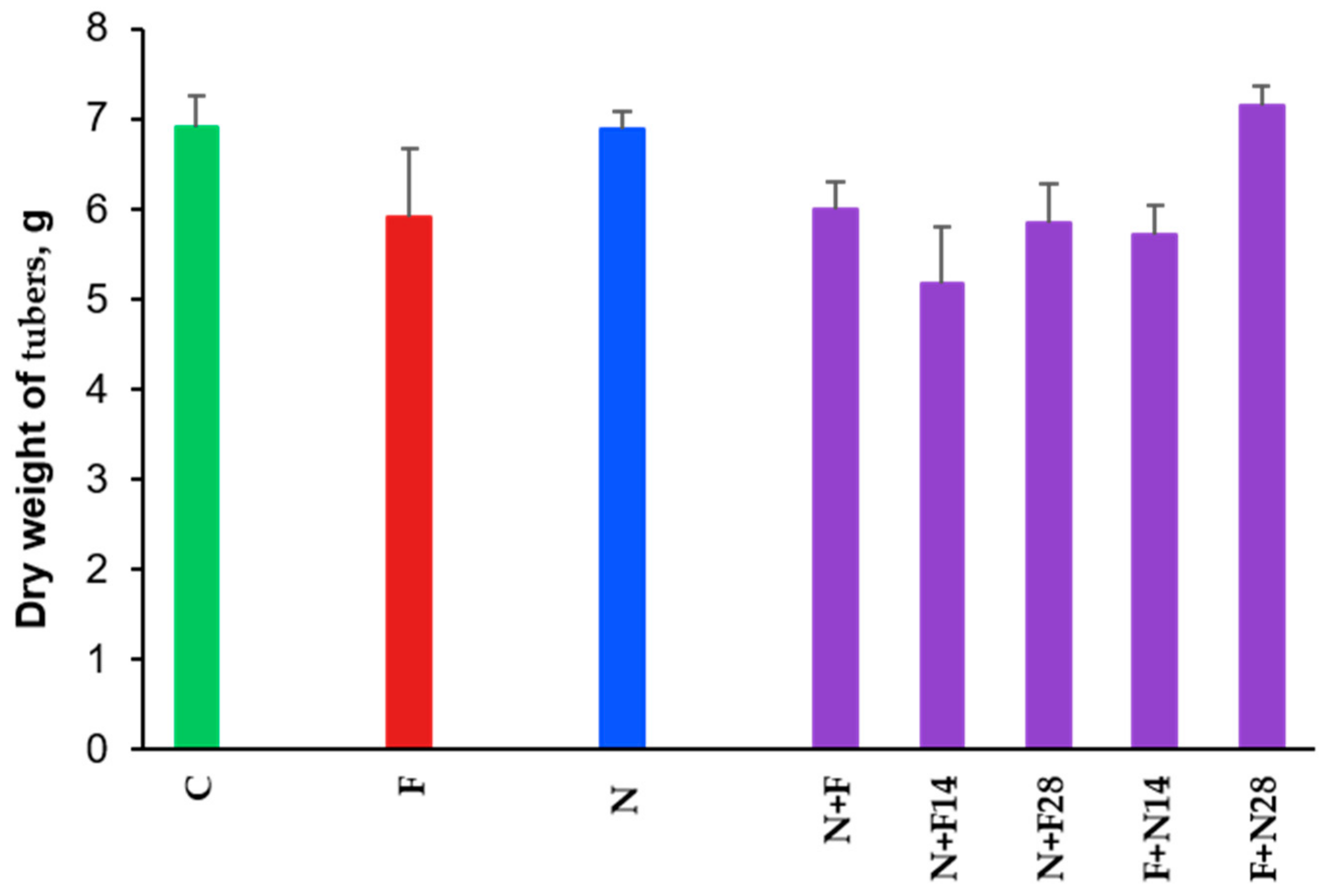
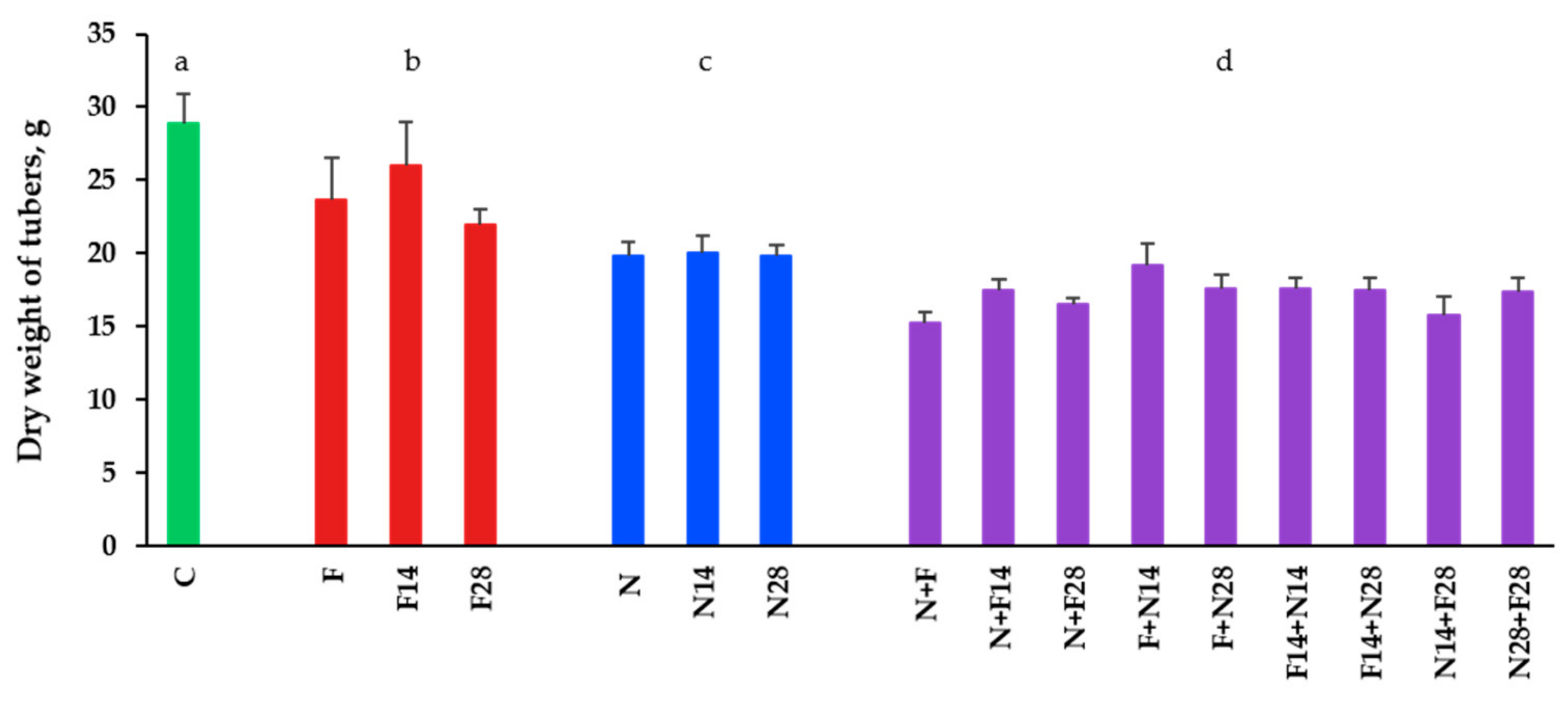
3.3. Impact on Tubers
3.4. Impact on Roots
3.5. Potting Medium
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Plant Parts | Nematode Damage | Sclerotia | Stem Canker | |||
|---|---|---|---|---|---|---|
| Stem | 0 | No damage | 0 | No sclerotium | 0 | No symptoms |
| 1 | 1–10 stripes | 1 | 1–10 sclerotia | 1 | Small lesions | |
| 2 | 11–20 stripes | 2 | 11–20 sclerotia | 2 | Large lesions | |
| 3 | >20 stripes | 3 | >20 sclerotia | 3 | Lesion surrounds stem | |
| 4 | Dead * | |||||
| Stolon | Lesions | Sclerotia | % Brown Stolons | |||
| 0 | No damage | 0 | No sclerotium | 0 | No symptoms | |
| 1 | 1–10 stripes | 1 | 1–10 sclerotia | 1 | Small lesions | |
| 2 | 11–20 stripes | 2 | 11–20 sclerotia | 2 | Large lesions | |
| 3 | >20 stripes | 3 | >20 sclerotia | 3 | Lesion surrounds stem | |
| Tubers | Nematode Damage | Sclerotia | “Elephant Hide” | |||
| 0 | No damage | 0 | No sclerotium | 0 | No symptoms | |
| 1 | 1–10 spots | 1 | 1–10 sclerotia | 1 | Few small | |
| 2 | 11–20 spots | 2 | 11–20 sclerotia | 2 | Cover more than ¼ | |
| 3 | >20 spots | 3 | >20 sclerotia | 3 | Cover more than ½ | |
| Roots | Nematode Damage | Sclerotia | “% Brown Roots” | |||
| 0 | No damage | 0 | No sclerotium | 0 | 0–9 | |
| 1 | Few stripes | 1 | 1–10 sclerotia | 1 | 10–24 | |
| 2 | Stripes on several roots | 2 | 11–20 sclerotia | 2 | 25–49 | |
| 3 | Stripes on most roots | 3 | >20 sclerotia | 3 | 50–100 | |
References
- Nilsson, I.; Rölin, Å.; van Shie, A. Odla Potatis: En Handbok; Hushållningssällskapet Skaraborg: Skara, Sweden, 2012; p. 237. [Google Scholar]
- Terre, F.N.d.P.d.P.d.P.d. A Practical Guide to Diseases, Pests and Disorders of the Potato: Identification Guide and Data Sheets; FN3PT GNIS ARVALIS: Paris, France, 2011. [Google Scholar]
- Hartel, P.G. Microbial Processes | Environmental Factors. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 448–455. [Google Scholar] [CrossRef]
- Braga, R.M.; Dourado, M.N.; Araújo, W.L. Microbial interactions: Ecology in a molecular perspective. Braz. J. Microbiol. 2016, 47, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Decraemer, W.; Hunt, D.J. Structure and classification. In Plant Nematology, 2nd ed.; Perry, R.N., Moens, M., Eds.; CAB International: Wallingford, UK, 2013; pp. 3–39. [Google Scholar]
- Dale, F.; Neilson, R. Research Review: Free-Living Nematodes and Spraing; British Potato Council: Oxford, UK, 2006. [Google Scholar]
- Back, M.A.; Haydock, P.P.J.; Jenkinson, P. Disease complexes involving plant parasitic nematodes and soilborne pathogens. Plant. Pathol. 2002, 51, 683–697. [Google Scholar] [CrossRef]
- Carling, D.E.; Leiner, R.H.; Westphale, P.C. Symptoms, signs and yield reduction associated with rhizoctonia disease of potato induced by tuber-borne inoculum of Rhizoctonia solani AG-3. Am. Potato J. 1989, 66, 693–701. [Google Scholar] [CrossRef]
- Muzhinji, N.; Woodhall, J.W.; Truter, M.; van der Waals, J.E. Elephant Hide and Growth Cracking on Potato Tubers Caused by Rhizoctonia solani AG3-PT in South Africa. Plant. Dis. 2014, 98, 570. [Google Scholar] [CrossRef] [PubMed]
- Björsell, P.; Edin, E.; Viketoft, M. Interactions between some plant-parasitic nematodes and Rhizoctonia solani in potato fields. Appl. Soil Ecol. 2017, 113, 151–154. [Google Scholar] [CrossRef]
- Kotcon, J.B.; Rouse, D.I.; Mitchell, J.E. Interactions of Verticillium dahliae, Colletotricum coccodes, Rhizoctonia solani and Pratylenchus penetrans in the earlt dying syndrome of Russet Burbank potatoes. Phytopathology 1985, 75, 68–74. [Google Scholar] [CrossRef]
- Viketoft, M.; Andersson, A.; Edin, E. Cultivar Effects on the Interaction between Free-Living Plant-Parasitic Nematodes and the Fungal Pathogen Rhizoctonia solani in Potato. Am. J. Potato Res. 2017, 94, 314–322. [Google Scholar] [CrossRef]
- Back, M.; Haydock, P.; Jenkinson, P. Interactions between the potato cyst nematode Globodera rostochiensis and diseases caused by Rhizoctonia solani AG3 in potatoes under field conditions. Eur. J. Plant. Pathol. 2006, 114, 215–223. [Google Scholar] [CrossRef]
- Back, M.; Jenkinson, P.; Deliopoulos, T.; Haydock, P. Modifications in the potato rhizosphere during infestations of Globodera rostochiensis and subsequent effects on the growth of Rhizoctonia solani. Eur. J. Plant. Pathol. 2010, 128, 459–471. [Google Scholar] [CrossRef]
- Bhattarai, S.; Haydock, P.P.J.; Back, M.A.; Hare, M.C.; Lankford, W.T. Interactions between the potato cyst nematodes, Globodera pallida, G. rostochiensis, and soil-borne fungus, Rhizoctonia solani (AG3), diseases of potatoes in the glasshouse and the field. Nematology 2009, 11, 631–640. [Google Scholar] [CrossRef]
- Bhattarai, S.; Haydock, P.P.J.; Back, M.A.; Hare, M.C.; Lankford, W.T. Interactions between field populations of the potato cyst nematode Globodera pallida and Rhizoctonia solani diseases of potatoes under controlled environment and glasshouse conditions. Nematology 2010, 12, 783–790. [Google Scholar] [CrossRef]
- Liu, B.; Shen, W.; Wei, H.; Smith, H.; Louws, F.J.; Steadman, J.R.; Correll, J.C. Rhizoctonia communities in soybean fields and their relation with other microbes and nematode communities. Eur. J. Plant. Pathol. 2016, 144, 671–686. [Google Scholar] [CrossRef]
- Lovell, D.J.; Powers, S.J.; Welham, S.J.; Parker, S.R. A perspective on the measurement of time in plant disease epidemiology. Plant Pathol. 2004, 53, 705–712. [Google Scholar] [CrossRef]
- Bains, P.S.; Bennypaul, H.S.; Lynch, D.R.; Kawchuk, L.M.; Schaupmeyer, C.A. Rhizoctonia disease of potatoes (Rhizoctonia solani): Fungicidal efficacy and cultivar susceptibility. Am. J. Potato Res. 2002, 79, 99–106. [Google Scholar] [CrossRef]
- Djébali, N.; Belhassen, T. Field study of the relative susceptibility of eleven potato (Solanum tuberosum L.) varieties and the efficacy of two fungicides against Rhizoctonia solani attack. Crop. Prot. 2010, 29, 998–1002. [Google Scholar] [CrossRef]
- Edin, E.; Viketoft, M. Free-Living Plant-Parasitic Nematodes do not Affect the Efficiency of Seed Tuber Fungicide Treatment against Rhizoctonia solani. Am. J. Potato Res. 2017, 1–8. [Google Scholar] [CrossRef]
- Viketoft, M.; Palmborg, C.; Sohlenius, B.; Huss-Danell, K.; Bengtsson, J. Plant species effects on soil nematode communities in experimental grasslands. Appl. Soil Ecol. 2005, 30, 90–103. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Nlme: Linear and Nonlinear Mixed Effects Models; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Lenth, R. Emmeans: Estimated Marginal Means, aka Least-Squares Means. Available online: https://rdrr.io/cran/emmeans/ (accessed on 11 March 2019).
- Bernard, E.C.; Laughlin, C.W. Relative susceptibility of selected cultivars of potato to Pratylenchus Penetrans. J. Nematol. 1976, 8, 239–242. [Google Scholar]
- Holgado, R.; Magnusson, C. Nematodes as a Limiting Factor in Potato Production in Scandinavia. Potato Res. 2012, 55, 269–278. [Google Scholar] [CrossRef][Green Version]
- Morgan, G.D.; Stevenson, W.R.; Macguidwin, A.E.; Kelling, K.A.; Binning, L.K.; Zhu, J. Plant pathogen population dynamics in potato fields. J. Nematol. 2002, 34, 189–193. [Google Scholar] [PubMed]
- Leonetti, P.; Costanza, A.; Zonno, M.; Molinari, S.; Altomare, C. How fungi interact with nematode to activate the plant defence response to tomato plants. Commun. Agric. Appl. Biol. Sci. 2014, 79, 357–362. [Google Scholar] [PubMed]
- Nordmeyer, D.; Sikora, R.A. Effect of a Culture Filtrate From Fusariuma Venaceum On the Penetration of Heterodera Daverti Into Roots of Trifolium Subterraneum. Nematologica 1983, 29, 88–94. [Google Scholar] [CrossRef]
- Davis, E.L.; MacGuidwin, A.E. Lesion Nematode Disease; The American Phytopathological Society (APS): St. Paul, MN, USA, 2000. [Google Scholar] [CrossRef]
- Woodhall, J.W.; Lees, A.K.; Edwards, S.G.; Jenkinson, P. Infection of potato by Rhizoctonia solani: Effect of anastomosis group. Plant Pathol. 2008, 57, 897–905. [Google Scholar] [CrossRef]
- Muzhinji, N.; Truter, M.; Woodhall, J.W.; van der Waals, J.E. Anastomosis Groups and Pathogenicity of Rhizoctonia solani and Binucleate Rhizoctonia from Potato in South Africa. Plant Dis. 2015, 99, 1790–1802. [Google Scholar] [CrossRef] [PubMed]
- Bång, U. Rhizoctonia Solani—Marksmitta Finns Det, Vilka Stammar Förekommer? Slutrapport SLF: Toronto, ON, Canada, 2008; projekt number 0242016. [Google Scholar]
- Lehtonen, M.; Wilson, P.; Ahvenniemi, P.; Valkonen, J. Formation of canker lesions on stems and black scurf on tubers in experimentally inoculated potato plants by isolates of AG2-1, AG3 and AG5 of Rhizoctonia solani: A pilot study and literature review. Agric. Food Sci. 2009, 18, 223–233. [Google Scholar] [CrossRef]
- Larkin, R.P.; Halloran, J.M. Management Effects of Disease-Suppressive Rotation Crops on Potato Yield and Soilborne Disease and Their Economic Implications in Potato Production. Am. J. Potato Res. 2014, 91, 429–439. [Google Scholar] [CrossRef]
| Nematode Addition (N) | ||||
|---|---|---|---|---|
| Fungus (F) | Not added | Start | Day 14 | Day 28 |
| Not added | Control * | N * | N14 | N28 |
| Start | F * | N + F * | F + N14 * | F + N28 * |
| Day 14 | F14 | N + F14 * | F14 + N14 | F14 + N28 |
| Day 28 | F28 | N + F28 * | N14 + F28 | N28 + F28 |
| Treatment | No. of Stems | Stem Biomass | Stolon Biomass | Root Biomass | No. of Tubers |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| C | 1.38 (0.17) | 1.99 (0.08) | 0.19 (0.02) a | 0.35 (0.01) | 4.00 (0.35) |
| F | 1.88 (0.28) | 1.87 (0.15) | 0.05 (0.11) b | 0.35 (0.03) | 4.25 (0.52) |
| N | 1.63 (0.35) | 9.94 (0.01) | 0.18 (0.02) a | 0.33 (0.02) | 4.50 (0.64) |
| N + F | 1.25 (0.15) | 1.80 (0.07) | 0.05 (0.01) b | 0.31 (0.03) | 4.13 (0.87) |
| N + F14 | 1.38 (0.17) | 1.55 (0.24) | 0.03 (0.01) b | 0.24 (0.05) | 4.13 (0.67) |
| N + F28 | 1.75 (0.34) | 1.98 (0.17) | 0.16 (0.03) a | 0.41 (0.04) | 4.00 (0.56) |
| F + N14 | 2.63 (0.30) | 1.83 (0.12) | 0.06 (0.01) b | 0.31 (0.03) | 5.75 (0.84) |
| F + N28 | 1.88 (0.33) | 1.74 (0.09) | 0.05 (0.01) b | 0.31 (0.02) | 4.00 (0.43) |
| Experiment 2 | |||||
| C | 1.63 (0.2) | 4.52 (0.2) | 0.21 (0.03) ab | 0.84 (0.09) ab | 6.50 (0.6) |
| F | 1.50 (0.3) | 3.95 (0.2) | 0.09 (0.02) abc | 0.78 (0.08) ab | 4.63 (0.6) |
| F14 | 2.00 (0.3) | 4.49 (0.3) | 0.11 (0.06) abc | 0.80 (0.06) ab | 5.29 (1.0) |
| F28 | 2.13 (0.1) | 4.20 (0.3) | 0.15 (0.03) abc | 0.90 (0.08) a | 7.00 (1.1) |
| N | 2.13 (0.3) | 4.00 (0.2) | 0.16 (0.03) abc | 0.65 (0.06) ab | 7.25 (1.1) |
| N14 | 2.13 (0.1) | 4.05 (0.2) | 0.16 (0.03) abc | 0.61 (0.05) ab | 8.25 (0.6) |
| N28 | 1.88 (0.3) | 4.14 (0.2) | 0.23 (0.02) a | 0.80 (0.08) ab | 6.88 (0.5) |
| N + F | 2.00 (0.3) | 4.13 (0.4) | 0.10 (0.03) abc | 0.57 (0.05) b | 8.50 (1.4) |
| N + F14 | 2.00 (0.0) | 4.32 (0.2) | 0.08 (0.03) bc | 0.73 (0.07) ab | 5.88 (0.6) |
| N + F28 | 2.29 (0.3) | 3.86 (0.3) | 0.17 (0.02) abc | 0.60 (0.08) ab | 5.29 (1.2) |
| F + N14 | 2.38 (0.3) | 4.24 (0.2) | 0.10 (0.02) abc | 0.71 (0.07) ab | 6.75 (1.1) |
| F + N28 | 1.75 (0.2) | 3.95 (0.1) | 0.13 (0.02) abc | 0.60 (0.06) ab | 6.63 (0.8) |
| F14 + N14 | 1.75 (0.2) | 3.94 (0.1) | 0.06 (0.02) c | 0.67 (0.06) ab | 7.25 (1.4) |
| F14 + N28 | 1.88 (0.2) | 4.03 (0.3) | 0.09 (0.03) c | 0.55 (0.06) b | 7.00 (1.4) |
| N14 + F28 | 1.88 (0.1) | 3.76 (0.2) | 0.14 (0.02) abc | 0.64 (0.04) ab | 6.00 (1.2) |
| N28 + F28 | 2.38 (0.3) | 4.18 (0.1) | 0.14 (0.02) abc | 0.80 (0.07) ab | 7.13 (1.1) |
| Treatment | Stem | Tubers | Stolons I | Roots | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stem Canker | Sclerotia | Nematode Damage | Elephant Hide | Black Scurf | Nematode Damage | Sclerotia | Lesions | % Brown Stolons | Sclerotia | Lesions | % Brown Roots | |
| Experiment 1 | ||||||||||||
| C | 0 II | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 100 III |
| F | 87 | 40 | 0 | 32 b | 68 ab | 0 | 25 | 0 | 75 | 88 | 0 | 100 |
| N | 0 | 15 | 92 | 0 | 0 | 0 | 0 | 88 | 38 | 0 | 63 | 100 |
| N + F | 90 | 80 | 100 | 45 ab | 42 ab | 0 | 25 | 50 | 88 | 100 | 100 | 100 |
| N + F14 | 100 | 82 | 64 | 39 ab | 42 ab | 0 | 38 | 63 | 63 | 75 | 63 | 88 |
| N + F28 | 64 | 57 | 79 | 78 a | 81 a | 0 | 75 | 75 | 100 | 88 | 100 | 100 |
| F + N14 | 100 | 57 | 71 | 39 b | 39 b | 0 | 13 | 25 | 75 | 100 | 75 | 100 |
| F + N28 | 67 | 40 | 60 | 34 b | 56 ab | 0 | 0 | 50 | 100 | 75 | 25 | 100 |
| Treatment | Stem | Tubers | Stolons a | Roots | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stem Canker | Sclerotia | Nematode Damage | Elephant Hide | Black scurf | Nematode Damage | Sclerotia | Lesions | % Brown Stolons | Sclerotia | Lesions | % Brown Roots | ||
| Experiment 2 | |||||||||||||
| C | 0 I | 0 II | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 18 III |
| F | 77 | 69 | 77 | 0 | 54 | 51 | 0 | 50 | 0 | 24 | 75 | 0 | 23 |
| F14 | 86 | 64 | 93 | 0 | 78 | 76 | 0 | 86 | 0 | 32 | 100 | 0 | 24 |
| F28 | 88 | 59 | 94 | 0 | 70 | 84 | 0 | 100 | 0 | 42 | 100 | 0 | 36 |
| N | 0 | 0 | 0 | 100 | 0 | 0 | 33 | 0 | 100 | 11 | 0 | 100 | 34 |
| N14 | 0 | 0 | 0 | 100 | 0 | 0 | 44 | 0 | 100 | 5 | 0 | 100 | 36 |
| N28 | 0 | 0 | 0 | 73 | 0 | 0 | 20 | 0 | 100 | 6 | 0 | 100 | 27 |
| N + F | 75 | 69 | 88 | 94 | 37 | 62 | 41 | 88 | 75 | 30 | 100 | 100 | 36 |
| N + F14 | 88 | 63 | 94 | 100 | 66 | 66 | 36 | 100 | 100 | 30 | 100 | 100 | 31 |
| N + F28 | 93 | 60 | 100 | 100 | 70 | 68 | 32 | 100 | 100 | 29 | 100 | 100 | 29 |
| F + N14 | 84 | 58 | 79 | 100 | 85 | 67 | 35 | 75 | 100 | 31 | 100 | 100 | 31 |
| F + N28 | 57 | 36 | 43 | 86 | 19 | 26 | 9 | 38 | 100 | 17 | 38 | 100 | 29 |
| F14 + N14 | 86 | 50 | 93 | 100 | 72 | 74 | 45 | 63 | 88 | 33 | 63 | 100 | 34 |
| F14 + N28 | 87 | 67 | 73 | 100 | 75 | 80 | 21 | 38 | 100 | 31 | 38 | 100 | 31 |
| N14 + F28 | 87 | 53 | 80 | 100 | 60 | 77 | 27 | 88 | 100 | 34 | 88 | 100 | 36 |
| N28 + F28 | 84 | 58 | 89 | 89 | 54 | 58 | 5 | 100 | 100 | 28 | 100 | 100 | 31 |
| Treatment | No. in Stems | No. in Tubers | No. in Roots | No. in Potting Medium | ||||
|---|---|---|---|---|---|---|---|---|
| PPN | FFN | PPN | FFN | PPN | FFN | PPN | FFN | |
| Experiment 1 | ||||||||
| N | 31.1 (14.3) | 0.0 (0.0) b | 0.00 (0.00) | 0.00 (0.00) | 746.5 (283.1) | 0.0 (0.0) b | 0.05 (0.02) | 0.00 (0.00) |
| N + F | 25.6 (7.3) | 28.9 (16.0) ab | 0.02 (0.02) | 0.00 (0.00) | 419.9 (190.7) | 157.9 (103.7) ab | 0.01 (0.01) | 0.15 (0.05) |
| N + F14 | 67.2 (38.1) | 64.5 (15.6) a | 0.20 (0.10) | 0.06 (0.05) | 756.1 (232.1) | 91.5 (42.5) b | 0.04 (0.02) | 0.28 (0.16) |
| N + F28 | 26.6 (15.1) | 10.5 (3.0) b | 0.13 (0.06) | 0.02 (0.02) | 580.7 (155.4) | 32.8 (10.5) b | 0.05 (0.02) | 0.03 (0.01) |
| F + N14 | 54.0 (20.3) | 29.4 (17.0) ab | 0.05 (0.03) | 0.03 (0.03) | 473.5 (81.0) | 472.9 (124.7) a | 0.06 (0.03) | 0.47 (0.20) |
| F + N28 | 20.6 (6.0) | 64.1 (20.3) a | 0.19 (0.06) | 0.04 (0.04) | 175.1 (53.3) | 245.4 (99.6) ab | 0.01 (0.01) | 0.11 (0.02) |
| Experiment 2 | ||||||||
| N | 122.1 (32.3) | - | 1.63 (0.66) | - | 742.8 (112.6) | - | 0.25 (0.11) | - |
| N14 | 132.3 (50.2) | - | 0.48 (0.14) | - | 1957.9 (317.1) | - | 0.23 (0.07) | - |
| N28 | 7.8 (3.3) | - | 0.12 (0.15) | - | 438.5 (112.1) | - | 0.23 (0.15) | - |
| N + F | 130.3 (33.5) | - | 3.87 (0.04) | - | 1391.3 (424.6) | - | 0.13 (0.04) | - |
| N + F14 | 160.4 (44.5) | - | 3.39 (0.03) | - | 719.0 (126.4) | - | 0.07 (0.03) | - |
| N + F28 | 69.6 (17.9) | - | 0.61 (0.16) | - | 956.5 (152.07) | - | 0.19 (0.04) | - |
| F + N14 | 105.2 (23.1) | - | 6.94 (2.65) | - | 1793.9 (452.2) | - | 0.28 (0.10) | - |
| F + N28 | 46.1 (22.7) | - | 0.21 (0.10) | - | 520.3 (68.0) | - | 0.04 (0.02) | - |
| F14 + N14 | 68.4 (16.8) | - | 1.57 (0.52) | - | 1479.0 (224.8) | - | 0.39 (0.11) | - |
| F14 + N28 | 14.5 (7.4) | - | 0.34 (0.17) | - | 960.8 (316.7) | - | 0.15 (0.06) | - |
| N14 + F28 | 60.3 (12.0) | - | 2.50 (1.71) | - | 973.6 (265.7) | - | 0.27 (0.10) | - |
| N28 + F28 | 4.6 (2.9) | - | 0.29 (0.17) | - | 421.9 (147.1) | - | 0.09 (0.05) | - |
| Treatment | Stem Lesion | No. of P pg−1 Stem | Tuber Lesion | Probability (%) Tuber Lesion | No. of Pp g−1 Tuber | No. of Pp g−1 Root | No. of Pp g−1 Potting Medium |
|---|---|---|---|---|---|---|---|
| N, N + F, N + F14, N+ F28 | 11.3 (9.2–13.9) a | 103 (60–159) a | 5.0 (2.6–9.9) a | 34 (24–45) a | 1.8 (0.9–2.9) a | 800 (631–1016) b | 0.11 (0.05–0.19) ab |
| N14, N14 + F, N14 + F14, N14 + F28 | 12.3 (10.1–15.1) a | 76 (40–124) a | 7.6 (3.4–16.8) a | 39 (29–50) a | 1.6 (0.8–2.6) a | 1244 (927–1670) a | 0.21 (0.12–0.32) a |
| N28, N28 + F, N28 + F14, N28 + F28 | 3.8 (3.0–4.8) b | 6 (0.1–24) b | 1.3 (0.6–2.6) b | 12 (7–19) b | 0.1 (0.0–0.5) b | 396 (264–595) c | 0.05 (0.01–0.11) b |
| Treatment | Stolons Nectrotic Lesions | No. of Sclerotia on Stolons | Probability (%) Elephant Hide | Probability (%) Black Scurf | No. of Pp g−1 Tuber |
|---|---|---|---|---|---|
| C | 0 | 0 | 0 | 0 | 0 |
| N, N14, N28 | 23.4 (14.9–37.0) a | 0 | 0 | 0 | 0.4 (0.0–1.0) b |
| F, F + N, F + N14, F + N28 | 7.3 (4.5–11.8) b | 1.9 (1.2–3.2) b | 42 (31–55) b | 58 (43–72) b | 2.0 (1.0–3.3) a |
| F14, F14 + N, F14 + N14, F14 + N28 | 5.8 (3.5–9.5) b | 2.4 (1.5–3.9) b | 76 (65–84) a | 80 (68–89) a | 1.2 (0.5–2.3) ab |
| F28, F28 + N, F28 + N14, F28 + N28 | 13.7 (8.6–21.8) a | 7.6 (4.9–11.7) a | 69 (57–80) a | 87 (76–93) a | 0.5 (0.1–1.3) b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edin, E.; Gulsher, M.; Andersson Franko, M.; Englund, J.-E.; Flöhr, A.; Kardell, J.; Viketoft, M. Temporal Interactions between Root-Lesion Nematodes and the Fungus Rhizoctonia Solani Lead to Reduced Potato Yield. Agronomy 2019, 9, 361. https://doi.org/10.3390/agronomy9070361
Edin E, Gulsher M, Andersson Franko M, Englund J-E, Flöhr A, Kardell J, Viketoft M. Temporal Interactions between Root-Lesion Nematodes and the Fungus Rhizoctonia Solani Lead to Reduced Potato Yield. Agronomy. 2019; 9(7):361. https://doi.org/10.3390/agronomy9070361
Chicago/Turabian StyleEdin, Eva, Mehreen Gulsher, Mikael Andersson Franko, Jan-Eric Englund, Adam Flöhr, Jonas Kardell, and Maria Viketoft. 2019. "Temporal Interactions between Root-Lesion Nematodes and the Fungus Rhizoctonia Solani Lead to Reduced Potato Yield" Agronomy 9, no. 7: 361. https://doi.org/10.3390/agronomy9070361
APA StyleEdin, E., Gulsher, M., Andersson Franko, M., Englund, J.-E., Flöhr, A., Kardell, J., & Viketoft, M. (2019). Temporal Interactions between Root-Lesion Nematodes and the Fungus Rhizoctonia Solani Lead to Reduced Potato Yield. Agronomy, 9(7), 361. https://doi.org/10.3390/agronomy9070361





