Biofertilizer Production for Agronomic Application and Evaluation of Its Symbiotic Effectiveness in Soybeans
Abstract
:1. Introduction
2. Materials and Methods
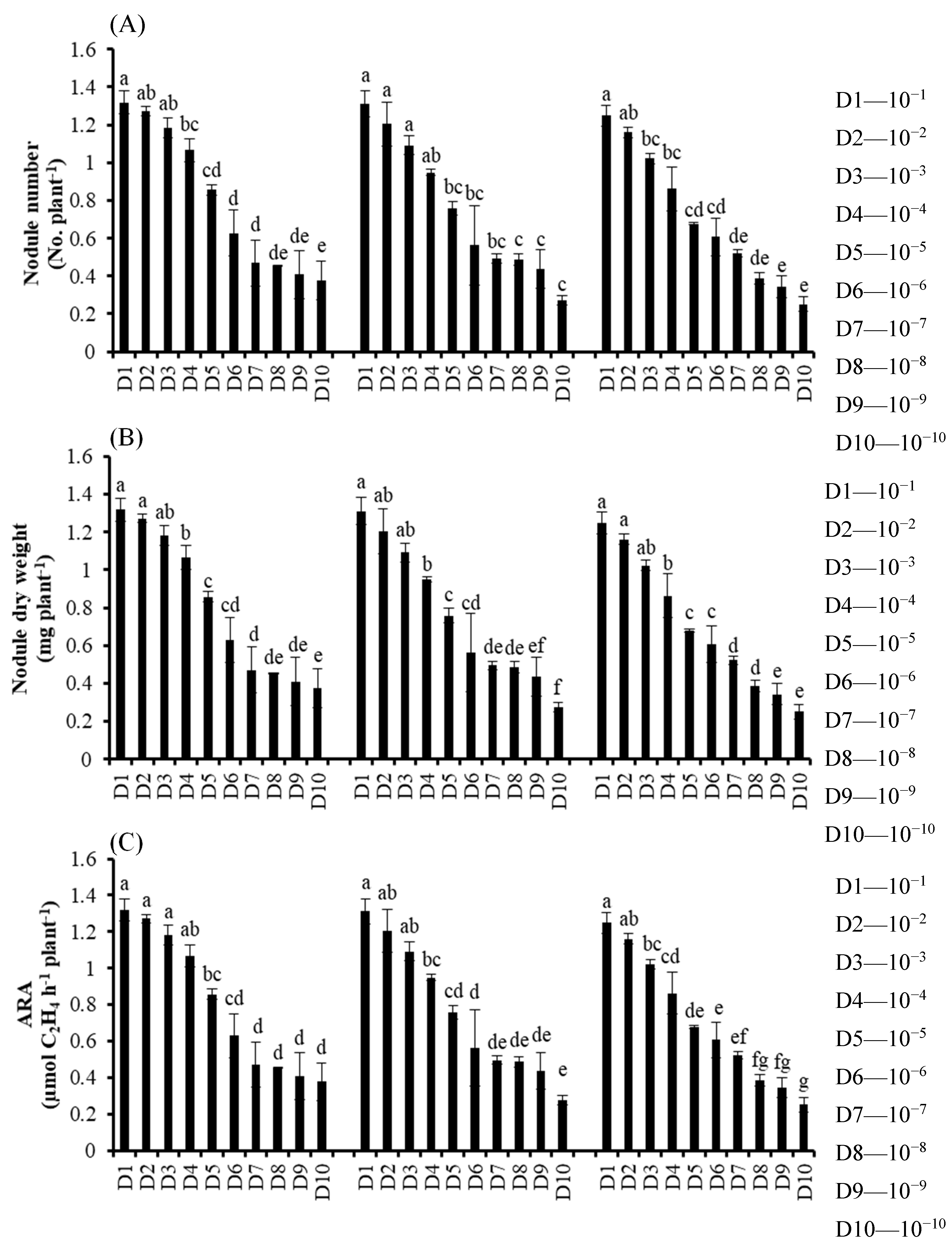
2.1. Expt. I. Effect of Single, Dual and Combined Inoculation of SAY3-7, BLY3-8 and P4 on Symbiotic Effectiveness of Different Soybean Varieties
2.2. Experiment II: Physicochemical Properties of Peat Soil and Biofertilizer Production Using the Peat Soil
2.3. Experiment III: Evaluation of Symbiotic Effectiveness of Biofertilizer on Yezin-3 (Rj4) and Yezin-6 (non-Rj) Soybean Varieties at Different Growth Stages
2.4. Statistical Analysis
3. Results
3.1. Experiment I. Effect of Single, Dual and Combined Inoculation of SAY3-7, BLY3-8 and P4 on Ssymbiotic Effectiveness of Different Soybean Varieties
3.2. Expteriment II. Physicochemical Properties of Peat Soil and Biofertilizer Production Using the Peat Soil
3.3. Experiment III. Evaluation of Ssymbiotic Effectiveness of Biofertilizer on Yezin-3 (Rj4) and Yezin-6 (non-Rj) Soybean Varieties at Different Growth Stages
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vessey, J.K. Plant growth promoting rhizobacteria as bio-fertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.A. Future of bio-fertilizers in Indian agriculture: An overview. Int. J. Agric. Food Res. 2015, 3, 10–23. [Google Scholar] [CrossRef]
- DAR. Rhizobium biofertilizer. In The Result of Research of Agricultural Research, Golden Jubilee; Department of Agricultural Research: Yezin, Myanmar, 2004; pp. 114–118. [Google Scholar]
- Schroth, M.N.; Hancock, J.G. Selected topics in biological control. Ann. Rev. Microbiol. 1981, 35, 453–476. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.M. Biological control of soil borne plant pathogens in the rhizosphere with bacteria. Ann. Rev. Phytopathol. 1988, 26, 379–407. [Google Scholar] [CrossRef]
- Bharathi, V.; Sudhakar, R.; Parimala, K.; Reddy, V.A. Evaluation of bioagents and biofertilizers for the management of seed and seedling diseases of Sesamum indicum (Sesame). eSci. J. Plant Pathol. 2013, 2, 179–186. [Google Scholar]
- Lehr, N.A.; Schrey, S.D.; Hampp, R.; Tarkka, M.T. Root inoculation with a forest soil. Streptomycete leads to locally and systemically increased resistance against phytopathogens in Norway Spruce. New Phytol. 2008, 177, 965–976. [Google Scholar] [PubMed]
- Rothrock, C.S.; Gottlieb, D. Role of antibiosis in antagonism of Streptomyces hygroscopicus var. Geldans to Rhizoctonia solani in Soil. Can. J. Microbiol. 1984, 30, 1440–1447. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clement, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Herridge, D.; Maw, J.B.; Thein, M.M.; Rupela, O.P.; Boonkerd, N.; Thao, T.Y.; Deaker, R.; Hartley, E.; Gemell, G. Expanding production and use of legume inoculants in Myanmar and Vietnam. In Proceedings of the 14th Australian Agronomy Conference, Adelaide, Australia, 21–25 September 2008. [Google Scholar]
- Than, M.M.; San, K.K.; Thein, M.M. Evaluation of effective rhizobial strains for commercial legume inoculants. J. Agric. For. Livest. Fish. Sci. 2006, 6, 264–280. [Google Scholar]
- Topre, S.D.; Panikar, S.S.; Mahajani, S.U. Biofertilizer: A novel approach for agriculture. J. Agric. Biotechnol. Sustain. Dev. 2011, 3, 205–208. [Google Scholar]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2107, 24, 3315–3335. [Google Scholar] [CrossRef]
- Than, H.; Aung, N.N.; Kyi, P.P. Response of rhizobial peat inoculants on five important legumes. In Proceedings of the 18th Congress Myanmar Agricultural Science Research Division, Yezin, Myanmar, 1987; pp. 1–12. [Google Scholar]
- Smith, D.L.; Hume, D.J. Comparison of assay methods for N2 fixation utilizing white bean and soybean. Can. J. Plant Sci. 1987, 67, 11–19. [Google Scholar] [CrossRef]
- Ishizuka, J.; Suemasu, Y.; Mizogami, K. Preference of Rj-soybean cultivars for Bradyrhizobium japonicum for nodulation. Soil Sci Plant Nutr. 1991, 37, 15–21. [Google Scholar] [CrossRef]
- Ishizuka, J.; Yokoyama, A.; Suemasu, Y. Relationship between serotypes of Bradyrhizobium japonicum and their compatibility with Rj-cultivars for nodulation. Soil Sci Plant Nutr. 1991, 37, 23–30. [Google Scholar] [CrossRef]
- Htwe, A.Z.; Saeki, Y.; Moe, K.; Yamakawa, T. Determining nodulation regulatory (Rj) genes Myanmar soybean cultivars and their symbiotic effectiveness with Bradyrhizobium japonicum USDA110. Am. J. Plant Sci. 2015, 6, 2799–2810. [Google Scholar] [CrossRef]
- Soe, K.M.; Yamakawa, T.; Hashimoto, S.; Sarr, P. Phylogenetic diversity of indigenous soybean bradyrhizobia from different agro-climatic regions in Myanmar. Sci. Asia 2013, 39, 574–583. [Google Scholar]
- Htwe, A.Z.; Moh, S.M.; Moe, K.; Yamakawa, T. Effects of co-inoculation of Bradyrhizobium japonicum SAY3-7 and Streptomyces griseoflavus P4 on plant growth, nodulation, nitrogen fixation, nutrient uptake, and yield of soybean in a field condition. Soil Sci. Plant Nutr. 2018, 64, 222–229. [Google Scholar] [CrossRef]
- Htwe, A.Z.; Moh, S.M.; Moe, K.; Yamakawa, T. Effects of co-inoculation of Bradyrhizobium elkanii BLY3-8 and Streptomyces griseoflavus P4 on Rj4 soybean varieties. Soil Sci. Plant Nutr. 2018, 64, 449–454. [Google Scholar] [CrossRef]
- Nakano, Y.; Yamakawa, T.; Ikeda, M.; Ishizuka, J. Nodulation of Rj-soybean varieties with Rhizobium fredii USDA193 under limited supply of nutrients. Soil Sci. Plant Nutr. 1997, 43, 929–932. [Google Scholar] [CrossRef]
- Htwe, A.Z.; Yamakawa, T.; Sarr, P.S.; Sakata, T. Diversity and distribution of soybean-nodulation bradyrhizobia isolated from major soybean-growing regions in Myanmar. Afr. J. Microbiol. Res. 2015, 9, 2183–2196. [Google Scholar]
- Kuykendall, L.D. Isolation and identification of genetically marked strains of nitrogen-fixing microsymbionts of soybeans. In Practical Symbiotic Nitrogen Fixation Methodology; Elkan, G.H., Ed.; Marcel Dekker: New York, NY, USA, 1987; pp. 205–220. [Google Scholar]
- Shimizu, M.; Nakagawa, Y.; Sato, Y.; Furumai, T.; Igarashi, Y.; Onaka, H.; Yoshida, R.; Kunoh, H. Studies on endophytic actinomycetes (I) Streptomyces sp. isolate from rhododendron and its antifungal activity. J. Gen. Plant Pathol. 2000, 66, 360–366. [Google Scholar] [CrossRef]
- Haider, J.; Hussam, A.K.M.A.; Ikeda, M.; Yamakawa, T.; Ishizuka, J. Effects of nitrate application on growth, nodulation and nitrogen fixation of nitrate-tolerant mutant soybean. Soil Sci. Plant Nutr. 1991, 37, 521–529. [Google Scholar] [CrossRef]
- Ohyama, T.; Ito, M.; Kobayashi, K.; Araki, S.; Yasuyoshi, S.; Sasaki, O.; Yamazaki, T.; Soyama, K.; Tanemura, R.; Mizuno, Y.; et al. Analytical procedures of N, P, K contents in plant and manure materials using H2SO4–H2O2 Kjeldahl digestion method. Jpn. Bull. Facul. Agric. Niigata Univ. 1991, 43, 110–120. [Google Scholar]
- Cataldo, D.A.; Schrader, L.E.; Youngs, V.L. Analysis by digestion and colrimetric assay of total nitrogen in plant tissues high in nitrate. Crop Sci. 1974, 14, 854–856. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Nitrogen availability indexes for submerged rice soils. Adv. Agron. 1983, 36, 415–451. [Google Scholar]
- Truog, E. The determination of the readily available phosphorus in soils. J. Am. Soc. Agron. 1930, 22, 874–882. [Google Scholar] [CrossRef]
- Muramoto, J.; Goto, I.; Ninaki, M. Rapid analysis of exchangeable cation and cation exchange capacity (CEC) of soils by shaking extraction method. Soil Sci Plant Nutr. 1992, 63, 210–215. [Google Scholar]
- Somasegaran, P.; Hoben, H.J. Methods in Legume-Rhizobium Technology. Available online: https://www.ctahr.hawaii.edu/bnf/Downloads/Training/Rhizobium%20technology/Title%20Page.PDF (accessed on 12 February 2019).
- Moore, S.; Stein, W.H. A Modified ninhydrin reagent for the photometric determination of amino acids and realted compounds. J. Biol. Chem. 1954, 211, 907–913. [Google Scholar]
- Cataldo, D.A.; Haroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Young, E.G.; Conway, C.F. On the estimation of Allantoin by the Rimini-Schryver reaction. J. Biol. Chem. 1942, 142, 839–853. [Google Scholar]
- Herridge, D.F.; Peoples, M.B. Ureide assay for measuring nitrogen fixation by nodulated soybean calibrated by 15N methods. Plant Physiol. 1990, 93, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Sessitsch, A.; Howieson, J.G.; Perret, X.; Antoun, H.; Martínez-Romero, E. Advances in rhizobium research. Crit. Rev. Plant Sci. 2002, 21, 323–378. [Google Scholar] [CrossRef]
- Doolotkeldieva, T.; Bobusheva, S.; Konurbaeva, M. Effects of Streptomyces biofertilizer to soil fertility and rhizosphere’s functional biodiversity of agricultural plants. Adv. Microbiol. 2015, 5, 555–571. [Google Scholar] [CrossRef]
- Santiago, C.D.; Yagi, S.; Ijima, M.; Nashimoto, T.; Sawada, M.; Ikeda, S.; Asano, K.; Orikasa, Y.; Ohwada, T. Bacterial compatibility in combined inoculations enhances the growth of potato seedlings. Microbes Environ. 2017, 32, 14–23. [Google Scholar] [CrossRef]
- Tang-um, J.; Niamsup, H. Chitinase production and antifungal potential of endophytic Streptomyces strain P4. Maejo Int. J. Sci. Technol. 2012, 6, 95–104. [Google Scholar]
- Htwe, A.Z.; Yamakawa, T. Enhanced plant growth and/or nitrogen fixation by leguminous and non-leguminous crops after single or dual inoculation of Streptomyces griseoflavus P4 with Bradyhizobium strains. Afr. J. Microbiol. Res. 2015, 9, 2337–2344. [Google Scholar]
- Thein, M.M.; Hein, M. Rhizobial inoculants production and their on-farm use in Myanmar. Extending nitrogen fixation research to farmer’s fields. In Proceedings of the International Workshop on Managing Legume Nitrogen Fixation in The Cropping Suystems of Asia, Hyderabad, India, 20–24 August 1996. [Google Scholar]
- Jitacksorn, S.; Sadowsky, M.J. Nodulation gene regulation and Quorum sensing control density- dependent suppression and restriction of nodulation in the Bradyrhizobium japonicum-soybean symbiosis. Appl. Environ. Microbiol. 2008, 74, 3749–3756. [Google Scholar] [CrossRef]
- Yamakawa, T.; Fukushima, Y. Low inoculum densities of Bradyrhizobium japonicum USDA110 is effective on production of soybean (Glycine max L. Merr.) cultivar Fukuyutaka.). J. Faculty Agric. Kyushu Univ. 2014, 59, 45–53. [Google Scholar]
- Albareda, M.; Rodrı’guez-Navarro, D.N.; Temprano, F.J. Soybean inoculation: Dose, N fertilizer supplementation and rhizobia persistence in soil. Field Crops Res. 2009, 113, 352–356. [Google Scholar] [CrossRef]
- Soe, K.M.; Yamakawa, T. Evaluating the effects of Streptomyces griseoflavus P4 on dry weight of different crops and examine phytohormones activity in term of indole acdic acid (IAA) production. Myanmar Agric. Res. J. 2018, 4, 165–17145. [Google Scholar]
- Fukaki, H.; Okushima, Y.; Tasaka, M. Auxin-mediated lateral root formation in higher plants. Int. Rev. Cytol. 2007, 256, 111–137. [Google Scholar]
- Soe, K.M.; Myint, S.S.; Win, M.M.; Aung, T.T.; San, K.K.; Myint, S.S. Co-inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 inoculants to improve plant Growth, seed Yield of soybean cultivars and soil fertility improvement. Myanmar Agric. Res. J. 2018, 4, 154–164. [Google Scholar]
- Ntambo, M.S.; Chilinda, I.S.; Taruvinga, A.; Hafeez, S.; Anwar, T.; Sharif, R.; Chambi, C.; Kies, L. The effect of rhizobium inoculation with nitrogen fertilizer on growth and yield of soybeans (Glycine max L.). Int. J. Biosci. 2017, 10, 163–172. [Google Scholar]
- Ulzen, J.; Abaidoo, R.C.; Mensah, N.E.; Masso, C.; AbdelGadir, A.H. Bradyrhizobium inoculants enhance grain Yields of soybean and cowpea in Northern Ghana. Front. Plant Sci. 2016, 7, 1770. [Google Scholar] [CrossRef]
- Kozieł, M.; Gebala, B.; Martyniuk, S. Response of soybean to seed inoculation with Bradyrhizobium japonicum and with mixed inoculants of B. japonicum and Azotobacter chroococcum. Pol. J. Microbiol. 2013, 62, 457–460. [Google Scholar] [PubMed]
- Soe, K.M.; Yamakawa, T. Evaluation of effective Myanmar Bradyrhizobium strains isolated from Myanmar soybean and effects of coinoculation with Streptomyces griseoflavus P4 for nitrogen fixation. Soil Sci. Plant Nutr. 2013, 59, 361–370. [Google Scholar] [CrossRef]
- Soe, K.M.; Bhromsiri, A.; Karladee, D.; Yamakawa, T. Effects of endophytic actinomycetes and Bradyrhizobium japonicum strains on growth, nodulation, nitrogen fixation and seed weight of different soybean varieties. Soil Sci. Plant Nutr. 2012, 58, 319–325. [Google Scholar] [CrossRef]
- Klubeck, B.P.; Hendrickson, L.L.; Zablotowicz, R.M.; Skwara, J.E.; Varsa, E.C.; Smith, S.; Isleib, T.G.; Maya, J.; Valdes, M.; Dazzo, F.B.; et al. Competitiveness of selected Bradyrhizobium japonicum strains in Midwestern USA soils. Soil Sci. Soc. Am. J. 1988, 52, 662–666. [Google Scholar] [CrossRef]
- Soe, K.M.; Bhromsiri, A.; Karladee, D. Effect of selected endophytic actinomycetes (Steptomyces sp.) and bradyrhizobia from Myanmar on growth, nodulation, nitrogen fixation and yield of different soybean varieties. CMU J. Nat. Sci. 2010, 9, 95–109. [Google Scholar]
- Soe, K.M.; Yamakawa, T. Low-density co-inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to enhance symbiosis and seed yield in soybean varieties. Am. J. Plant Sci. 2013, 4, 1879–1892. [Google Scholar] [CrossRef]
- Mishra, P.K.; Bisht, S.C.; Mishra, S.; Selvakumar, G.; Bisht, J.K.; Gupta, H.S. Coinoculation of Rhizobium Leguminosarum-PR1 with a cold tolerant Pseudomonas sp. improves iron acquisition, nutrient uptake and growth of filed pea (Pisum Sativum L.). J. Plant Nutr. 2012, 35, 243–256. [Google Scholar] [CrossRef]
- Somasegaran, P.; Hoben, H.J. Handbook for Rhizobia: Methods in Legumes-Rhizobium Technology; Springer: New York, NY, USA, 1994; pp. 1–450. [Google Scholar]
- Senaratne, R.; Amornpinol, C.; Hardarson, G. Effect of combined nitrogen on nitrogen fixation of soybean (Glycine max L. Merril) as affected by cultivar and rhizobial strain. Plant Siol 1987, 103, 45–50. [Google Scholar] [CrossRef]
- Htwe, A.Z.; Yamakawa, T.; Moe, K.; Dien, D.C. Symbiotic effectiveness of different indigenous Bradyrhizobium strains on selected Rj-genes harboring Myanmar soybean cultivars. Afr. J. Microbiol. Res. 2015, 9, 2345–2353. [Google Scholar]
| Variety | Treatment | NN (No. plant−1) | NDW (mg plant−1) | RDW (g plant−1) | SDW (g plant−1) | ARA (µmol C2H4 h−1 plant−1) |
|---|---|---|---|---|---|---|
| Yezin-3 | Control | 0.0 ± 0.0 b | 0.00 ± 0.00 c | 0.33 ± 0.04 a | 0.61 ± 0.03 b | 0.00 ± 0.00 c |
| P4 | 0.0 ± 0.0 b | 0.00 ± 0.00 c | 0.33 ± 0.01 a | 0.64 ± 0.02 b | 0.00 ± 0.00 c | |
| SAY3-7 | 11.5 ± 3.2 a | 54.43 ± 4.42 a | 0.30 ± 0.06 a | 0.82 ± 0.03 a | 1.04 ± 0.09 b | |
| BLY3-8 | 14.0 ± 6.1 a | 33.50 ± 0.64 b | 0.28 ± 0.02 a | 0.85 ± 0.05 a | 0.90 ± 0.09 b | |
| SAY3-7 + BLY3-8 | 10.3 ± 2.6 a | 50.10 ± 4.49 a | 0.31 ± 0.01 a | 0.84 ± 0.02 a | 1.32 ± 0.09 a | |
| SAY3-7 + P4 | 11.8 ± 4.4 a | 51.00 ± 5.51 a | 0.33 ± 0.06 a | 0.92 ± 0.10 a | 1.09 ± 0.04 b | |
| BLY3-8 + P4 | 13.8 ± 3.5 a | 35.47 ± 3.59 b | 0.32 ± 0.05 a | 0.83 ± 0.04 a | 0.98 ± 0.04 b | |
| SAY3-7 + BLY3-8 + P4 | 13.8 ± 2.3 a | 50.37 ± 1.77 a | 0.26 ± 0.02 a | 0.82 ± 0.00 a | 1.34 ± 0.06 a | |
| Yezin-6 | Control | 0.0 ± 0.0 b | 0.00 ± 0.00 d | 0.41 ± 0.04 a | 0.69 ± 0.06 b | 0.00 ± 0.00 c |
| P4 | 0.0 ± 0.0 b | 0.00 ± 0.00 d | 0.43 ± 0.05 a | 0.84 ± 0.04 ab | 0.00 ± 0.00 c | |
| SAY3-7 | 16.0 ± 2.9 a | 58.70 ± 2.87 b | 0.47 ± 0.04 a | 1.04 ± 0.04 a | 1.61 ± 0.02 b | |
| BLY3-8 | 15.0 ± 1.6 a | 47.03 ± 0.71 c | 0.39 ± 0.06 a | 1.04 ± 0.11 a | 1.47 ± 0.15 b | |
| SAY3-7 + BLY3-8 | 11.7 ± 1.7 a | 65.70 ± 5.19 ab | 0.45 ± 0.05 a | 0.98 ± 0.01 a | 2.22 ± 0.05 a | |
| SAY3-7 + P4 | 15.7 ± 5.2 a | 57.60 ± 3.05 b | 0.37 ± 0.02 a | 0.94 ± 0.03 a | 1.73 ± 0.23 b | |
| BLY3-8 + P4 | 14.3 ± 3.7 a | 46.20 ± 3.16 c | 0.46 ± 0.11 a | 1.05 ± 0.13 a | 1.51 ± 0.06 b | |
| SAY3-7 + BLY3-8 + P4 | 18.3 ± 3.4 a | 71.87 ± 3.03 a | 0.47 ± 0.04 a | 0.94 ± 0.01 a | 2.25 ± 0.10 a |
| Physicochemical Properties | Values |
|---|---|
| Soil pH (Soil: H2O; 1:2.5) | 7.11 |
| Total N (%) | 0.80 |
| Total P2O5 (%) | 0.09 |
| Available N (mg N/ 100 g soil) | 150.26 |
| Available P (mg P2O5/ 100 g soil) | 4.25 |
| CEC (cmolc Kg−1) | 20.47 |
| Exc. Ca (cmolc Kg−1) | 25.61 |
| Exc. Mg (cmolc Kg−1) | 1.08 |
| Months after Producing | P4 | Bradyrhizobium |
|---|---|---|
| Plate Count (cfu g−1) | MPN (Cells g−1) | |
| 1 | 1.7 × 1010 | 7 × 109 |
| 2 | 2 × 1010 | 7 × 109 |
| 3 | 3.2 × 1011 | 7 × 109 |
| 4 | 2.8 × 1011 | 7 × 109 |
| 5 | 4 × 1010 | 7 × 109 |
| 6 | 2 × 1010 | 7 × 109 |
| 7 | 1.2 × 109 | 7 × 109 |
| 8 | 4 × 108 | 7 × 109 |
| 9 | 4 × 108 | 7 × 109 |
| 10 | 3 × 108 | 7 × 109 |
| 11 | 1.8 × 108 | 7 × 109 |
| 12 | 1 × 108 | 7 × 109 |
| Treatment | NN (No. plant−1) | NDW (mg plant−1) | RDW (g plant−1) | SDW (g plant−1) | ARA (µmol C2H4 h−1 plant−1) |
|---|---|---|---|---|---|
| Variety | |||||
| Yezin-3 | 10.3 ± 1.7 A | 26.98 ± 4.33 A | 0.46 ± 0.07 A | 1.81 ± 0.11 A | 0.62 ± 0.12 A |
| Yezin-6 | 12.2 ± 2.1 A | 27.57 ± 5.54 A | 0.47 ± 0.06 A | 1.61 ± 0.11 B | 0.60 ± 0.15 A |
| Treatment of Yezin-3 | |||||
| Control | 11.7 ± 0.5 a | 22.97 ± 2.19 b | 0.40 ± 0.01 b | 1.72 ± 0.04 b | 0.50 ± 0.07 b |
| Biofertilizer | 12.7 ± 1.9 a | 31.00 ± 0.73 a | 0.51 ± 0.04 a | 1.91 ± 0.05 a | 0.73 ± 0.01 a |
| Treatment of Yezin-6 | |||||
| Control | 9.3 ± 2.4 a | 22.40 ± 0.96 b | 0.41 ± 0.01 b | 1.51 ± 0.05 c | 0.46 ± 0.04 b |
| Biofertilizer | 11.3 ± 1.7 a | 32.73 ± 2.67 a | 0.52 ± 0.03 a | 1.71 ± 0.03 b | 0.73 ± 0.07 a |
| Source of variance (Pr > F) | |||||
| Variety | ns | ns | ns | ** | ns |
| Treatment | ns | ** | ** | ** | ** |
| Variety × Treatment | ns | ns | ns | ns | ns |
| CV% | 8.64 | 9.34 | 4.67 | 2.79 | 8.64 |
| Treatment | NN (No. plant−1) | NDW (mg plant−1) | RDW (g plant−1) | SDW (mg plant−1) | RUI (%) | % Ndfa |
|---|---|---|---|---|---|---|
| Variety | ||||||
| Yezin-3 | 58.0 ± 5.7 B | 370.00 ± 76.16 A | 1.50 ± 0.26 B | 7.39 ± 0.35 A | 80.64 ± 7.10 B | 88.56 ± 10.60 B |
| Yezin-6 | 83.2 ± 11.6 A | 408.33 ± 41.40 A | 1.96 ± 0.12 A | 7.51 ± 0.31 A | 86.42 ± 1.75 A | 97.20 ± 2.62 A |
| Treatment of Yezin-3 | ||||||
| Control | 52.7 ± 1.7 c | 313.33 ± 49.22 b | 1.31 ± 0.05 b | 7.05 ± 0.04 b | 73.57 ± 0.90 c | 78.01 ± 1.34 c |
| Biofertilizer | 63.3 ± 2.4 bc | 426.67 ± 52.49 ab | 1.69 ± 0.24 ab | 7.72 ± 0.10 a | 87.71 ± 0.22 a | 99.12 ± 0.33 a |
| Treatment of Yezin-6 | ||||||
| Control | 72.7 ± 5.3 b | 376.67 ± 4.71 ab | 1.85 ± 0.04 a | 7.22 ± 0.14 b | 84.81 ± 0.16 b | 94.80 ± 0.23 b |
| Biofertilizer | 93.7 ± 4.5 a | 440.00 ± 37.42 a | 2.07 ± 0.06 a | 7.80 ± 0.03 a | 88.03 ± 0.98 a | 99.60 ± 1.47 a |
| Source of Variance (Pr > F) | ||||||
| Variety | ** | ns | ** | ns | ** | ** |
| Treatment | ** | ** | * | ** | ** | ** |
| Variety × Treatment | ns | ns | ns | ns | ** | ** |
| CV% | 7.26 | 10.32 | 9.79 | 1.38 | 1.06 | 1.42 |
| Treatment | N (mg plant−1) | P (mg plant−1) | K (mg plant−1) | Ca (mg plant−1) | Mg (mg plant−1) |
|---|---|---|---|---|---|
| Varieties | |||||
| Yezin-3 | 33.88 ± 7.16 A | 1.13 ± 0.07 A | 0.38 ± 0.02 A | 0.37 ± 0.06 A | 0.18 ± 0.02 A |
| Yezin-6 | 38.10 ± 5.82 A | 1.12 ± 0.11 A | 0.39 ± 0.04 A | 0.37 ± 0.04 A | 0.16 ± 0.01 A |
| Treatment of Yezin-3 | |||||
| Control | 27.27 ± 2.92 b | 1.15 ± 0.04 a | 0.36 ± 0.03 ab | 0.33 ± 0.05 a | 0.18 ± 0.02 a |
| Biofertilizer | 40.50 ± 2.51 ab | 1.11 ± 0.09 a | 0.41 ± 0.02 ab | 0.41 ± 0.04 a | 0.19 ± 0.01 a |
| Treatment of Yezin-6 | |||||
| Control | 32.82 ± 2.41 ab | 1.06 ± 0.08 a | 0.35 ± 0.01 b | 0.38 ± 0.03 a | 0.16 ± 0.01 a |
| Biofertilizer | 43.37 ± 2.50 a | 1.17 ± 0.11 a | 0.43 ± 0.01 a | 0.36 ± 0.04 a | 0.16 ± 0.01 a |
| Source of Variance (Pr > F) | |||||
| Variety | ns | ns | ns | ns | ns |
| Treatment | ** | ns | ** | ns | ns |
| Variety × Treatment | ns | ns | ns | ns | ns |
| CV% | 13.2 | 9.05 | 5.7 | 15.7 | 8.64 |
| Treatment | N (mg plant−1) | P (mg plant−1) | K (mg plant−1) | Ca (mg plant−1) | Mg (mg plant−1) |
|---|---|---|---|---|---|
| Varieties | |||||
| Yezin-3 | 158.59 ± 6.63 A | 26.31 ± 0.68 A | 2.09 ± 0.23 B | 0.95 ± 0.11 B | 1.20 ± 0.07 B |
| Yezin-6 | 142.16 ± 9.69 B | 27.40 ± 0.99 A | 2.72 ± 0.10 A | 1.31 ± 0.15 A | 1.38 ± 0.06 A |
| Treatment of Yezin-3 | |||||
| Control | 153.15 ± 0.87 ab | 23.03 ± 0.46 b | 1.76 ± 0.01 b | 0.72 ± 0.02 b | 1.05 ± 0.06 c |
| Biofertilizer | 164.02 ± 5.29 a | 29.60 ± 0.78 a | 2.42 ± 0.13 ab | 1.19 ± 0.09 ab | 1.35 ± 0.08 ab |
| Treatment of Yezin-6 | |||||
| Control | 141.11 ± 8.58 b | 23.70 ± 1.28 b | 2.38 ± 0.06 ab | 1.07 ± 0.18 ab | 1.22 ± 0.07 bc |
| Biofertilizer | 143.21 ± 10.58 b | 31.09 ± 0.34 a | 3.06 ± 0.05 a | 1.55 ± 0.04 a | 1.54 ± 0.01 a |
| Source of Variance (Pr > F) | |||||
| Variety | ** | ns | ** | * | ** |
| Treatment | ** | ** | ** | ** | ** |
| Variety × Treatment | ns | ns | ns | ns | ns |
| CV% | 5.89 | 5.61 | 10.18 | 15.05 | 6.67 |
| Treatment | N (mg plant−1) | P (mg plant−1) | K (mg plant−1) | Ca (mg plant−1) | Mg (mg plant−1) |
|---|---|---|---|---|---|
| Varieties | |||||
| Yezin-3 | 192.24 ± 29.23 B | 9.87 ± 3.43 A | 1.49 ± 0.36 B | 1.29 ± 0.25 A | 0.89 ± 0.16 A |
| Yezin-6 | 238.10 ± 28.44 A | 9.87 ± 3.92 A | 1.73 ± 0.41 A | 1.41 ± 0.30 A | 0.82 ± 0.18 A |
| Treatment of Yezin-3 | |||||
| Control | 163.53 ± 3.14 c | 9.66 ± 1.34 a | 1.28 ± 0.12 b | 1.21 ± 0.11 a | 0.87 ± 0.07 a |
| Biofertilizer | 220.94 ± 7.16 b | 10.08 ± 0.44 a | 1.70 ± 0.14 a | 1.38 ± 0.03 a | 0.90 ± 0.2 a |
| Treatment of Yezin-6 | |||||
| Control | 212.24 ± 11.44 b | 9.54 ± 1.78 a | 1.64 ± 0.29 a | 1.34 ± 0.15 a | 0.78 ± 0.11 a |
| Biofertilizer | 263.97 ± 12.23 a | 10.20 ± 0.60 a | 1.81 ± 0.14 a | 1.47 ± 0.22 a | 0.86 ± 0.08 a |
| Source of Variance (Pr > F) | |||||
| Variety | ** | ns | ** | ns | ns |
| Treatment | ** | ns | ** | ns | ns |
| Variety × Treatment | ns | ns | ns | ns | ns |
| CV% | 4.26 | 8.21 | 5.84 | 10.03 | 8.34 |
| Treatment | Pods (No. plant−1) | Seed Per Pod (No. pod−1) | 100 Seed Weight (g) | Seed Yield (g plant−1) |
|---|---|---|---|---|
| Variety | ||||
| Yezin-3 | 20.8 ± 3.0 A | 1.8 ± 0.1 A | 13.48 ± 0.95 A | 5.07 ± 0.41 B |
| Yezin-6 | 20.3 ± 2.4 A | 1.9 ± 0.1 A | 14.51 ± 0.89 A | 5.84 ± 0.59 A |
| Treatment of Yezin-3 | ||||
| Control | 19.3 ± 1.7 a | 1.8 ± 0.1 a | 13.75 ± 0.71 a | 4.66 ± 0.06 b |
| Biofertilizer | 22.3 ± 3.3 a | 1.9 ± 0.1 a | 13.22 ± 1.07 a | 5.31 ± 0.11 b |
| Treatment of Yezin-6 | ||||
| Control | 18.7 ± 1.9 a | 2.0 ± 0.1 a | 14.31 ± 0.93 a | 5.47 ± 0.25 b |
| Biofertilizer | 22.0 ± 1.4 a | 2.0 ± 0.1 a | 14.71 ± 0.80 a | 6.36 ± 0.30 a |
| Source of Variance (Pr > F) | ||||
| Variety | ns | ns | ns | ** |
| Treatment | ns | ns | ns | ** |
| Variety × Treatment | ns | ns | ns | ns |
| CV% | 13.57 | 7.95 | 7.86 | 5.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Htwe, A.Z.; Moh, S.M.; Moe, K.; Yamakawa, T. Biofertilizer Production for Agronomic Application and Evaluation of Its Symbiotic Effectiveness in Soybeans. Agronomy 2019, 9, 162. https://doi.org/10.3390/agronomy9040162
Htwe AZ, Moh SM, Moe K, Yamakawa T. Biofertilizer Production for Agronomic Application and Evaluation of Its Symbiotic Effectiveness in Soybeans. Agronomy. 2019; 9(4):162. https://doi.org/10.3390/agronomy9040162
Chicago/Turabian StyleHtwe, Aung Zaw, Seinn Moh Moh, Kyi Moe, and Takeo Yamakawa. 2019. "Biofertilizer Production for Agronomic Application and Evaluation of Its Symbiotic Effectiveness in Soybeans" Agronomy 9, no. 4: 162. https://doi.org/10.3390/agronomy9040162
APA StyleHtwe, A. Z., Moh, S. M., Moe, K., & Yamakawa, T. (2019). Biofertilizer Production for Agronomic Application and Evaluation of Its Symbiotic Effectiveness in Soybeans. Agronomy, 9(4), 162. https://doi.org/10.3390/agronomy9040162





