Harnessing Soil Microbes to Improve Plant Phosphate Efficiency in Cropping Systems
Abstract
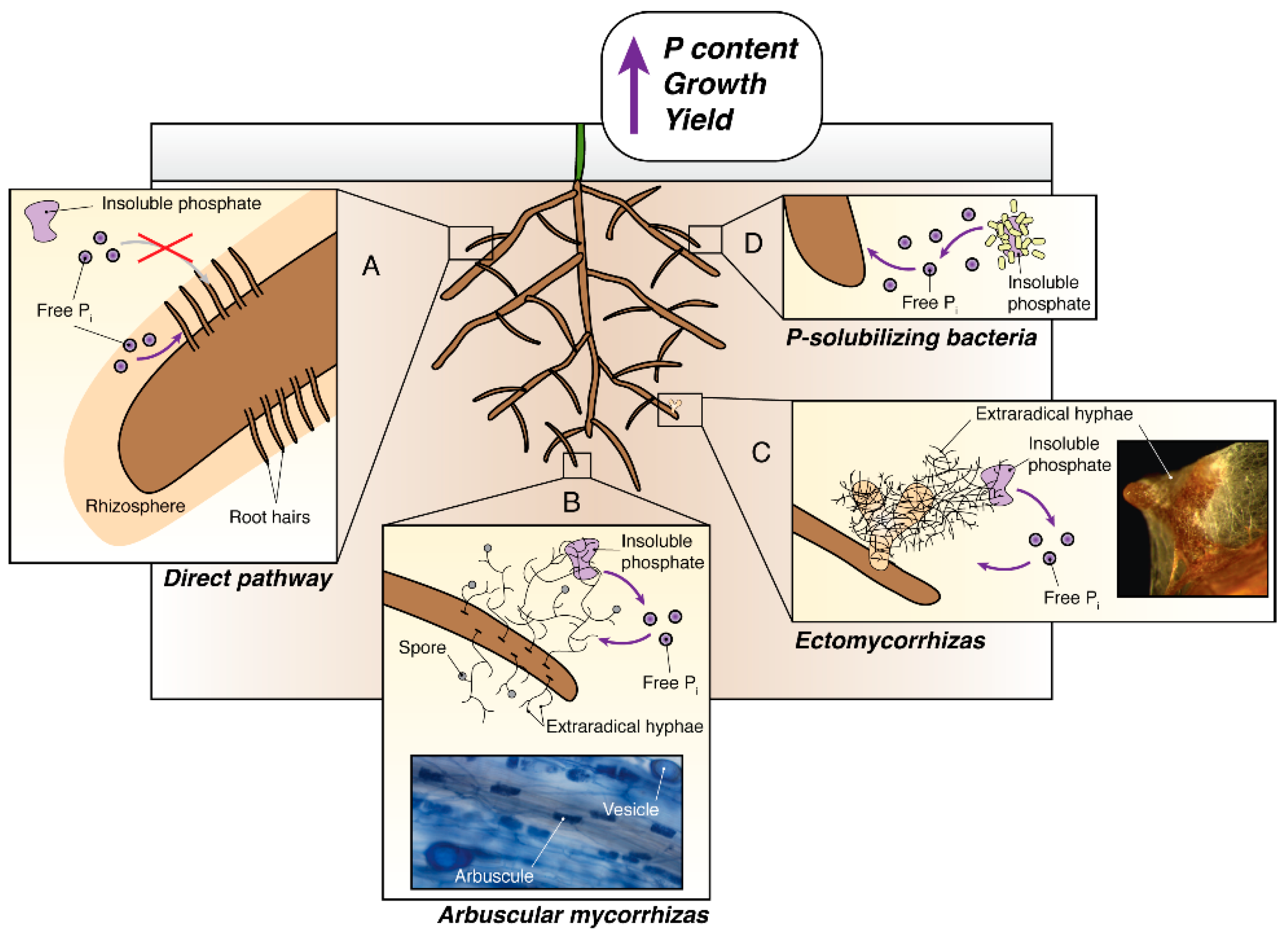
1. Introduction
2. The Use of Arbuscular Mycorrhizal Symbiosis in Agriculture to Improve Phosphate Uptake
3. The Use of Ectomycorrhizal Fungi to Improve Phosphate Uptake for Lignocellulosic Biofuel Crops
4. Phosphate Solubilizing Bacteria and Their Potential to Increase the Phosphate Acquisition of Crops
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Kruse, J.; Abraham, M.; Amelung, W.; Baum, C.; Bol, R.; Kühn, O.; Lewandowski, H.; Niederberger, J.; Oelmann, Y.; Rüger, C.; et al. Innovative methods in soil phosphorus research: A review. J. Plant Nutr. Soil Sci. 2015, 178, 43–88. [Google Scholar] [CrossRef] [PubMed]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Matar, A.; Torrent, J.; Ryan, J. Soil and fertilizer phosphorus and crop responses in the dryland Mediterranean zone. In Advances in Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1992; pp. 81–146. ISBN 978-1-4612-2844-8. [Google Scholar]
- Dalai, R.C. Soil organic phosphorus. Adv. Agron. 1977, 29, 83–117. [Google Scholar]
- Turner, B.L.; Papházy, M.J.; Haygarth, P.M.; McKelvie, I.D. Inositol phosphates in the environment. Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.W.B.; Tiessen, H. Dynamics of soil organic phosphorus. Biogeochemistry 1987, 4, 41–60. [Google Scholar] [CrossRef]
- Cade-Menun, B.J.; Carter, M.R.; James, D.C.; Liu, C.W. Phosphorus forms and chemistry in the soil profile under long-term conservation tillage: A phosphorus-31 nuclear magnetic resonance study. J. Environ. Qual. 2010, 39, 1647–1656. [Google Scholar] [CrossRef]
- Ha, S.; Tran, L.-S. Understanding plant responses to phosphorus starvation for improvement of plant tolerance to phosphorus deficiency by biotechnological approaches. Crit. Rev. Biotechnol. 2014, 34, 16–30. [Google Scholar] [CrossRef]
- Laliberté, E.; Turner, B.L.; Costes, T.; Pearse, S.J.; Wyrwoll, K.-H.; Zemunik, G.; Lambers, H. Experimental assessment of nutrient limitation along a 2-million-year dune chronosequence in the south-western Australia biodiversity hotspot. J. Ecol. 2012, 100, 631–642. [Google Scholar] [CrossRef]
- Kouas, S.; Labidi, N.; Debez, A.; Abdelly, C. Effect of P on nodule formation and N fixation in bean. Agron. Sustain. Dev. 2005, 25, 389–393. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Bieleski, R.L. Phosphate pools, phosphate transport, and phosphate availability. Annu. Rev. Plant Physiol. 1973, 24, 225–252. [Google Scholar] [CrossRef]
- Jain, A.; Poling, M.D.; Karthikeyan, A.S.; Blakeslee, J.J.; Peer, W.A.; Titapiwatanakun, B.; Murphy, A.S.; Raghothama, K.G. Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiol. 2007, 144, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Chacón-López, A.; Cruz-Ramírez, A.; Nieto-Jacobo, F.; Sánchez-Calderón, L.; Herrera-Estrella, L.; López-Bucio, J.; Dubrovsky, J.G. Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant Cell Physiol. 2005, 46, 174–184. [Google Scholar]
- Carvalho, F.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Naresh Kumar, G.; Parekh, L.J.; Poole, P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- King, K.W.; Williams, M.R.; Macrae, M.L.; Fausey, N.R.; Frankenberger, J.; Smith, D.R.; Kleinman, P.J.A.; Brown, L.C. Phosphorus transport in agricultural subsurface drainage: A review. J. Environ. Qual. 2015, 44, 467–485. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Dhillon, J.; Torres, G.; Driver, E.; Figueiredo, B.; Raun, W.R. World phosphorus use efficiency in cereal crops. Agron. J. 2017, 109, 1670–1677. [Google Scholar] [CrossRef]
- Rutherford, P.M.; Dudas, M.J.; Arocena, J.M. Trace elements and fluoride in phosphogypsum leachates. Environ. Technol. 1995, 16, 343–354. [Google Scholar] [CrossRef]
- Garcia, K.; Delaux, P.-M.; Cope, K.R.; Ané, J.-M. Molecular signals required for the establishment and maintenance of ectomycorrhizal symbioses. New Phytol. 2015, 208, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Duff, S.M.G.; Sarath, G.; Plaxton, W.C. The role of acid phosphatases in plant phosphorus metabolism. Physiol. Plant. 1994, 90, 791–800. [Google Scholar] [CrossRef]
- Jones, D.L. Organic acids in the rhizosphere—A critical review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Giehl, R.F.H.; von Wiren, N. Root nutrient foraging. Plant Physiol. 2014, 166, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A.; Lambers, H.; Smith, S.E.; Westoby, M. Costs of acquiring phosphorus by vascular land plants: Patterns and implications for plant coexistence. New Phytol. 2018, 217, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.; Fusseder, A.; Beck, E. Development and replenishment of the P-depletion zone around the primary root of maize during the vegetation period. Plant Soil 1987, 101, 247–255. [Google Scholar] [CrossRef]
- Smith, S.E.; Anderson, I.C.; Smith, F.A. Mycorrhizal associations and phosphorus acquisition: From cells to ecosystems. Annu. Plant Rev. 2015, 48, 409–439. [Google Scholar]
- Bates, T.R.; Lynch, J.P. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ. 1996, 19, 529–538. [Google Scholar] [CrossRef]
- Rausch, C.; Bucher, M. Molecular mechanisms of phosphate transport in plants. Planta 2002, 216, 23–37. [Google Scholar] [CrossRef]
- Sawers, R.J.H.; Svane, S.F.; Quan, C.; Grønlund, M.; Wozniak, B.; Gebreselassie, M.-N.; González-Muñoz, E.; Chávez Montes, R.A.; Baxter, I.; Goudet, J.; et al. Phosphorus acquisition efficiency in arbuscular mycorrhizal maize is correlated with the abundance of root-external hyphae and the accumulation of transcripts encoding PHT1 phosphate transporters. New Phytol. 2017, 214, 632–643. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qiu, Y.-L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, N.; Radhakrishnan, G.V.; Delaux, P.M. What have we learnt from studying the evolution of the arbuscular mycorrhizal symbiosis? Curr. Opin. Plant Biol. 2018, 44, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Sepp, S.K.; Davison, J.; Jairus, T.; Vasar, M.; Moora, M.; Zobel, M.; Öpik, M. Non-random association patterns in a plant-mycorrhizal fungal network reveal host–symbiont specificity. Mol. Ecol. 2019, 28, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Timonen, S.; Marschner, P. Mycorrhizosphere concept. In Microbial Activity in the Rhizoshere; Mukerji, K.G., Manoharachary, C., Singh, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 155–172. ISBN 978-3-540-29420-7. [Google Scholar]
- Priyadharsini, P.; Rojamala, K.; Ravi, R.K.; Muthuraja, R.; Nagaraj, K.; Muthukumar, T. Mycorrhizosphere: The extended rhizosphere and its significance. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Choudhary, D.K., Varma, A., Tuteja, N., Eds.; Springer: Singapore, 2016; pp. 97–124. ISBN 978-981-10-2854-0. [Google Scholar]
- Harrison, M.J. Signaling in the arbuscular mycorrhizal symbiosis. Annu. Rev. Microbiol. 2005, 59, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Breuillin-Sessoms, F.; Floss, D.S.; Gomez, S.K.; Pumplin, N.; Ding, Y.; Levesque-Tremblay, V.; Noar, R.D.; Daniels, D.A.; Bravo, A.; Eaglesham, J.B.; et al. Suppression of arbuscule degeneration in Medicago truncatula phosphate transporter4 mutants is dependent on the ammonium transporter 2 family protein AMT2;3. Plant Cell 2015, 27, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.C.; Keck, M.; Gödde, V.; Niehaus, K.; Küster, H.; Hohnjec, N. Knockdown of the symbiotic sucrose synthase MtSucS1 affects arbuscule maturation and maintenance in mycorrhizal roots of Medicago truncatula. Plant Physiol. 2010, 152, 1000–1014. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.; Hart, M.M.; Bago, A.; et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef]
- Luginbuehl, L.H.; Menard, G.N.; Kurup, S.; Van Erp, H.; Radhakrishnan, G.V.; Breakspear, A.; Oldroyd, G.E.D.; Eastmond, P.J. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 2017, 356, 1175–1178. [Google Scholar] [CrossRef]
- Bravo, A.; Brands, M.; Wewer, V.; Dörmann, P.; Harrison, M.J. Arbuscular mycorrhiza-specific enzymes FatM and RAM2 fine-tune lipid biosynthesis to promote development of arbuscular mycorrhiza. New Phytol. 2017, 214, 1631–1645. [Google Scholar] [CrossRef]
- Zheng, C.; Ji, B.; Zhang, J.; Zhang, F.; Bever, J.D. Shading decreases plant carbon preferential allocation towards the most beneficial mycorrhizal mutualist. New Phytol. 2015, 205, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Lendenmann, M.; Thonar, C.; Barnard, R.L.; Salmon, Y.; Werner, R.A.; Frossard, E.; Jansa, J. Symbiont identity matters: Carbon and phosphorus fluxes between Medicago truncatula and different arbuscular mycorrhizal fungi. Mycorrhiza 2011, 21, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.; Doidy, J.; Zimmermann, S.D.; Wipf, D.; Courty, P.-E. Take a trip through the plant and fungal transportome of mycorrhiza. Trends Plant Sci. 2016, 21, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; ISBN 978-0-12-370526-6. [Google Scholar]
- Guerrero-Galán, C.; Houdinet, G.; Calvo-Polanco, M.; Bonaldi, K.E.; Garcia, K.; Zimmermann, S.D. The role of plant transporters in mycorrhizal symbioses. Adv. Bot. Res. 2018, 87, 303–342. [Google Scholar]
- Paszkowski, U.; Kroken, S.; Roux, C.; Briggs, S.P. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 13324–13329. [Google Scholar] [CrossRef] [PubMed]
- Rausch, C.; Daram, P.; Brunner, S.; Jansa, J.; Laloi, M.; Leggewie, G.; Amrhein, N.; Bucher, M. A phosphate transporter expressed in arbuscule-containing cells in potato. Nature 2001, 414, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, M.E.; Stinchcombe, J.R. Multiple mutualist effects on genomewide expression in the tripartite association between Medicago truncatula, nitrogen-fixing bacteria and mycorrhizal fungi. Mol. Ecol. 2016, 25, 4946–4962. [Google Scholar] [CrossRef]
- Kafle, A.; Garcia, K.; Wang, X.; Pfeffer, P.E.; Strahan, G.D.; Bücking, H. Nutrient demand and fungal access to resources control the carbon allocation to the symbiotic partners in tripartite interactions of Medicago truncatula. Plant Cell Environ. 2019, 42, 270–284. [Google Scholar] [CrossRef]
- Ossler, J.N.; Zielinski, C.A.; Heath, K.D. Tripartite mutualism: Facilitation or trade-offs between rhizobial and mycorrhizal symbionts of legume hosts. Am. J. Bot. 2015, 102, 1332–1341. [Google Scholar] [CrossRef]
- Desbrosses, G.J.; Stougaard, J. Root nodulation: A Paradigm for how plant-microbe symbiosis influences host developmental pathways. Cell Host Microbe 2011, 10, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.A.; Kucey, R.M.N. Carbon flow in plant microbial associations. Science 1981, 213, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Liu, J.; Ma, K.; Ciais, P.; Polasky, S. Reducing human nitrogen use for food production. Sci. Rep. 2016, 6, 30104. [Google Scholar] [CrossRef] [PubMed]
- Udvardi, M.; Poole, P.S. Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, S.; Schulze, J.; Tran, L.-S.P. Comparative analysis of the symbiotic efficiency of Medicago truncatula and Medicago sativa under phosphorus deficiency. Int. J. Mol. Sci. 2013, 14, 5198–5213. [Google Scholar] [CrossRef] [PubMed]
- Schulze, J. How are nitrogen fixation rates regulated in legumes? J. Plant Nutr. Soil Sci. 2004, 167, 125–137. [Google Scholar] [CrossRef]
- Sulieman, S.; Van Ha, C.; Schulze, J.; Tran, L.-S.P. Growth and nodulation of symbiotic Medicago truncatula at different levels of phosphorus availability. J. Exp. Bot. 2013, 64, 2701–2712. [Google Scholar] [CrossRef]
- Thuita, M.; Vanlauwe, B.; Mutegi, E.; Masso, C. Reducing spatial variability of soybean response to rhizobia inoculants in farms of variable soil fertility in Siaya County of western Kenya. Agric. Ecosyst. Environ. 2018, 261, 153–160. [Google Scholar] [CrossRef]
- Ulzen, J.; Abaidoo, R.C.; Ewusi-Mensah, N.; Masso, C. On-farm evaluation and determination of sources of variability of soybean response to Bradyrhizobium inoculation and phosphorus fertilizer in northern Ghana. Agric. Ecosyst. Environ. 2018, 267, 23–32. [Google Scholar] [CrossRef]
- Bournaud, C.; James, E.K.; de Faria, S.M.; Lebrun, M.; Melkonian, R.; Duponnois, R.; Tisseyre, P.; Moulin, L.; Prin, Y. Interdependency of efficient nodulation and arbuscular mycorrhization in Piptadenia gonoacantha, a Brazilian legume tree. Plant Cell Environ. 2018, 41, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Püschel, D.; Janoušková, M.; Voříšková, A.; Gryndlerová, H.; Vosátka, M.; Jansa, J. Arbuscular mycorrhiza stimulates biological nitrogen fixation in two Medicago spp. through improved phosphorus acquisition. Front. Plant Sci. 2017, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, Q.; Chen, F.; Yan, X.; Liao, H. Effects of co-inoculation with arbuscular mycorrhizal fungi and rhizobia on soybean growth as related to root architecture and availability of N and P. Mycorrhiza 2011, 21, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ibiang, Y.B.; Mitsumoto, H.; Sakamoto, K. Bradyrhizobia and arbuscular mycorrhizal fungi modulate manganese, iron, phosphorus, and polyphenols in soybean (Glycine max (L.) Merr.) under excess zinc. Environ. Exp. Bot. 2017, 137, 1–13. [Google Scholar] [CrossRef]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Mortimer, P.E.; Le Roux, M.R.; Pérez-Fernández, M.A.; Benedito, V.A.; Kleinert, A.; Xu, J.; Valentine, A.J. The dual symbiosis between arbuscular mycorrhiza and nitrogen fixing bacteria benefits the growth and nutrition of the woody invasive legume Acacia cyclops under nutrient limiting conditions. Plant Soil 2013, 366, 229–241. [Google Scholar] [CrossRef]
- Eulenstein, F.; Tauschke, M.; Behrendt, A.; Monk, J.; Schindler, U.; Lana, A.M.; Monk, S. The application of mycorrhizal fungi and organic fertilisers in horticultural potting soils to improve water use efficiency of crops. Horticulturae 2017, 3, 8. [Google Scholar] [CrossRef]
- Ortas, I. The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. Fields Crops Res. 2012, 125, 35–48. [Google Scholar] [CrossRef]
- Cely, M.V.T.; de Oliveira, A.G.; de Freitas, V.F.; de Luca, M.B.; Barazetti, A.R.; dos Santos, I.M.O.; Gionco, B.; Garcia, G.V.; Prete, C.E.C.; Andrade, G. Inoculant of arbuscular mycorrhizal fungi (Rhizophagus clarus) increase yield of soybean and cotton under field conditions. Front. Microbiol. 2016, 7, 720. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, D.; Rai, R.K.; Mishra, S.D.; Raja, A.; Purakayastha, T.J.; Varghese, E. Influence of phosphorus and biofertilizers on soybean and wheat root growth and properties. Field Crops Res. 2014, 166, 1–9. [Google Scholar] [CrossRef]
- Niwa, R.; Koyama, T.; Sato, T.; Adachi, K.; Tawaraya, K.; Sato, S.; Hirakawa, H.; Yoshida, S.; Ezawa, T. Dissection of niche competition between introduced and indigenous arbuscular mycorrhizal fungi with respect to soybean yield responses. Sci. Rep. 2018, 8, 7419. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, E.; Heijden, M.G.A.; Rillig, M.C.; Kiers, T.E. Mycorrhizal fungal establishment in agricultural soils: Factors determining inoculation success. New Phytol. 2012, 197, 1104–1109. [Google Scholar] [CrossRef]
- Ryan, M.H.; Graham, J.H. Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol. 2018, 220, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.H.; Graham, J.H.; Morton, J.B.; Kirkegaard, J.A. Research must use a systems agronomy approach if management of the arbuscular mycorrhizal symbiosis is to contribute to sustainable intensification. New Phytol. 2019. Available online: https://nph.onlinelibrary.wiley.com/doi/10.1111/nph.15600 (accessed on 18 January 2019). [CrossRef] [PubMed]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Camenzind, T.; Cavagnaro, T.R.; Degrune, F.; Hohmann, P.; Lammel, D.R.; Mansour, I.; Roy, J.; van der Heijden, M.G.A.; et al. Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytol. 2019. Available online: https://nph.onlinelibrary.wiley.com/doi/10.1111/nph.15602 (accessed on 18 January 2019).
- Wang, X.; Zhao, S.; Bücking, H. Arbuscular mycorrhizal growth responses are fungal specific but do not differ between soybean genotypes with different phosphate efficiency. Ann. Bot. 2016, 118, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Smith, F.A.; Dickson, S.; Holloway, R.E.; Smith, S.E. Plant growth depressions in arbuscular mycorrhizal symbioses: Not just caused by carbon drain? New Phytol. 2008, 178, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Mäder, P.; Kaiser, F.; Adholeya, A.; Singh, R.; Uppal, H.S.; Sharma, A.K.; Srivastava, R.; Sahai, V.; Aragno, M.; Wiemken, A.; et al. Inoculation of root microorganisms for sustainable wheat–rice and wheat–black gram rotations in India. Soil Biol. Biochem. 2011, 43, 609–619. [Google Scholar] [CrossRef]
- Somerville, C.; Youngs, H.; Taylor, C.; Davis, S.C.; Long, S.P. Feedstocks for lignocellulosic biofuels. Science 2010, 329, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Song, W.; Buhain, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sustain. Energy Rev. 2015, 42, 712–725. [Google Scholar] [CrossRef]
- Guo, M.; Li, C.; Facciotto, G.; Bergante, S.; Bhatia, R.; Comolli, R.; Ferré, C.; Murphy, R. Bioethanol from poplar clone Imola: An environmentally viable alternative to fossil fuel? Biotechnol. Biofuels 2015, 8, 134. [Google Scholar] [CrossRef]
- Macaya-Sanz, D.; Chen, J.; Kalluri, U.C.; Muchero, W.; Tschaplinski, T.J.; Gunter, L.E.; Simon, S.J.; Biswal, A.K.; Bryan, A.C.; Payyavula, R.; et al. Agronomic performance of Populus deltoides trees engineered for biofuel production. Biotechnol. Biofuels 2017, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Richards, B.K.; Stoof, C.R.; Cary, I.J.; Woodbury, P.B. Reporting on marginal lands for bioenergy feedstock production: A modest proposal. BioEnergy Res. 2014, 7, 1060–1062. [Google Scholar] [CrossRef]
- Altieri, M.A. Agroecology: The science of natural resource management for poor farmers in marginal environments. Agric. Ecosyst. Environ. 2002, 93, 1–24. [Google Scholar] [CrossRef]
- Mitchell, C. New cultural treatments and yield optimisation. Biomass Bioenergy 1995, 9, 11–34. [Google Scholar] [CrossRef]
- Guénon, R.; Bastien, J.-C.; Thiébeau, P.; Bodineau, G.; Bertrand, I. Carbon and nutrient dynamics in short-rotation coppice of poplar and willow in a converted marginal land, a case study in central France. Nutr. Cycl. Agroecosyst. 2016, 106, 293–309. [Google Scholar] [CrossRef]
- Becquer, A.; Guerrero-Galán, C.; Eibensteiner, J.L.; Houdinet, G.; Bücking, H.; Zimmermann, S.D.; Garcia, K. The ectomycorrhizal contribution to tree nutrition. Adv. Bot. Res. 2019, 89, 77–126. [Google Scholar]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2017, 108, 1028–1046. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Aime, M.C.; Grigoriev, I.V.; Martin, F.; Stajich, J.E.; Blackwell, M. The fungal tree of life: From molecular systematics to genome-scale phylogenies. Microbiol. Spectr. 2017, 5. Available online: http://www.asmscience.org/content/journal/microbiolspec/10.1128/microbiolspec.FUNK-0053-2016 (accessed on 15 September 2017). [CrossRef]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The structure, distribution, and biomass of the world’s forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Martin, F.; Duplessis, S.; Ditengou, F.; Lagrange, H.; Voiblet, C.; Lapeyrie, F. Developmental cross talking in the ectomycorrhizal symbiosis: Signals and communication genes. New Phytol. 2001, 151, 145–154. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.; Lindahl, B.D. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Marmeisse, R.; Girlanda, M. Mycorrhizal fungi and the soil carbon and nutrient cycling. In Environmental and Microbial Relationships; Druzhinina, I.S., Kubicek, C.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 189–203. ISBN 978-3-319-29532-9. [Google Scholar]
- Tatry, M.-V.; El Kassis, E.; Lambilliotte, R.; Corratgé, C.; van Aarle, I.; Amenc, L.K.; Alary, R.; Zimmermann, S.; Sentenac, H.; Plassard, C. Two differentially regulated phosphate transporters from the symbiotic fungus Hebeloma cylindrosporum and phosphorus acquisition by ectomycorrhizal Pinus pinaster. Plant J. 2009, 57, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.; Haider, M.Z.; Delteil, A.; Corratgé-Faillie, C.; Conéjero, G.; Tatry, M.-V.; Becquer, A.; Amenc, L.; Sentenac, H.; Plassard, C.; et al. Promoter-dependent expression of the fungal transporter HcPT1.1 under Pi shortage and its spatial localization in ectomycorrhiza. Fungal Genet. Biol. 2013, 58–59, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Becquer, A.; Garcia, K.; Plassard, C. HcPT1.2 participates in Pi acquisition in Hebeloma cylindrosporum external hyphae of ectomycorrhizas under high and low phosphate conditions. Plant Signal Behav. 2018, 13, e1525997. [Google Scholar] [CrossRef] [PubMed]
- Torres-Aquino, M.; Becquer, A.; Le Guernevé, C.; Louche, J.; Amenc, L.K.; Staunton, S.; Quiquampoix, H.; Plassard, C. The host plant Pinus pinaster exerts specific effects on phosphate efflux and polyphosphate metabolism of the ectomycorrhizal fungus Hebeloma cylindrosporum: A radiotracer, cytological staining and 31P NMR spectroscopy study. Plant Cell Environ. 2017, 40, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Becquer, A.; Garcia, K.; Amenc, L.; Rivard, C.; Doré, J.; Trives-Segura, C.; Szponarski, W.; Russet, S.; Baeza, Y.; Lassalle-Kaiser, B.; et al. The Hebeloma cylindrosporum HcPT2 Pi transporter plays a key role in ectomycorrhizal symbiosis. New Phytol. 2018, 220, 1185–1199. [Google Scholar] [CrossRef]
- Loth-Pereda, V.; Orsini, E.; Courty, P.-E.; Lota, F.; Kohler, A.; Diss, L.; Blaudez, D.; Chalot, M.; Nehls, U.; Bucher, M.; et al. Structure and expression profile of the phosphate Pht1 transporter gene family in mycorrhizal Populus trichocarpa. Plant Physiol. 2011, 156, 2141–2154. [Google Scholar] [CrossRef]
- Rousseau, J.V.D.; Sylvia, D.M.; Fox, A.J. Contribution of ectomycorrhiza to the potential nutrient-absorbing surface of pine. New Phytol. 1994, 128, 639–644. [Google Scholar] [CrossRef]
- Torres Aquino, M.; Plassard, C. Dynamics of ectomycorrhizal mycelial growth and P transfer to the host plant in response to low and high soil P availability. FEMS Microbiol. Ecol. 2004, 48, 149–156. [Google Scholar] [CrossRef]
- Chen, W.; Koide, R.T.; Adams, T.S.; DeForest, J.L.; Cheng, L.; Eissenstat, D.M. Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc. Nalt. Acad. Sci. USA 2016, 113, 8741–8746. [Google Scholar] [CrossRef]
- Brandes, B.; Godbold, D.L.; Kuhn, A.J.; Jentschke, G. Nitrogen and phosphorus acquisition by the mycelium of the ectomycorrhizal fungus Paxillus involutus and its effect on host nutrition. New Phytol. 1998, 140, 735–743. [Google Scholar] [CrossRef]
- Jentschke, G.; Brandes, B.; Kuhn, A.J.; Schröder, W.H.; Godbold, D.L. Interdependence of phosphorus, nitrogen, potassium and magnesium translocation by the ectomycorrhizal fungus Paxillus involutus. New Phytol. 2001, 149, 327–337. [Google Scholar] [CrossRef]
- Colpaert, J.V.; Van Tichelen, K.K.; Van Assche, J.A.; Van Laere, A. Short-term phosphorus uptake rates in mycorrhizal and non-mycorrhizal roots of intact Pinus sylvestris seedlings. New Phytol. 1999, 143, 589–597. [Google Scholar] [CrossRef]
- Jones, M.D.; Durall, D.M.; Tinker, P.B. A comparison of arbuscular and ectomycorrhizal Eucalyptus coccifera: Growth response, phosphorus uptake efficiency and external hyphal production. New Phytol. 1998, 140, 125–134. [Google Scholar] [CrossRef]
- Van Tichelen, K.K.; Colpaert, V.J. Kinetics of phosphate absorption by mycorrhizal and non-mycorrhizal Scots pine seedlings. Physiol. Plant. 2000, 110, 96–103. [Google Scholar] [CrossRef]
- Plassard, C.; Louche, J.; Ali, M.A.; Duchemin, M.; Legname, E.; Cloutier-Hurteau, B. Diversity in phosphorus mobilisation and uptake in ectomycorrhizal fungi. Ann. For. Sci. 2011, 68, 33–43. [Google Scholar] [CrossRef]
- Rineau, F.; Shah, F.; Smits, M.M.; Persson, P.; Johansson, T.; Carleer, R.; Troein, C.; Tunlid, A. Carbon availability triggers the decomposition of plant litter and assimilation of nitrogen by an ectomycorrhizal fungus. ISME J. 2013, 7, 2010–2022. [Google Scholar] [CrossRef] [PubMed]
- Tunlid, A.; Floudas, D.; Koide, R.; Rineau, F. Soil organic matter decomposition mechanisms in ectomycorrhizal fungi. In Molecular Mycorrhizal Symbiosis; Wiley-Blackwell: Hoboken, NJ, USA, 2016; pp. 257–275. ISBN 9781118951446. [Google Scholar]
- Antibus, R.K.; Sinsabaugh, R.L.; Linkins, A.E. Phosphatase activities and phosphorus uptake from inositol phosphate by ectomycorrhizal fungi. Can. J. Bot. 1992, 70, 794–801. [Google Scholar] [CrossRef]
- Colpaert, J.V.; Van Laere, A.; Van Tichelen, K.K.; Van Assche, J.A. The use of inositol hexaphosphate as a phosphorus source by mycorrhizal and non-mycorrhizal Scots Pine (Pinus sylvestris). Funct. Ecol. 1997, 11, 407–415. [Google Scholar] [CrossRef]
- Perez-Moreno, J.; Read, J.D. Mobilization and transfer of nutrients from litter to tree seedlings via the vegetative mycelium of ectomycorrhizal plants. New Phytol. 2000, 145, 301–309. [Google Scholar] [CrossRef]
- Perez-Moreno, J.; Read, D.J. Exploitation of pollen by mycorrhizal mycelial systems with special reference to nutrient recycling in boreal forests. Proc. Biol. Sci. 2001, 268, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moreno, J.; Read, D.J. Nutrient transfer from soil nematodes to plants: A direct pathway provided by the mycorrhizal mycelial network. Plant Cell Environ. 2002, 24, 1219–1226. [Google Scholar] [CrossRef]
- Tibbett, M.; Sanders, F.E. Ectomycorrhizal symbiosis can enhance plant nutrition through improved access to discrete organic nutrient patches of high resource quality. Ann. Bot. 2002, 89, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.; Stenlid, J.; Olsson, S.; Finlay, R. Translocation of 32P between interacting mycelia of a wood-decomposing fungus and ectomycorrhizal fungi in microcosm systems. New Phytol. 1999, 144, 183–193. [Google Scholar] [CrossRef]
- Fontaine, L.; Thiffault, N.; Paré, D.; Fortin, J.-A.; Piché, Y. Phosphate-solubilizing bacteria isolated from ectomycorrhizal mycelium of Picea glauca are highly efficient at fluorapatite weathering. Botany 2016, 94, 1183–1193. [Google Scholar] [CrossRef]
- Trappe, J.M. Selection of fungi for ectomycorrhizal inoculation in nurseries. Annu. Rev. Phytopathol. 1977, 15, 203–222. [Google Scholar] [CrossRef]
- Marx, D.H. Ectomycorrhizal fungus inoculations: A tool for improving forestation practices. Trop. Mycorrhiza Res. 1980, 118, 13–71. [Google Scholar]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef]
- Banik, S.; Dey, B.K. Available phosphate content of an alluvial soil as influenced by inoculation of some isolated phosphate-solubilizing micro-organisms. Plant Soil 1982, 69, 353–364. [Google Scholar] [CrossRef]
- Banik, S.; Dey, B.K. Phosphate-solubilizing potentiality of the microorganisms capable of utilizing aluminium phosphate as a sole phosphate source. Zent. Mikrobiol. 1983, 138, 17–23. [Google Scholar] [CrossRef]
- Gupta, R.; Singal, R.; Shankar, A.; Kuhad, R.C.; Saxena, R.K. A modified plate assay for screening phosphate solubilizing microorganisms. J. Gen. Appl. Microbiol. 1994, 40, 255–260. [Google Scholar] [CrossRef]
- Vazquez, P.; Holguin, G.; Puente, M.E.; Lopez-Cortes, A.; Bashan, Y. Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol. Fertil. Soils 2000, 30, 460–468. [Google Scholar] [CrossRef]
- Sadiq, H.M.; Jahangir, G.Z.; Nasir, I.A.; Iqtidar, M.; Iqbal, M. Isolation and characterization of phosphate-solubilizing bacteria from rhizosphere soil. Biotechnol. Biotechnol. Equip. 2013, 27, 4248–4255. [Google Scholar] [CrossRef]
- Tani, A.; Akita, M.; Murase, H.; Kimbara, K. Culturable bacteria in hydroponic cultures of moss Racomitrium japonicum and their potential as biofertilizers for moss production. J. Biosci. Bioeng. 2011, 112, 32–39. [Google Scholar] [CrossRef]
- Bar-Yosef, B.; Rogers, R.D.; Wolfram, J.H.; Richman, E. Pseudomonas cepacia–Mediated rock phosphate solubilization in kaolinite and montmorillonite suspensions contribution from the agricultural research organization series 626/98. Soil Sci. Soc. Am. J. 1999, 63, 1703–1708. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, W.; Ge, Y. Exopolysaccharide: A novel important factor in the microbial dissolution of tricalcium phosphate. World J. Microbiol. Biotechnol. 2008, 24, 1059–1065. [Google Scholar] [CrossRef]
- Hwangbo, H.; Park, R.D.; Kim, Y.W.; Rim, Y.S.; Park, K.H.; Kim, T.H.; Suh, J.S.; Kim, K.Y. 2-Ketogluconic acid production and phosphate solubilization by Enterobacter intermedium. Curr. Microbiol. 2003, 47, 87–92. [Google Scholar]
- Shahid, M.; Hameed, S.; Imran, A.; Ali, S.; van Elsas, J.D. Root colonization and growth promotion of sunflower (Helianthus annuus L.) by phosphate solubilizing Enterobacter sp. Fs-11. World J. Microbiol. Biotechnol. 2012, 28, 2749–2758. [Google Scholar] [CrossRef]
- Thaller, M.C.; Berlutti, F.; Schippa, S.; Iori, P.; Passariello, C.; Rossolini, G.M. Heterogeneous patterns of acid phosphatases containing low-molecular-mass polypeptides in members of the family Enterobacteriaceae. Int. J. Syst. Evol. Microbiol. 1995, 45, 255–261. [Google Scholar] [CrossRef]
- Ohtake, H.; Wu, H.; Imazu, K.; Anbe, Y.; Kato, J.; Kuroda, A. Bacterial phosphonate degradation, phosphite oxidation and polyphosphate accumulation. Resour. Conserv. Recycl. 1996, 18, 125–134. [Google Scholar] [CrossRef]
- Katznelson, H.; Bose, B. Metabolic activity and phosphate-dissolving capability of bacterial isolates from wheat roots, rhizosphere, and non-rhizosphere soil. Can. J. Microbiol. 1959, 5, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.K.; Chakrabartty, P.K. Solubilization of inorganic phosphate by Rhizobium. Folia Microbiol. 1993, 38, 325–330. [Google Scholar] [CrossRef]
- Bajpai, P.D.; Sundara Rao, W.V.B. Phosphate solubilising bacteria. Soil Sci. Plant Nutr. 1971, 17, 44–45. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Kumar, G.N.; Parekh, L.J. Effect of buffering on the phosphate-solubilizing ability of microorganisms. World J. Microbiol. Biotechnol. 1998, 14, 669–673. [Google Scholar] [CrossRef]
- Kirk, G.J.D.; Santos, E.E.; Findenegg, G.R. Phosphate solubilization by organic anion excretion from rice (Oryza sativa L.) growing in aerobic soil. Plant Soil 1999, 211, 11–18. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, P.; Wang, T.; Wang, M.; Chen, M.; Chen, N.; Pan, L.; Chi, X. Isolation and characterization of halotolerant phosphate-solubilizing microorganisms from saline soils. 3 Biotech 2018, 8, 461. [Google Scholar] [CrossRef]
- Park, K.-H.; Lee, C.-Y.; Son, H.-J. Mechanism of insoluble phosphate solubilization by Pseudomonas fluorescens RAF15 isolated from ginseng rhizosphere and its plant growth-promoting activities. Lett. Appl. Microbiol. 2009, 49, 222–228. [Google Scholar] [CrossRef]
- Kim, K.Y.; McDonald, G.A.; Jordan, D. Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol. Fertil. Soils 1997, 24, 347–352. [Google Scholar] [CrossRef]
- Ragot, S.A.; Kertesz, M.A.; Mészáros, É.; Frossard, E.; Bünemann, E.K. Soil phoD and phoX alkaline phosphatase gene diversity responds to multiple environmental factors. FEMS Microbiol. Ecol. 2016, 93, fiw212. [Google Scholar] [CrossRef]
- Richardson, A.E.; Hadobas, P.A. Soil isolates of Pseudomonas spp. that utilize inositol phosphates. Can. J. Microbiol. 1997, 43, 509–516. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of Phosphate Solubilization and Its Potential Applications for Improving Plant Growth-Promoting Bacteria—First International Meeting on Microbial Phosphate Solubilization; Velázquez, E., Rodríguez-Barrueco, C., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 15–21. [Google Scholar]
- Wei, K.; Sun, T.; Tian, J.; Chen, Z.; Chen, L. Soil microbial biomass, phosphatase and their relationships with phosphorus turnover under mixed inorganic and organic nitrogen addition in a Larix gmelinii plantation. For. Ecol. Manag. 2018, 422, 313–322. [Google Scholar] [CrossRef]
- Anderson, O.R.; Juhl, A.R.; Bock, N. Effects of organic carbon enrichment on respiration rates, phosphatase activities, and abundance of heterotrophic bacteria and protists in organic-rich Arctic and mineral-rich temperate soil samples. Polar Biol. 2018, 41, 11–24. [Google Scholar] [CrossRef]
- Spohn, M.; Treichel, N.S.; Cormann, M.; Schloter, M.; Fischer, D. Distribution of phosphatase activity and various bacterial phyla in the rhizosphere of Hordeum vulgare L. depending on P availability. Soil Biol. Biochem. 2015, 89, 44–51. [Google Scholar] [CrossRef]
- Shen, L.; Wu, X.-Q.; Zeng, Q.-W.; Liu, H.-B. Regulation of soluble phosphate on the ability of phytate mineralization and β-propeller phytase gene expression of Pseudomonas fluorescens JZ-DZ1, a phytate-mineralizing Rhizobacterium. Curr. Microbiol. 2016, 73, 915–923. [Google Scholar] [CrossRef]
- Zhao, K.; Penttinen, P.; Zhang, X.; Ao, X.; Liu, M.; Yu, X.; Chen, Q. Maize rhizosphere in Sichuan, China, hosts plant growth promoting Burkholderia cepacia with phosphate solubilizing and antifungal abilities. Microbiol. Res. 2014, 169, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.; Xu, G.; Tian, X.; Xie, H.; Yang, X.; Cao, C. Root colonization and growth promotion of soybean, wheat and Chinese cabbage by Bacillus cereus YL6. PLoS ONE 2018, 13, e0200181. [Google Scholar]
- Kumar, S.; Bauddh, K.; Barman, S.C.; Singh, R.P. Amendments of microbial biofertilizers and organic substances reduces requirement of urea and DAP with enhanced nutrient availability and productivity of wheat (Triticum aestivum L.). Ecol. Eng. 2014, 71, 432–437. [Google Scholar] [CrossRef]
- Valetti, L.; Iriarte, L.; Fabra, A. Growth promotion of rapeseed (Brassica napus) associated with the inoculation of phosphate solubilizing bacteria. Appl. Soil Ecol. 2018, 132, 1–10. [Google Scholar] [CrossRef]
- Biswas, J.K.; Banerjee, A.; Rai, M.; Naidu, R.; Biswas, B.; Vithanage, M.; Dash, M.C.; Sarkar, S.K.; Meers, E. Potential application of selected metal resistant phosphate solubilizing bacteria isolated from the gut of earthworm (Metaphire posthuma) in plant growth promotion. Geoderma 2018, 330, 117–124. [Google Scholar] [CrossRef]
- Nassal, D.; Spohn, M.; Eltlbany, N.; Jacquiod, S.; Smalla, K.; Marhan, S.; Kandeler, E. Effects of phosphorus-mobilizing bacteria on tomato growth and soil microbial activity. Plant Soil 2018, 427, 17–37. [Google Scholar] [CrossRef]
- Samaddar, S.; Chatterjee, P.; Truu, J.; Anandham, R.; Kim, S.; Sa, T. Long-term phosphorus limitation changes the bacterial community structure and functioning in paddy soils. Appl. Soil Ecol. 2019, 134, 111–115. [Google Scholar] [CrossRef]
- Research, G.V. Biofertilizers Market Size, Share and Trends Analysis Report by Product (Nitrogen Fixing, Phophate Solubilizing), by Application (Seed Treatment, Soil Treatment), and Segment Forecasts, 2012–2022; Grand View Research: San Francisco, CA, USA, February 2018; Report ID: 978-1-68038-038-5. [Google Scholar]
- Kafle, A.; Garcia, K.; Peta, V.; Yakha, J.; Soupir, A.; Bücking, H. Beneficial plant microbe interactions and their effect on nutrient uptake, yield and stress resistance of soybeans. In Soybean—The Basis of Yield, Biomass and Productivity; Kasai, M., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Gibert, A.; Tozer, W.; Westoby, M. Plant performance response to eight different types of symbiosis. New Phytol. 2019. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kafle, A.; Cope, K.R.; Raths, R.; Krishna Yakha, J.; Subramanian, S.; Bücking, H.; Garcia, K. Harnessing Soil Microbes to Improve Plant Phosphate Efficiency in Cropping Systems. Agronomy 2019, 9, 127. https://doi.org/10.3390/agronomy9030127
Kafle A, Cope KR, Raths R, Krishna Yakha J, Subramanian S, Bücking H, Garcia K. Harnessing Soil Microbes to Improve Plant Phosphate Efficiency in Cropping Systems. Agronomy. 2019; 9(3):127. https://doi.org/10.3390/agronomy9030127
Chicago/Turabian StyleKafle, Arjun, Kevin R. Cope, Rachel Raths, Jaya Krishna Yakha, Senthil Subramanian, Heike Bücking, and Kevin Garcia. 2019. "Harnessing Soil Microbes to Improve Plant Phosphate Efficiency in Cropping Systems" Agronomy 9, no. 3: 127. https://doi.org/10.3390/agronomy9030127
APA StyleKafle, A., Cope, K. R., Raths, R., Krishna Yakha, J., Subramanian, S., Bücking, H., & Garcia, K. (2019). Harnessing Soil Microbes to Improve Plant Phosphate Efficiency in Cropping Systems. Agronomy, 9(3), 127. https://doi.org/10.3390/agronomy9030127





