Evaluation of Soybean Plant Introductions for Traits that can Improve Emergence under Varied Soil Moisture Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Germplasm
2.2. Seed Source
2.3. Greenhouse Experiment to Measure Primary Root Length and Emergence
Experimental Design and Statistical Analyses
2.4. Laboratory Experiment to Measure Time Taken for Radicle Emergence
Experimental Design and Statistical Analyses
3. Results
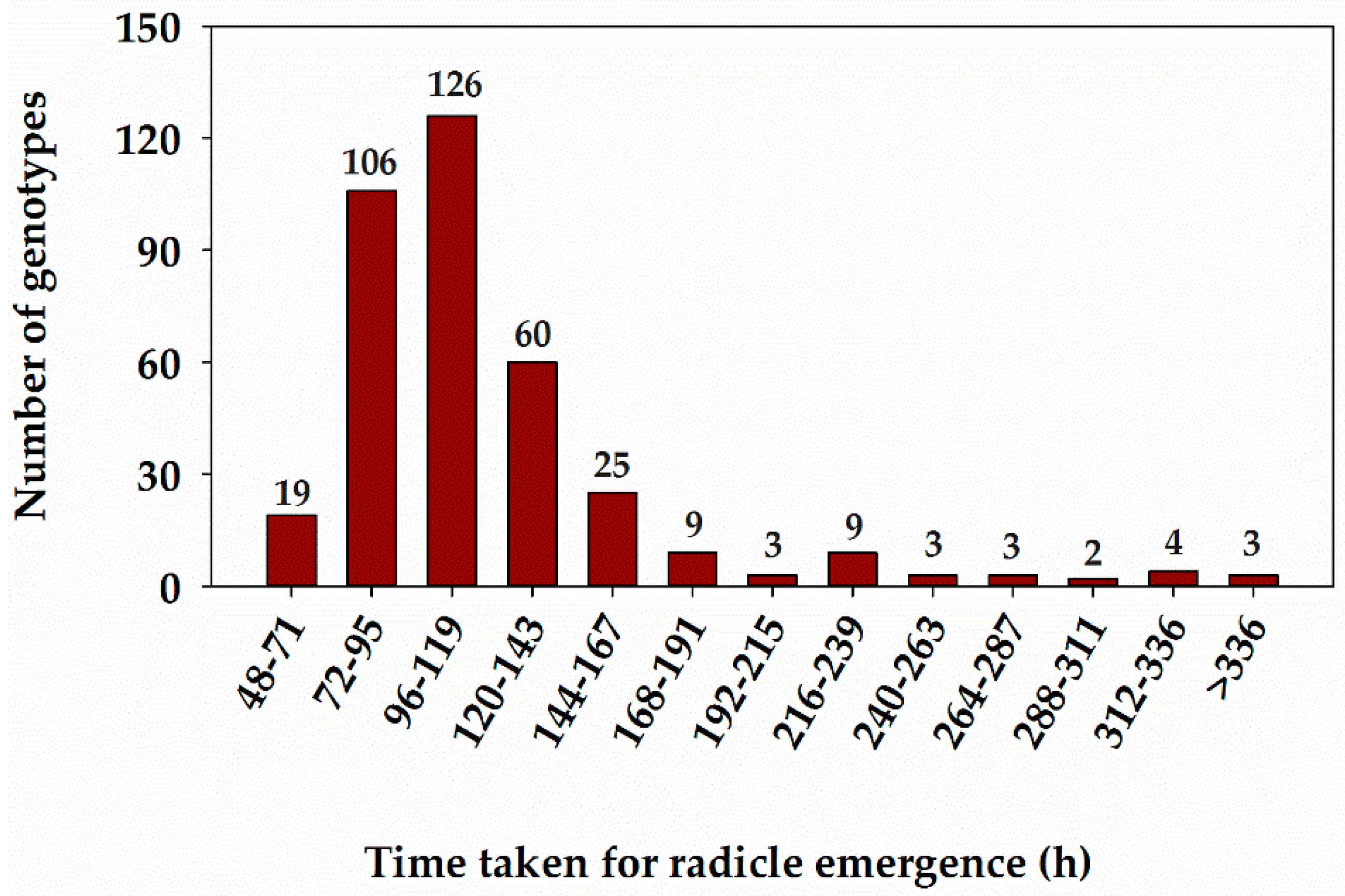
3.1. Genetic Variability for Primary Root Length and Time Taken for Radicle Emergence
3.2. Relationships among Emergence, Primary Root Length, and Seed Weight of Soybean Genotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- SoyStats 2018. A Reference Guide to Important Soybean Facts and Figures. American Soybean Association. Available online: http://soystats.com/ (accessed on 01 February 2019).
- Palmer, J.; Dunphy, J.E.; Reese, P. Managing Drought Stressed Soybeans in the Southeast; NC Cooperative Extension Service: Raleigh, NC, USA, 1996. [Google Scholar]
- Specht, J.E.; Hume, D.J.; Kumudini, S.V. Soybean yield potential – a genetic and physiological perspective. Crop Sci. 1999, 39, 1560–1570. [Google Scholar] [CrossRef]
- Purcell, L.C.; Specht, J.E. Physiological traits for ameliorating drought stress. In Soybean: Improvement, Production, and Uses, Agronomy Monograph 16, 3rd ed.; Boerma, H.R., Specht, J.E., Eds.; ASA/SSA/SSSA: Madison, WI, USA, 2004; pp. 569–620. [Google Scholar]
- Zipper, S.C.; Qiu, J.; Kucharik, C.J. Drought effects on US maize and soybean production: spatiotemporal patterns and historical changes. Environ. Res. Lett. 2016, 11, 094021. [Google Scholar] [CrossRef]
- Sloane, R.J.; Patterson, R.P.; Carter, T.E., Jr. Field drought tolerance of a soybean plant introduction. Crop Sci. 1990, 30, 118–123. [Google Scholar] [CrossRef]
- Carter, T.E., Jr.; De Souza, P.I.; Purcell, L.C. Recent advances in breeding for drought and aluminum resistance in soybean. In Proceedings of World Soybean Conference VI, Chicago, IL, USA, 4–7 August 1999; Kauffman, H., Ed.; Superior Print: Champaign, IL, USA, 1999; pp. 106–125. [Google Scholar]
- Pathan, M.S.; Lee, J.D.; Shannon, J.G.; Nguyen, H.T. Recent advances in breeding for drought and salt stress tolerance in soybean. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 739–773. [Google Scholar]
- Sinclair, T.R.; Purcell, L.C.; King, C.A.; Sneller, C.H.; Chen, P.; Vadez, V. Drought tolerance and yield increase of soybean resulting from improved N2 fixation. Field Crops Res. 2007, 101, 68–71. [Google Scholar] [CrossRef]
- Purcell, L.C.; King, C.A.; Ball, R.A. Soybean cultivar differences in ureides and the relationship to drought tolerant nitrogen fixation and manganese nutrition. Crop Sci. 2000, 40, 1062–1070. [Google Scholar] [CrossRef]
- Carter, T.E., Jr.; Todd, S.M.; Gillen, A.M. Registration of ‘USDA-N8002’ soybean cultivar with high yield and abiotic stress resistance traits. J. Plant Regist. 2016, 10, 238–245. [Google Scholar] [CrossRef]
- Sionit, N.; Kramer, P.J. Effect of water stress during different stages of growth of soybean. Agron. J. 1977, 69, 274–278. [Google Scholar] [CrossRef]
- Senaratna, T.; McKersie, B.D. Dehydration injury in germinating soybean (Glycine max L. Merr.) seeds. Plant Physiol. 1983, 72, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Georgia Soybean Production Guide 2018. The Univ. of Georgia College of Agricultural and Environmental Sciences. Available online: http://www.caes.uga.edu/content/dam/caes-website/extension-outreach/commodities/grains/docs/soybean/2018-Soybean-Production-Guide.pdf (accessed on 1 February 2019).
- Torrion, J.A.; Setiyono, T.D.; Cassman, K.G.; Ferguson, R.B.; Irmak, S.; Specht, J.E. Soybean root development relative to vegetative and reproductive phenology. Agron. J. 2012, 104, 1702–1709. [Google Scholar] [CrossRef]
- Hufstetler, E.V.; Boerma, H.R.; Carter, T.E.; Earl, H.G. Genotypic variation for three physiological traits affecting drought tolerance in soybean. Crop Sci. 2007, 47, 25–35. [Google Scholar] [CrossRef]
- King, C.A.; Purcell, L.C.; Brye, K.R. Differential wilting among soybean genotypes in response to water deficit. Crop Sci. 2009, 49, 290–298. [Google Scholar] [CrossRef]
- Dhanapal, A.P.; Ray, J.D.; Singh, S.K.; Hoyos-Villegas, V.; Smith, J.R.; Purcell, L.C.; King, C.A.; Cregan, P.; Song, Q.; Fritschi, F. Genome-wide association study (GWAS) of carbon isotope ratio (δ13C) in diverse soybean [Glycine max (L.) Merr.] genotypes. Theor. Appl. Genet. 2015, 128, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Dhanapal, A.P.; Ray, J.D.; Singh, S.K.; Hoyos-Villegas, V.; Smith, J.R.; Purcell, L.C.; King, C.A.; Fritschi, F.B. Genome-wide association analysis of diverse soybean genotypes reveals novel markers for nitrogen traits. Plant Genome 2015, 8. [Google Scholar] [CrossRef]
- Kaler, A.S.; Ray, J.D.; Schapaugh, W.T.; King, C.A.; Purcell, L.C. Genome-wide association mapping of canopy wilting in diverse soybean genotypes. Theor. Appl. Genet. 2017, 130, 2203–2217. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Sneller, C.H.; Purcell, L.C.; Sinclair, T.R.; King, C.A.; Ishibashi, T. Registration of soybean germplasm lines R01-416F and R01-581F for improved yield and nitrogen fixation under drought stress. J. Plant Reg. 2007, 1, 166–167. [Google Scholar] [CrossRef]
- Sadok, W.; Gilbert, M.E.; Raza, M.A.; Sinclair, T.R. Basis of slow-wilting phenotype in soybean PI 471938. Crop Sci. 2012, 52, 1261–1269. [Google Scholar] [CrossRef]
- Lee, G.J.; Carter, T.E., Jr.; Boerma, H.R.; Shannon, J.G.; Hood, M.; Hawbaker, M. Identification of soybean yield QTL in irrigated and rain-fed environments. In Agronomy Abstracts; ASA, CSSA, and SSSA: Madison, WI, USA, 2002. [Google Scholar]
- Uniform Soybean Tests Southern States 2017. USDA—Agricultural Research Service Crop Genetics Research Unit, Stoneville, MS. Available online: https://www.ars.usda.gov/ARSUserFiles/60661000/UniformSoybeanTests/2017SoyBook%20lockedREV.pdf (accessed on 1 February 2019).
- Kolasinska, K.; Szyrmer, J.; Dul, S. Relationship between laboratory seed quality tests and field emergence of common bean seed. Crop Sci. 2000, 40, 470–475. [Google Scholar] [CrossRef]
- International Rules for Seed Testing: Rules; International Seed Testing Association (ISTA): Zurich, Switzerland, 2004; p. 243.
- Fehr, W.R.; Caviness, C.E. Stages of Soybean Development; Special Report 80; Iowa Agricultural and Home Economics Experiment Station Publications, Iowa State University: Ames, IA, USA, 1977. [Google Scholar]
- McCarter, K.S. Analysis of covariance. In Applied Statistics in Agricultural, Biological, and Environmental Sciences; Glaz, B., Yeater, K.M., Eds.; ASA/CSSA/SSSA: Madison, WI, USA, 2018; pp. 235–277. [Google Scholar]
- Association of Official Seed Analysts (AOSA). Rules for Testing Seeds; Association of Official Seed Analysts (AOSA): Wichita, KS, USA, 2017. [Google Scholar]
- Taylor, H.M.; Burnett, E.; Booth, G.D. Taproot elongation rates of soybeans. Z. Acker Pflanzenbau Bd. 1978, 146, 33–39. [Google Scholar]
- Manavalan, L.P.; Guttikonda, S.K.; Tran, L.P.; Nguyen, H.T. Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 2009, 50, 1260–1276. [Google Scholar] [CrossRef] [PubMed]
- Fried, H.G.; Narayanan, S.; Fallen, B. Characterization of a soybean (Glycine max L. Merr.) germplasm collection for root traits. PLoS ONE 2018, 13, e0200463. [Google Scholar] [CrossRef] [PubMed]


| Trait | P Value | ||
|---|---|---|---|
| Moisture Level 1 | Genotype | Moisture Level*Genotype | |
| Primary root length (cm) | <0.0001 | 0.0164 | 1.000 |
| Time taken for radicle emergence (h) | N/A | <0.0001 | N/A |
| Variables | Slope (p-Value 1) | Intercept (p-Value) | R2 2 |
|---|---|---|---|
| y – emergence 3, covariate − primary root length 3 | 2.222 (p < 0.0001) | 41.47 (p < 0.0001) | 0.81 |
| y – emergence 4, covariate − primary root length 4 | 2.833 (p < 0.0001) | 49.28 (p < 0.0001) | 0.54 |
| y = emergence 5, covariate − primary root length 5 | 0.162 (p = 0.17) | 88.83 (p < 0.0001) | N/A |
| y = emergence 6, covariate − primary root length 6 | 2.49 (p < 0.0001) | 37.50 (p < 0.0001) | 0.70 |
| y = emergence 7, covariate − primary root length 7 | 4.150 (p < 0.0001) | 2.366 (p < 0.0001) | 0.81 |
| y = emergence 3, covariate - seed weight | 0.007 (p = 0.51) | 9.173 (p < 0.0001) | N/A |
| y = primary root length 3, covariate − seed weight | −0.131 (P = 0.15) | 52.16 (P < 0.0001) | N/A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, S.; Fallen, B. Evaluation of Soybean Plant Introductions for Traits that can Improve Emergence under Varied Soil Moisture Levels. Agronomy 2019, 9, 118. https://doi.org/10.3390/agronomy9030118
Narayanan S, Fallen B. Evaluation of Soybean Plant Introductions for Traits that can Improve Emergence under Varied Soil Moisture Levels. Agronomy. 2019; 9(3):118. https://doi.org/10.3390/agronomy9030118
Chicago/Turabian StyleNarayanan, Sruthi, and Benjamin Fallen. 2019. "Evaluation of Soybean Plant Introductions for Traits that can Improve Emergence under Varied Soil Moisture Levels" Agronomy 9, no. 3: 118. https://doi.org/10.3390/agronomy9030118
APA StyleNarayanan, S., & Fallen, B. (2019). Evaluation of Soybean Plant Introductions for Traits that can Improve Emergence under Varied Soil Moisture Levels. Agronomy, 9(3), 118. https://doi.org/10.3390/agronomy9030118





