Drought-Tolerant Barley: II. Root Tip characteristics in Emerging Roots
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Root Border Cell Number
2.3. Root Tip Mucilage Dimensions
2.4. Measurement of Root Hairs in Presence of Water
2.5. Root Tip and Root Elongation Zone Dimensions
2.6. Rate of Germination and Root Growth
2.7. Statistical Analysis
3. Results
3.1. Increased Mucilage on “Solar” Root Tips
3.2. Increased Border Cell Production on “Solar” Root Tips
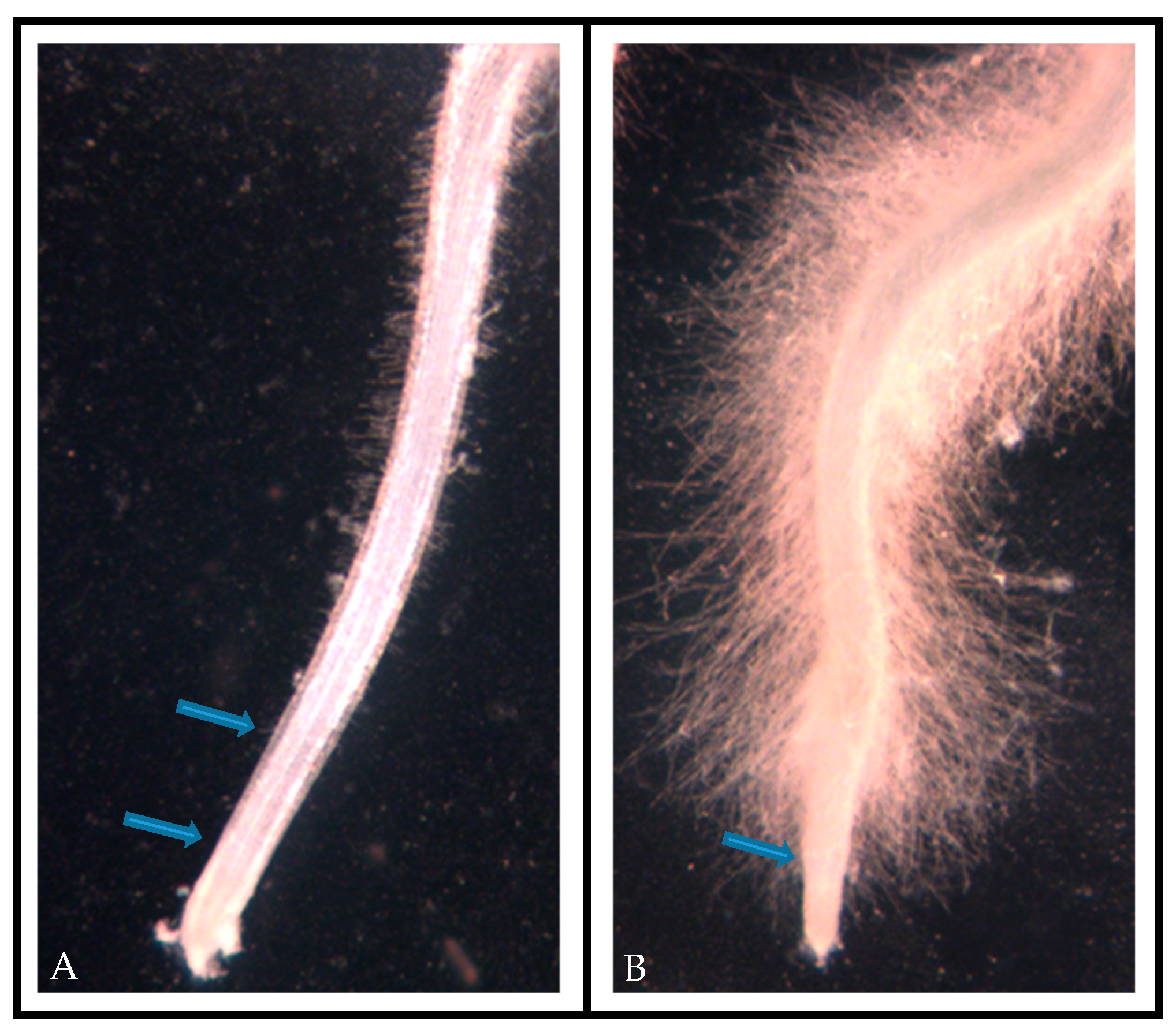
3.3. Altered Root Hair Development
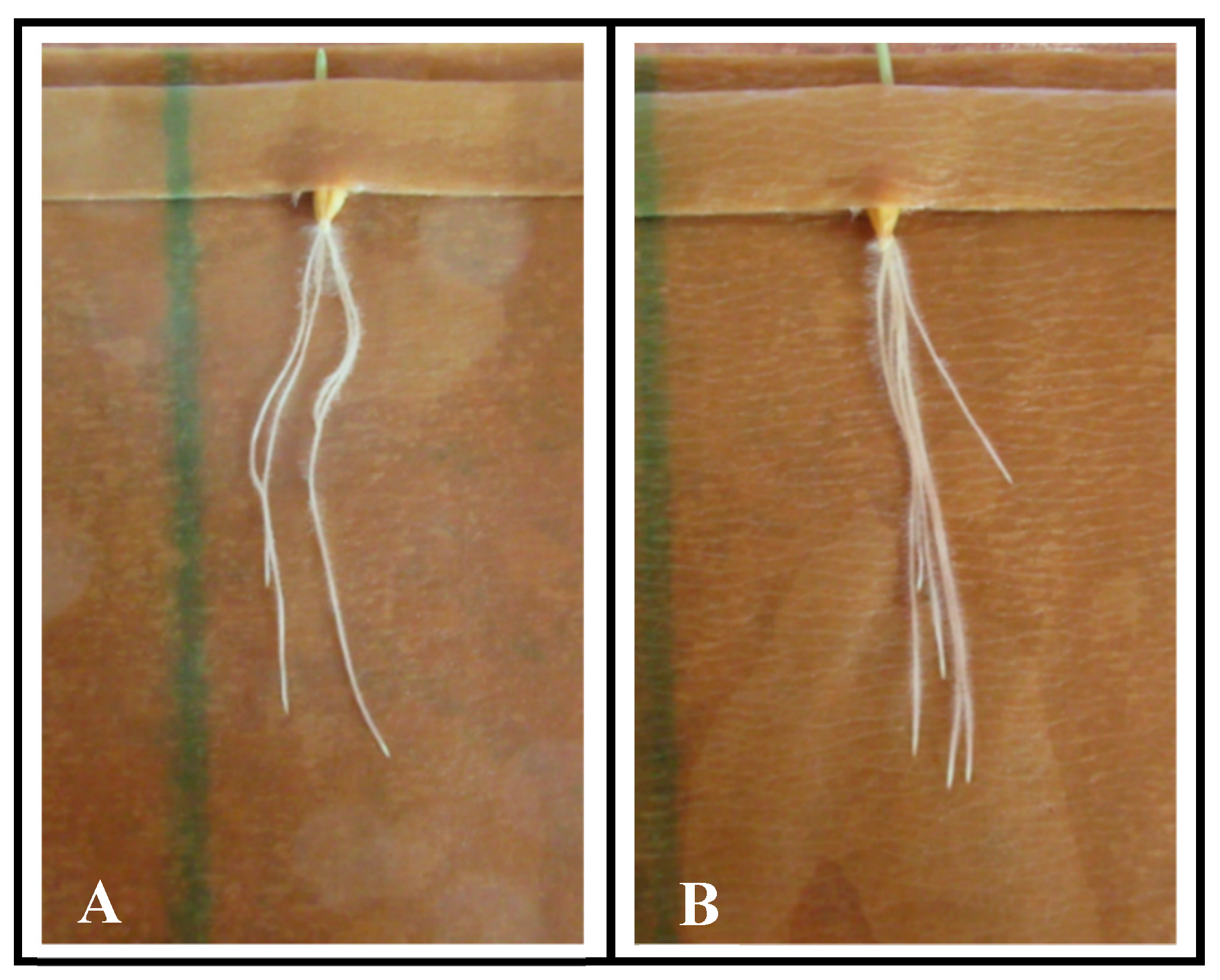
3.4. Altered Rate of Seed Germination in Drought Tolerant and Conventional Barley
3.5. Rate of Root Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reynolds, M.; Langridge, P. Physiological breeding. Curr. Opin. Plant Biol. 2016, 31, 162–171. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Royo, C.; Serret, M.D. Breeding for yield potential and stress adaptation in cereals. Crit. Rev. Plant Sci. 2008, 27, 377–412. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S.; Maatougui, S.; Michael, M.; Slash, M.; Haghparast, R.; Rahmanian, M.; Taheri, A.; Al-Yassin, A.; Benbelkacem, A.; Labdi, M. Plant breeding and climate changes. J. Agric. Sci. 2010, 148, 627–637. [Google Scholar] [CrossRef]
- Lopez-Castaneda, C.; Richards, R.; Farquhar, G. Variation in early vigor between wheat and barley. Crop Sci. 1995, 32, 472–479. [Google Scholar] [CrossRef]
- Gregory, P.J.; Tennant, D.; Belford, R.K. Root and shoot growth, and water and light use efficiency of barley and wheat crops grown on a shallow duplex soil in a mediterranean-type environment. Aus. J. Agric. Res. 1992, 43, 555–573. [Google Scholar] [CrossRef]
- York, L.M.; Nord, E.A.; Lynch, J.P. Integration of root phenes for soil resource acquisition. Frontiers in Plant Sci. 2013, 4, 1–15. [Google Scholar] [CrossRef]
- Palta, J.; Watt, M. Vigorous crop root systems: Form and function for improving the capture of water and nutrients. In Crop Physiology: Applications for Genetic Improvement and Agronomy, 1st ed.; Sadras, V.O., Calderini, D.F., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 309–325. [Google Scholar]
- Kirkegaard, J.A.; Lilley, J.M.; Howe, G.N.; Graham, J.M. Impact of subsoil water use on wheat yield. Aus. J. Agric. Res. 2007, 58, 303–315. [Google Scholar] [CrossRef]
- Ottman, M.J.; Sheedy, M.D.; Ramage, R.T. Seeding rates for barley bred for reduced water use. Cereal Res. Comm. 1990, 18, 179–184. [Google Scholar]
- McKinley, S. New low water, fertilizer barley variety released by University of Arizona. West. Farm Press 2007. Available online: https://www.farmprogress.com (accessed on 5 December 2018).
- Ramage, R.T.; Thompson, R.K.; Eslick, R.P. Release notice of composite cross XXXII. Barley Newsl. 1976, 19, 9–11. [Google Scholar]
- Ramage, R.T. Release of ‘Solum’ a Six-rowed Spring Barley Bred for Reduced Water Use Environments; University of Arizona Agricultural Experiment Station: Tucson, AZ, USA, 1992. [Google Scholar]
- Ottman, M.J. Release of ‘Solar’ a Six-rowed Spring Barley Bred for Reduced Water Use Environments; University of Arizona Agricultural Experiment Station: Tucson, AZ, USA, 2006. [Google Scholar]
- Carter, A.Y.; Ottman, M.J.; Hawes, M.C. Drought-Tolerant Barley: I Field observations of growth and development. Agronomy 2019, 9. (Accepted). [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to Image J: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A language and Environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.R-project.org (accessed on 24 March 2019).
- Wen, F.; Curlango-Rivera, G.; Huskey, D.A.; Xiong, Z.; Hawes, M.C. Visualization of extracellular DNA released during border cell separation from the root cap. Am. J. Bot. 2017, 104, 1–9. [Google Scholar] [CrossRef]
- Hawes, M.C.; McLain, J.; Ramirez-Andreotta, M.; Curlango-Rivera, G.; Flores-Lara, Y.; Brigham, L. Extracellular trapping of soil contaminants by root border cells: New insights into plant defense. Agronomy 2016, 6, 5. [Google Scholar] [CrossRef]
- Curlango-Rivera, G.; Huskey, D.A.; Mostafa, A.; Kessler, J.O.; Xiong, Z.; Hawes, M.C. Intraspecies variation in cotton border cell production: rhizosphere microbiome implications. Am. J. Bot. 2013, 100, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Griffin, G.J.; Hale, M.G.; Shay, F.J. Nature and quantity of sloughed organic matter produced by roots of axenic peanut plants. Soil Biol. Biochem. 1976, 8, 29–32. [Google Scholar] [CrossRef]
- Lynch, J.M.; Whipps, J.M. Substrate flow in the rhizosphere. Plant Soil 1990, 129, 1–10. [Google Scholar] [CrossRef]
- Odell, R.E.; Dumlao, M.R.; Samar, D.; Silk, W.K. Stage-dependent border cell and carbon flow from roots to rhizosphere. Am. J. Bot. 2008, 95, 441–446. [Google Scholar] [CrossRef]
- Huskey, D.A.; Curlango-Rivera, G.; Root, R.A.; Wen, F.; Amistadi, M.K.; Chorover, J.; Hawes, M.C. Trapping of lead by corn and pea root border cells. Plant Soil 2018, 430, 205–217. [Google Scholar] [CrossRef]
- Xie, W.; Xiong, W.; Pan, J.; Ali, T.; Cui, Q.; Guan, D.; Meng, J.; Mueller, N.D.; Lin, E.; Davis, S.J. Decreases in global beer supply due to extreme drought and heat. Nat. Plants 2018, 4, 964–997. [Google Scholar] [CrossRef]
- Kosovai, K.; Vitamvasi, P.; Urban, M.O.; Kholova, J.; Prasil, I.T. Breeding for enhanced drought resistance in barley and wheat—Drought-associated traits, genetic resources and their potential utilization in breeding programmes. Czech J. Genet. Plant Breed. 2014, 50, 247–261. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cel. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Fry, D.L.; Evans, A.L.; Sturrock, C.J.; Bullock, J.M.; Bardgett, R.D. Root architecture governs plasticity in response to drought. Plant Soil 2018, 433, 189–200. [Google Scholar] [CrossRef]
- Haling, R.E.; Brown, L.K.; Bengough, A.G.; Young, I.M.; Hallett, P.D.; White, P.J.; George, T.S. Root hairs improve root penetration, root soil contact, and phosphorus acquisition in soils of different strength. J Exp. Bot. 2013, 64, 3711–3721. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.K.; George, T.S.; Thompson, J.A.; Wright, G.; Lyon, J.; Dupuy, L.; Hubbard, S.F.; White, P.J. What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley (Hordeum vulgare)? Ann. Bot. 2012, 110, 319–328. [Google Scholar] [CrossRef]
- Li, T.; Lin, G.; Zhang, X.; Chen, Y.; Zhang, S.; Chen, B. Relative importance of an arbuscular mycorrhizal fungus (Rhizophagus intraradices) and root hairs in plant drought tolerance. Mycorrhiza 2014, 24, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Watson, B.S.; Bedair, M.F.; Urbanczyk-Wochniak, E.; Huhman, D.V.; Yang, D.S.; Allen, S.N.; Li, W.; Tang, Y.; Sumner, L.W. Integrated metabolomics and transcriptomics reveal enhanced specialized metabolism in Medicago truncatula root border cells. Plant Physiol. 2015, 167, 1699–1716. [Google Scholar] [CrossRef] [PubMed]
- Brigham, L.A.; Woo, H.H.; Wen, F.; Hawes, M.C. Meristem-specific suppression of mitosis and a global switch in gene expression in the root cap of pea by endogenous signals. Plant Physiol. 1998, 118, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Brigham, L.A.; Woo, H.H.; Nicoll, S.M.; Hawes, M.C. Differential expression of proteins and mRNAs from border cells and root tips of pea. Plant Physiol. 1995, 109, 457–463. [Google Scholar] [CrossRef]
- Nagahashi, G.; Douds, D.D., Jr. Isolated root caps, border cells, and mucilage from host roots stimulate hyphal branching of the arbuscular mycorrhizal fungus, Gigaspora gigantea. Mycol. Res. 2004, 108, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, G.; Douds, D.D., Jr. Rapid and sensitive bioassay to study signals between root exudates and arbuscular mycorrhizal fungi. Biotechnol. Tech. 1999, 13, 893–897. [Google Scholar] [CrossRef]
- Niemira, B.A.; Safir, G.R.; Hawes, M.C. Arbuscular mycorrhizal colonization and border cell production: A possible correlation. Phytopathology 1996, 86, 563–565. [Google Scholar]
- Bengough, A.G.; McKenzie, B.M. Sloughing of root cap cells decreases the frictional resistance to maize (Zea mays L.) root growth. J. Exp. Bot. 1997, 48, 885–893. [Google Scholar] [CrossRef]
- Lynch, J.P.; Chimungu, J.G.; Brown, K.M. Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement. J. Exp. Bot. 2014, 65, 6155–6166. [Google Scholar] [CrossRef]
- McCully, M.E. Roots in soil: Unearthing the complexities of roots and their rhizospheres. Ann. Rev. Plant Biol. 1999, 50, 695–718. [Google Scholar] [CrossRef] [PubMed]
- George, T.S.; Brown, L.K.; Ramsay, L. Understanding the genetic control and physiological traits associated with rhizosheath production by barley (Hordeum vulgare). New Phytol. 2014, 203, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Griffiths, B.; Bengough, A. Sloughing of cap cells and carbon exudation from maize seedling roots in compacted sand. New Phytol. 2000, 145, 477–482. [Google Scholar] [CrossRef]
- Ellis, R. Seed and seedling vigor in relation to crop growth and yield. Plant Growth Regul. 1992, 11, 249–255. [Google Scholar] [CrossRef]
- Pederson, L.H.; Jorgensen, P.E.; Poulsen, I. Effect of seed vigour and dormancy on field emergence, development and grain yield of winter wheat (Triticum aestivum L.) and winter barley (Hordeum vulgare L.). Seed Sci. Tech. 1993, 21, 159–178. [Google Scholar]
- Khajeh-Hosseini, M.; Lomholt, A.; Matthews, A. Mean germination time in the laboratory estimates the relative vigour and field performance of commercial seed lots of maize (Zea mays L.). Seed Sci. Tech. 2009, 37, 446–456. [Google Scholar] [CrossRef]
- Mavi, K.; Demir, I.; Matthews, S. Mean germination time estimates relative emergence of seed lots of three cucurbit crops under stressful conditions. Seed Sci. Tech. 2010, 38, 14–25. [Google Scholar] [CrossRef]
- Vettakkorumakankav, N.; Falk, D.; Saxena, P.; Fletcher, R.A. Crucial role for gibberellins in stress protection of plants. Plant Cell Physiol. 1999, 40, 542–548. [Google Scholar] [CrossRef]
- Spielmeyer, W.; Ellis, M.H.; Chandler, P.M. Semidwarf (sd-1), "green revolution" rice, contains a defective gibberellin 20-oxidase gene. Proc. Nat. Acad. Sci. USA 2002, 99, 9043–9048. [Google Scholar] [CrossRef]
- Colebrook, E.; Thomas, S.; Phillips, A.; Hedden, P. The role of gibberellin signaling in plant responses to abiotic stress. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Curlango-Rivera, G.; Duclos, D.V.; Ebolo, J.J.; Hawes, M.C. Transient exposure of root tips to primary and secondary metabolites: Impact on root growth and production of border cells. Plant Soil 2010, 332, 267–275. [Google Scholar] [CrossRef]
- Kapulnik, Y.; Resnick, N.; Mayzlish-Gati, E.; Kaplan, Y.; Wininger, S.; Hershenhorn, J.; Koltai, H. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. J. Exp. Bot. 2011, 62, 2915–2924. [Google Scholar] [CrossRef]
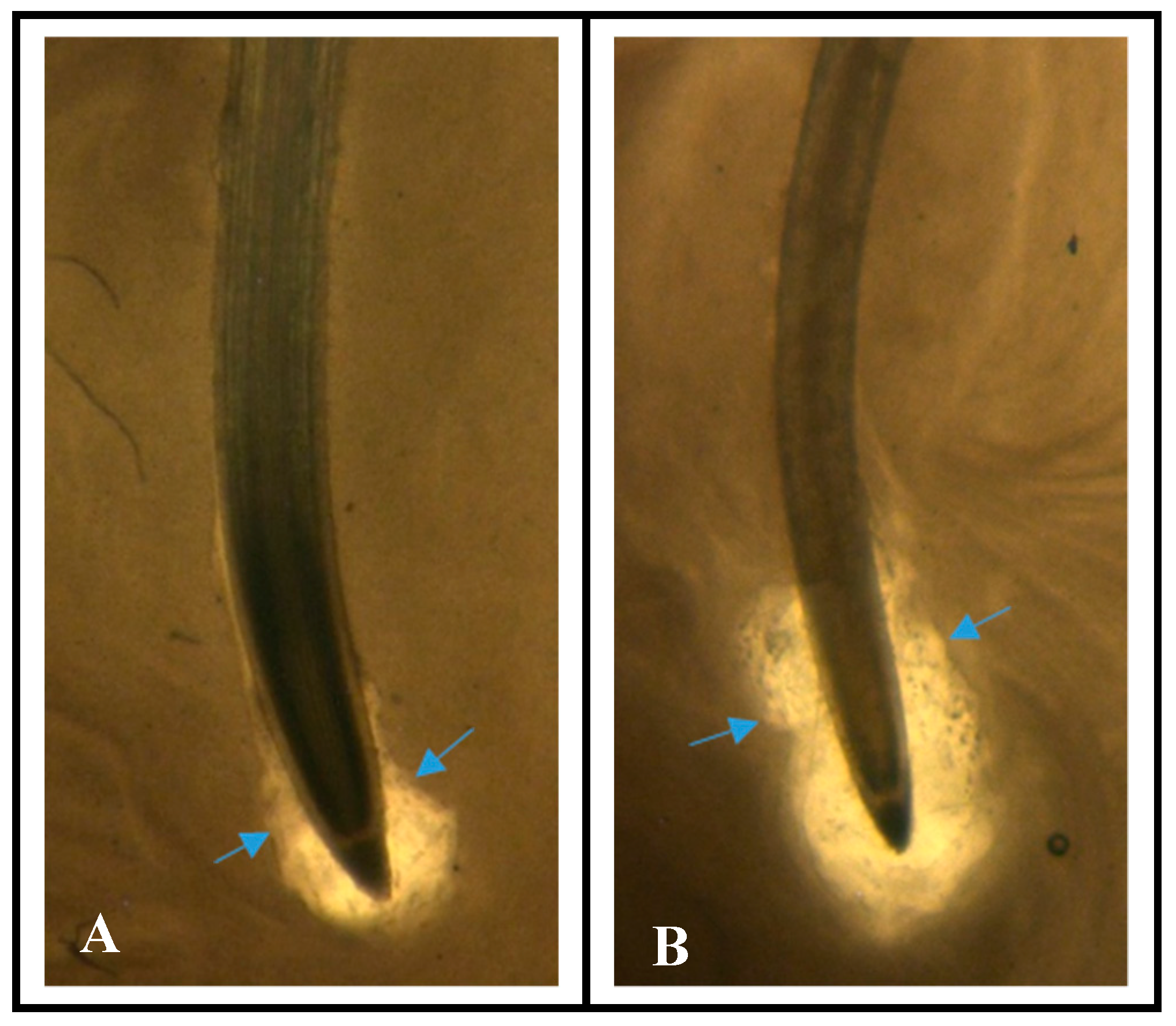
| Plant Variety | Root Tip Mucilage Area (mm2) |
|---|---|
| “Kopious” | 0.74 ± 0.28 a |
| “Cochise” | 0.80 ± 0.31 a |
| “Solar” z | 1.14 ± 0.46 b |
| “Solum” z | 0.92 ± 0.26 a |
| Plant Variety | Root Border Cell Number |
|---|---|
| “Kopious” y | 289 ± 28 a |
| “Cochise”y | 634 ± 183 b |
| “Solar”z | 472 ± 52 c |
| “Solum” z | 460 ± 95 c |
| Plant Variety | Number of Roots with Normal Root Hairs | Number of Roots with Extra Root Hairs | n | % Hairy Phenotype |
|---|---|---|---|---|
| “Kopious” y | 28 | 5 | 33 | 15 a |
| “Cochise” y | 44 | 8 | 52 | 15 a |
| “Solar” z | 18 | 19 | 37 | 51 b |
| “Solum” z | 48 | 6 | 54 | 11 a |
| Plant Variety | Root Tip + Elongation Zone (um) |
|---|---|
| “Kopious” | 2155.6 ± 199.3 a |
| “Cochise” | 2067.8 ± 317.8 a |
| “Solar” z | 1765.0 ± 228.4 b |
| “Solum” z | 1786.7 ± 314.9 b |
| Plant Variety | 24 h | 48 h | 72 h | 96 h | Total |
|---|---|---|---|---|---|
| “Solar” | 26/60 (43%) | 24/60 (40%) | 10/60 (17%) | 60/60 (100%) | |
| “Solum” | 27/60 (45%) | 19/60 (32%) | 13/60 (22%) | 59/60 (98%) | |
| “Cochise” | 0/60 | 16/60 (27%) | 22/60 (37%) | 19/60 (32%) | 57/60 (95%) |
| “Kopious” | 0/60 | 19/60 (32%) | 19/60 (32%) | 19/60 (32%) | 57/60 (95%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, A.Y.; Ottman, M.J.; Curlango-Rivera, G.; Huskey, D.A.; D’Agostini, B.A.; Hawes, M.C. Drought-Tolerant Barley: II. Root Tip characteristics in Emerging Roots. Agronomy 2019, 9, 220. https://doi.org/10.3390/agronomy9050220
Carter AY, Ottman MJ, Curlango-Rivera G, Huskey DA, D’Agostini BA, Hawes MC. Drought-Tolerant Barley: II. Root Tip characteristics in Emerging Roots. Agronomy. 2019; 9(5):220. https://doi.org/10.3390/agronomy9050220
Chicago/Turabian StyleCarter, Andrea Y., Michael J. Ottman, Gilberto Curlango-Rivera, David A. Huskey, Brooke A. D’Agostini, and Martha C. Hawes. 2019. "Drought-Tolerant Barley: II. Root Tip characteristics in Emerging Roots" Agronomy 9, no. 5: 220. https://doi.org/10.3390/agronomy9050220
APA StyleCarter, A. Y., Ottman, M. J., Curlango-Rivera, G., Huskey, D. A., D’Agostini, B. A., & Hawes, M. C. (2019). Drought-Tolerant Barley: II. Root Tip characteristics in Emerging Roots. Agronomy, 9(5), 220. https://doi.org/10.3390/agronomy9050220





