Effects of White Lupin and Groundnut on Fractionated Rhizosphere Soil P of Different P-Limited Soil Types in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Soils
2.2. Plant Growth Conditions
2.3. Plant and Soil Analyses
2.4. Data Analysis
3. Results
3.1. Plant Growth and P Uptake
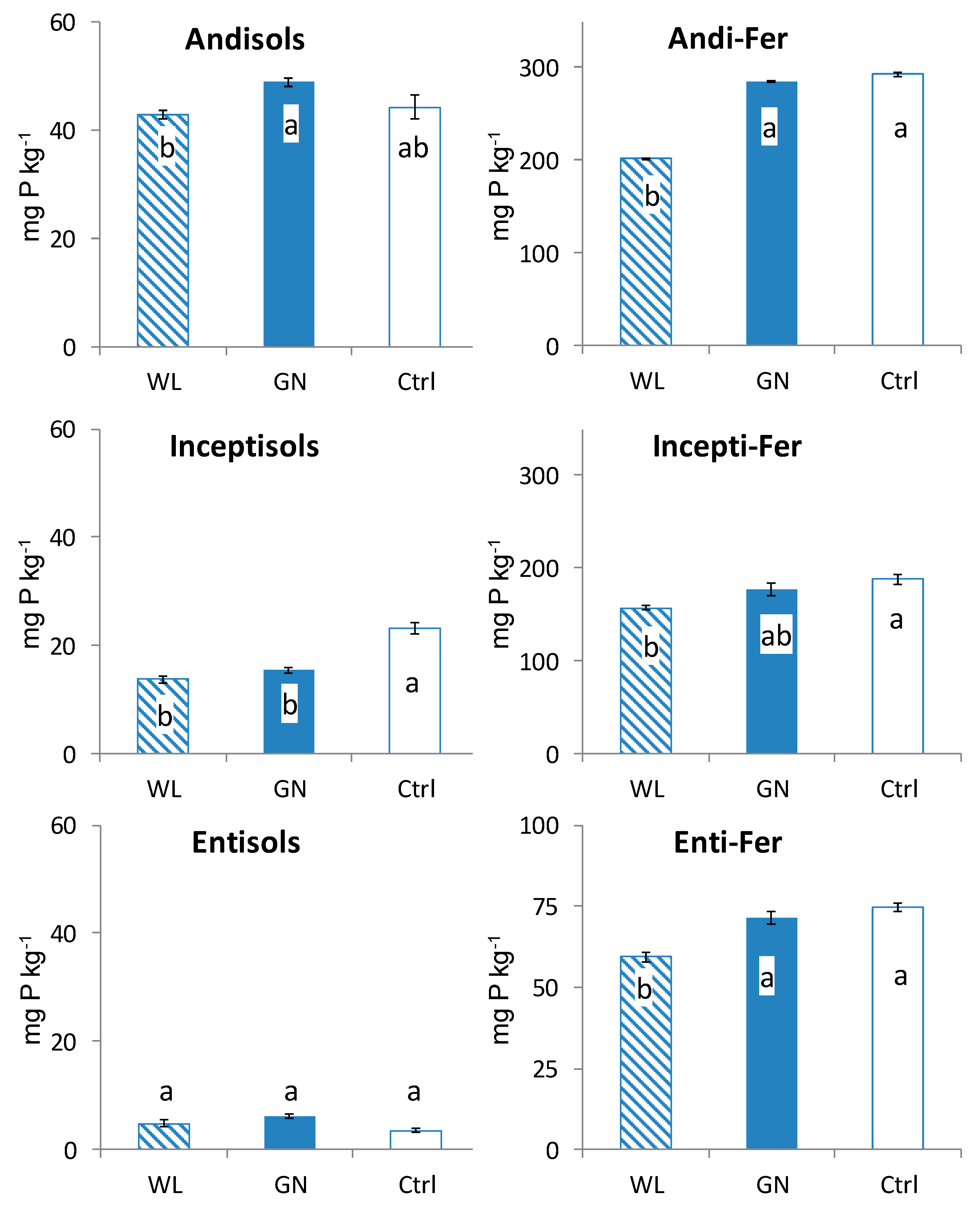
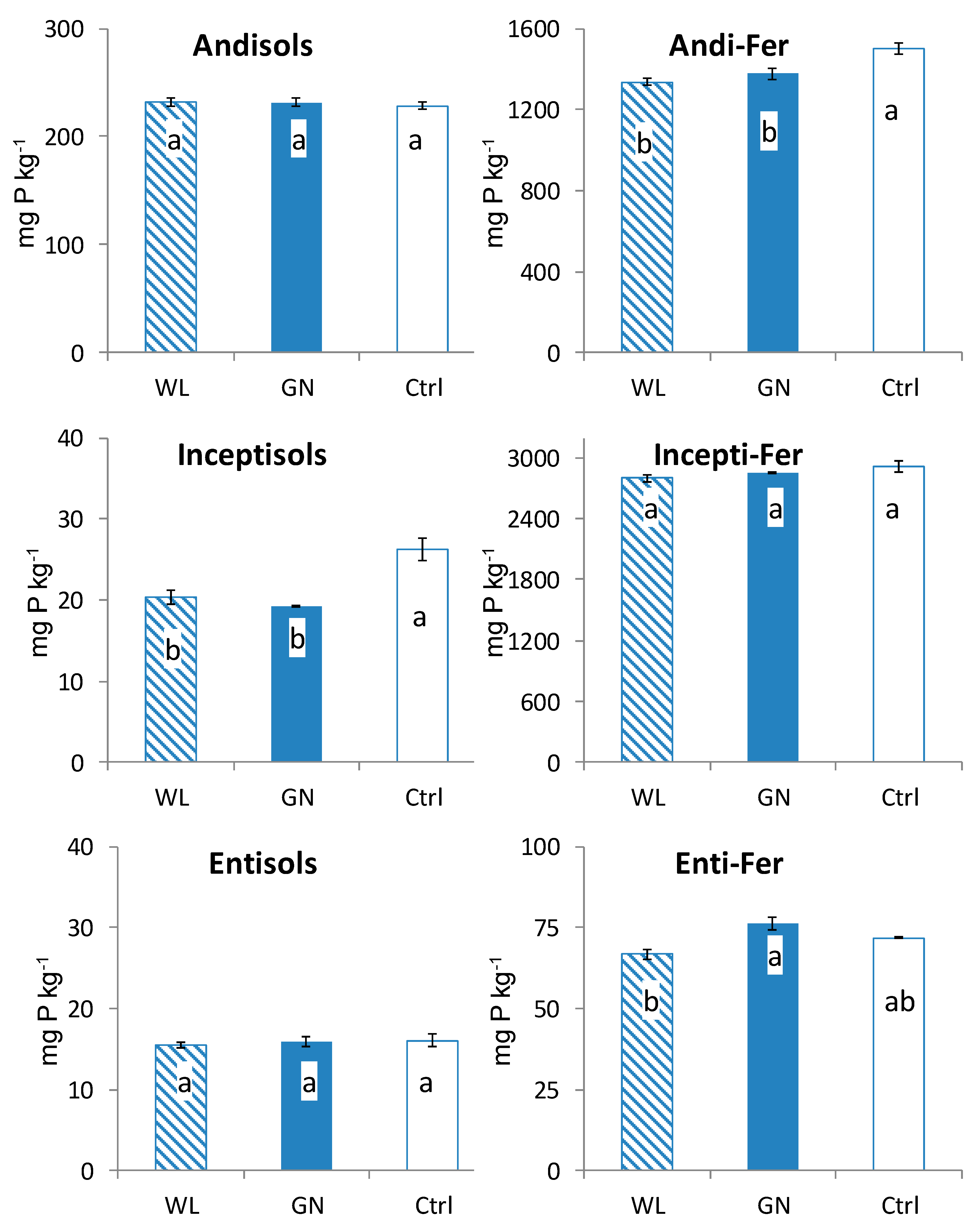
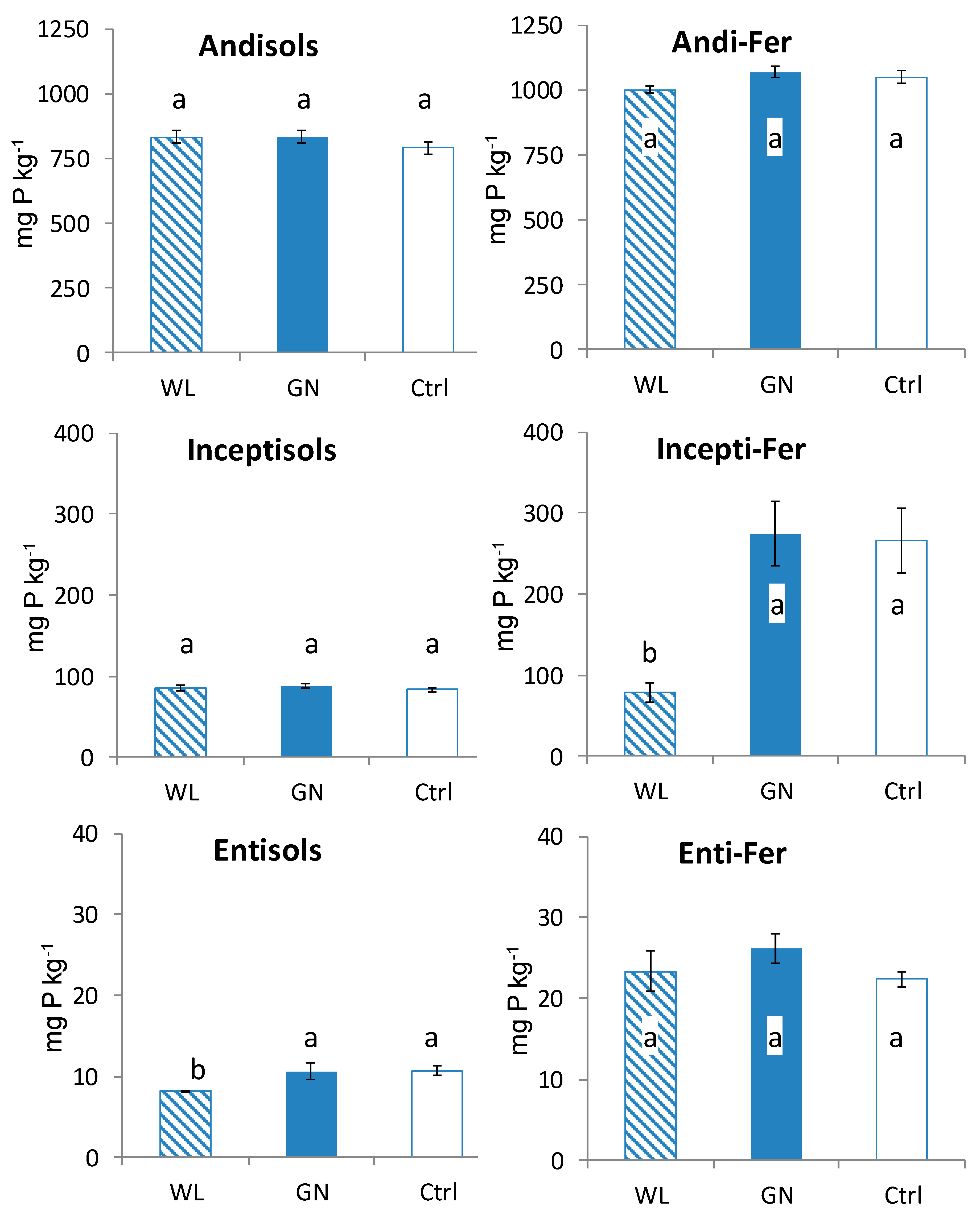
3.2. Fractionated P of Rhizosphere Soil
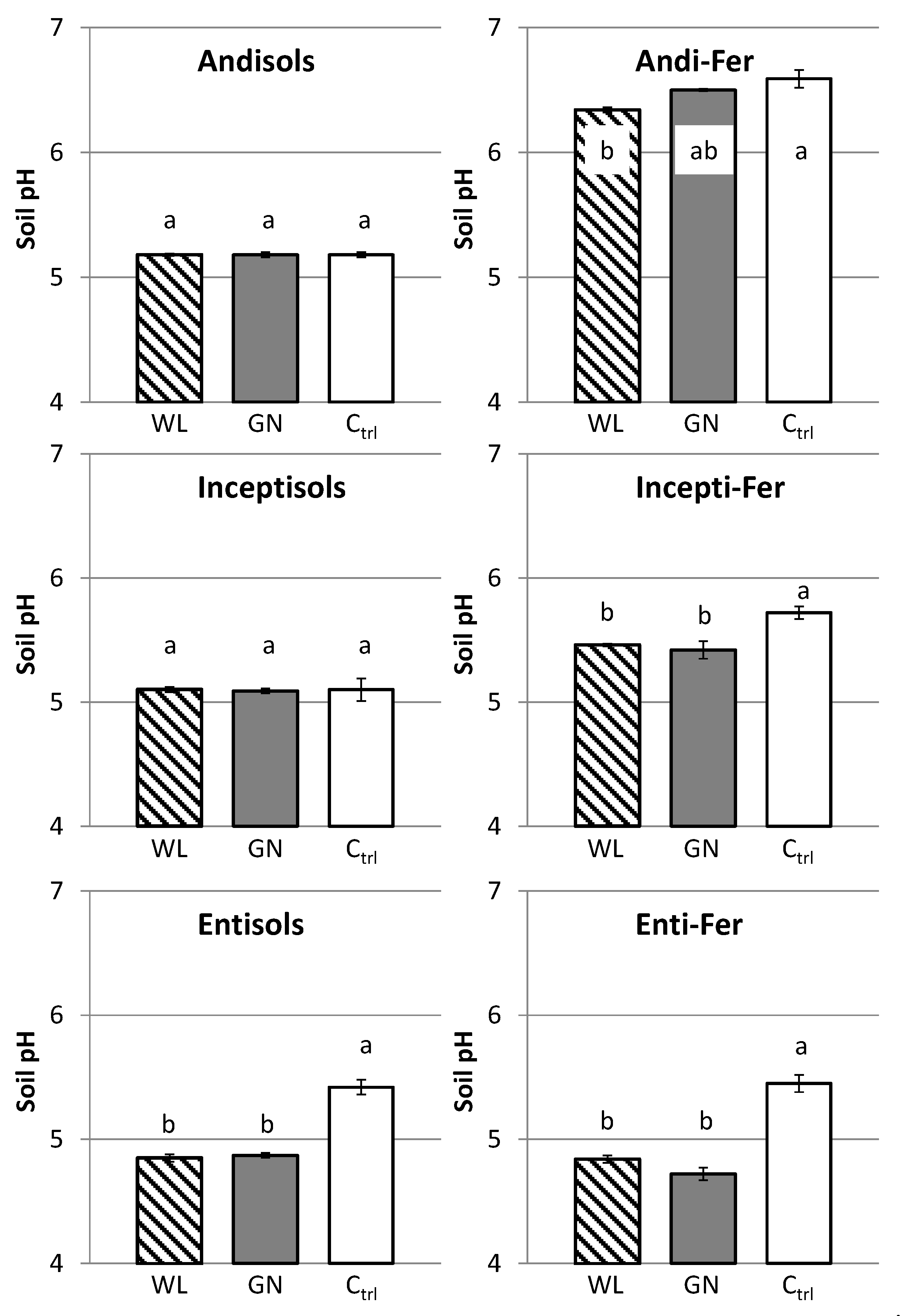
3.3. Rhizosphere Soil pH
4. Discussion
4.1. Comparison of Fractionated Rhizosphere Soil P Dynamics between WL and GN
4.2. Effect of Soil Physico-Chemical Properties on Rhizosphere Soil P Dynamics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holford, I.C.R. Soil phosphorus; its measurement, and its uptake by plants. Aust. J. Soil Res. 1997, 35, 227–239. [Google Scholar] [CrossRef]
- Takahashi, T.; Dahlgren, R.A. Nature, properties and function of aluminum–humus complexes in volcanic soils. Geoderma 2016, 263, 110–121. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Lambers, H.; Bolland, M.D.A.; Veneklaas, E.J. Phosphorus benefits of different legume crops to subsequent wheat grown in different soils of Western Australia. Plant Soil 2005, 271, 175–187. [Google Scholar] [CrossRef]
- Raghothama, K.G.; Karthikeyan, A.S. Phosphate acquisition. Plant Soil 2005, 274, 37–49. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Bouwman, A.F.; Beusen, A.H.W. Phosphorus demand for the 1970–2100 period: A scenario analysis of resource depletion. Glob. Environ. Chang. 2010, 20, 428–439. [Google Scholar] [CrossRef]
- Rose, T.J.; Hardiputra, B.; Rengel, Z. Wheat, canola and grain legume access to soil phosphorus fractions differs in soils with contrasting phosphorus dynamics. Plant Soil 2010, 326, 159–170. [Google Scholar] [CrossRef]
- Hassan, H.M.; Hasbullah, H.; Marschner, P. Growth and rhizosphere P pools of legume-wheat rotations at low P supply. Biol. Fertil. Soils 2013, 49, 41–49. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Lambers, H.; Bolland, M.D.A.; Veneklaas, E.J. Phosphorus uptake by grain legumes and subsequently grown wheat at different levels of residual phosphorus fertilizer. Aust. J. Agric. Res. 2005, 56, 1041–1047. [Google Scholar] [CrossRef]
- Lambers, H.; Bishop, J.G.; Hopper, S.D.; Laliberte, E.; Zúñiga-Feest, A. Phosphorus-mobilization ecosystem engineering: The roles of cluster roots and carboxylate exudation in young P-limited ecosystems. Ann. Bot. 2012, 110, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Marschner, P.; Zhang, F. Phosphorus pools and other soil properties in the rhizosphere of wheat and legumes growing in three soils in monoculture or as a mixture of wheat and legme. Plant Soil 2012, 360, 271–298. [Google Scholar] [CrossRef]
- Maltais-Landry, G. Legumes have a greater effect on rhizosphere properties (pH, organic acids and enzyme activity) but a smaller impact on soil P compared to other cover crops. Plant Soil 2015, 394, 139–154. [Google Scholar] [CrossRef]
- Turner, B.L.; McKelvie, I.D.; Haygarth, P.M. Characterisation of water-extractable soil organic phosphorus by phosphatase hydrolysis. Soil Biol. Biochem. 2002, 34, 27–35. [Google Scholar] [CrossRef]
- Gerke, J.; Romer, W.; Jungk, A. The excretion of citric and malic acid by proteoid roots of Lupinus albus L.; effects on soil solution concentrations of phosphate, iron, and aluminum in the proteoid rhizosphere in samples of an oxisol and a luvisol. J. Plant Nutr. Soil Sci. 1994, 157, 289–294. [Google Scholar]
- Shane, M.W.; Lambers, H.; Cawthray, G.R.; Kuhn, A.J.; Schurr, U. Impact of phosphorus mineral source (Al-P or Fe-P) and pH on cluster-root formation and carboxylate exudation in Lupinus albus L. Plant Soil 2008, 304, 169–178. [Google Scholar] [CrossRef]
- Ae, N.; Otani, T.; Makino, T.; Tazawa, J. Role of cell wall of groundnut roots in solubilizing sparingly soluble phosphorus in soil. Plant Soil 1996, 186, 197–204. [Google Scholar] [CrossRef]
- Shibata, R.; Yano, K. Phosphorus acquisition from non-labile sources in peanut and pigeonpea with mycorrhizal interaction. Appl. Soil Ecol. 2003, 24, 133–141. [Google Scholar] [CrossRef]
- Ae, N.; Arihara, J.; Okada, K.; Yoshihara, T.; Johansen, C. Phosphorus uptake by pigeon pea and its role in cropping system of the Indian subcontinent. Science 1990, 248, 447–480. [Google Scholar] [CrossRef]
- Gerke, J. The acquisition of phosphate by higher plants: Effect of carboxylate release by the roots. A critical review. J. Plant Nutr. Soil Sci. 2015, 178, 351–364. [Google Scholar] [CrossRef]
- Hinsinger, H. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Sugihara, S.; Tomita, Y.; Nishigaki, T.; Kilasara, M.; Wasaki, J.; Funakawa, S. Effects of different phosphorus-efficient lugumes and soil texture on fractionated rhizosphere soil phosphorus of strongly weathered soils. Biol. Fertil. Soils 2016, 52, 367–376. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.S.B.; Maruyama, H.; Masuda, G.; Wasaki, J. Interspecific facilitation of P acquisition in intercropping of maize with white lupin in two contrasting soils as influenced by different rates and forms of P supply. Plant Soil 2015, 390, 223–236. [Google Scholar] [CrossRef]
- Gunjigake, N.; Wada, K. Effects of phosphorus concentration and pH on phosphate retention by active aluminiumu and iron of Ando Soils. Soil Sci. 1981, 132, 347–352. [Google Scholar] [CrossRef]
- Otani, T.; Ae, N. The status of inorganic and organic phosphorus in some soils in relation to plant availability. Soil Sci. Plant Nutr. 1997, 43, 419–429. [Google Scholar] [CrossRef]
- Freese, D.; van der Zee, S.E.A.T.M.; van Riemsdijk, W.H. Comparison of different models for phosphate sorption as a function of the iron and aluminium oxides of soils. J. Soil Sci. 1992, 43, 729–738. [Google Scholar] [CrossRef]
- Takahashi, S.; Anwar, M.R. Wheat grain yield, phosphorus uptake and soil phosphorus fraction after 23 years of annual fertilizer application to an Andosol. Field Crop. Res. 2007, 101, 160–171. [Google Scholar] [CrossRef]
- Kinjo, K.; Tokashiki, Y.; Kitou, M. Chemical and Mineralogical properties and humic substances of soils cultivated with sugacane in Kita and Minami Daito Island, Japan. Res. Trop. Agric. 2009, 2, 80–84. (In Japanese) [Google Scholar]
- Otani, T.; Ae, N.; Tanaka, H. Phosphorus (P) uptake mechanisms of crops grown in soils gwoth low P status. Soil Sci. Plant Nutr. 1996, 42, 553–560. [Google Scholar] [CrossRef]
- Nwoke, O.C.; Vanlauwe, B.; Diels, J.; Sanginga, N.; Osounbi, O.; Merckx, R. Assessment of labile phosphorus fractions and adsorption characteristics in relation to soil properties of West African savanna soils. Agric. Ecosyst. Environ. 2003, 100, 285–294. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Lambers, H.; Bolland, M.D.A.; Veneklaas, E.J. Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant Soil 2006, 281, 109–120. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Wright, A.L. Soil phosphorus stocks and distribution in chemical fractions for long-term sugarcane, pasture, turfgrass, and forest systems in Florida. Nutr. Cycl. Agroecosyst. 2009, 83, 223–231. [Google Scholar] [CrossRef]
- Soil Survey Staff. Key to Soil Taxonomy, 10th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2006.
- Sekiya, K. Phosphoric acid. In Analysis Methods for Measuring Soil Fertility; Ishizawa, S., Ed.; Yokendo Co. Ltd.: Tokyo, Japan, 1970; pp. 251–253. [Google Scholar]
- Murphy, J.; Reley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Kitson, R.; Mellon, M.G. Colorimetric determination of phosphorus as molybdivanadophosporic acid. Ind. Eng. Chem. Anal. Ed. 1944, 16, 379–383. [Google Scholar] [CrossRef]
- Negassa, W.; Leinweber, P. How does the Hedley sequential phosphorus fractionation reflect impacts of land use and management on soil phosphorus: A review. J. Soil Sci. Plant Nutr. 2009, 172, 305–325. [Google Scholar] [CrossRef]
- Wissuwa, M.; Ae, N. Genotypic variation for phosphorus uptake from hardly soluble ironphosphate in groundnut (Arachis hypogaea L.). Plant Soil 1999, 206, 163–171. [Google Scholar] [CrossRef]
- Li, H.G.; Shen, J.B.; Zhang, F.S.; Marschner, P.; Cawthray, G.; Rengel, Z. Phosphorus uptake and rhizosphere properties of intercropped and monocropped maize, faba bean, and white lupin in acidic soil. Biol. Fertil. Soils 2010, 46, 79–91. [Google Scholar] [CrossRef]
- Wasaki, J.; Yamamura, T.; Shinano, T.; Osaki, M. Secreted acid phosphatase is expressed in cluster roots of lupin in response to phosphorus deficiency. Plant Soil 2003, 248, 129–136. [Google Scholar] [CrossRef]
- Ae, N.; Shen, R.F. Root cell-wall properties are proposed to contribute to phosphorus (P) mobilization by groundnut and pigeonpea. Plant Soil 2002, 245, 95–103. [Google Scholar] [CrossRef]
- Ohwaki, Y.; Hirata, H. Differences in carboxylic acid exudation among p-starved leguminous crops in relation to carboxylic acid contents in plant tissues and phospholipid level in roots. Soil Sci. Plant Nutr. 1992, 38, 235–243. [Google Scholar] [CrossRef]
- Wang, X.; Pearse, S.J.; Lambers, H. Cluster-root formation and carboxylate release in three Lupinus species as dependent on phosphorus supply, internal phosphorus concentration and relative growth rate. Ann. Bot. 2013, 112, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Le Bayon, R.C.; Weisskopf, L.; Martinoia, E.; Jansa, J.; Frossard, E.; Keller, F.; Follmi, K.B.; Gobat, J.M. Soil Phosphorus Uptake by Continuously Cropped Lupinus albus: A New Microcosm Design. Plant Soil 2006, 283, 309–321. [Google Scholar] [CrossRef]
- George, T.S.; Gregory, P.J.; Robinson, J.S.; Buresh, R.J. Changes in phosphorus concentrations and pH in the rhizosphere of some agroforestry and crop species. Plant Soil 2002, 246, 65–73. [Google Scholar] [CrossRef]
- Wagai, R.; Kajiura, M.; Asano, M.; Hiradate, S. Nature of soil organo-mineral assemblage examined by sequential density fractionation with and without sonication: Is allophanic soil different? Geoderma 2015, 241, 295–305. [Google Scholar] [CrossRef]
- Rasmussen, C.; Heckman, K.; Wieder, W.R.; Keiuluweit, M.; Lawrence, C.R. Beyond clay: Towards an improved set of variables for predicting soil organic matter content. Biogeochemistry 2018, 137, 297–306. [Google Scholar] [CrossRef]
- Turner, B.L.; Cade-Menun, B.J.; Condron, L.M.; Newman, S. Extraction of soil organic phosphorus. Talanta 2005, 66, 294–306. [Google Scholar] [CrossRef]
- Velásquez, G.; Ngo, P.T.; Rumpel, C.; Calabi-Floody, M.; Redel, Y.; Turner, B.L.; Condron, L.M.; Mora, M.L. Chemical nature of residual phosphorus in Andisols. Geoderma 2016, 271, 27–31. [Google Scholar] [CrossRef]
- Kar, G.; Hilger, D.; Schienau, J.J.; Peak, D. Effects of plant growth and time on phosphorus speciation in a manure-amended Prairie soil under controlled conditions. Rhizosphere 2017, 4, 1–8. [Google Scholar] [CrossRef]
| Soil pH | TC | TN | Clay (%) | Alo | Feo | Ald | Fed | Bray-P (mg P kg−1) | P Ads. Cap. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (H2O) | (KCl) | (g kg−1) | (g kg−1) | (mg P2O5 100 g−1) | |||||||
| Andisols | 5.3 | 4.6 | 94.3 | 6.5 | 27.9 | 63.8 | 29.6 | 21.5 | 55.5 | 11.7 | 2237.2 |
| Andi-Fer | 6.7 | 5.6 | 90.7 | 7.1 | 36.6 | 65.9 | 28.3 | 19.1 | 59.6 | 112.9 | 2072.3 |
| Inceptisosl | 5.1 | 3.2 | 18.4 | 1.9 | 30.7 | 2.9 | 1.6 | 5.4 | 35.3 | 9.4 | 686.5 |
| Incepti-Fer | 5.1 | 4.3 | 19.8 | 2.1 | 88.7 | 2.2 | 1.9 | 10.1 | 61.0 | 373.0 | 816.2 |
| Entisols | 5.5 | 4.1 | 2.4 | 0.2 | 4.4 | 0.5 | 0.4 | 0.1 | 4.4 | 6.5 | 130.6 |
| Enti-Fer | 5.5 | 3.4 | 2.6 | 0.3 | 6.0 | 1.5 | 0.6 | 1.1 | 4.8 | 119.2 | 130.2 |
| Biomass (g pot−1) | P Concentration (mg P g−1) | P Uptake (mg P pot−1) | |||||
|---|---|---|---|---|---|---|---|
| Above | Below | Above | Below | Above | Below | Total | |
| White Lupin | |||||||
| Anidsols | 1.58 cdB | 0.37 dB | 0.66 dA | 0.62 cA | 1.04 cB | 0.23 cB | 1.27 dB |
| (0.06) | (0.03) | (0.04) | (0.02) | (0.07) | (0.03) | (0.07) | |
| Andi-Fer | 7.24 aB | 2.46 aB | 3.32 bA | 2.06 bB | 23.85 bB | 5.09 bB | 28.94 bB |
| (0.43) | (0.22) | (0.17) | (0.11) | (1.25) | (0.59) | (1.75) | |
| Inceptisols | 0.63 dB | 0.43 dB | 0.84 dA | 0.71 cA | 0.53 cB | 0.29 cB | 0.82 dB |
| (0.10) | (0.16) | (0.03) | (0.03) | (0.10) | (0.10) | (0.18) | |
| Incepti-Fer | 8.34 aA | 2.14 abB | 5.08 aA | 3.36 aA | 42.61 aA | 7.27 aB | 49.87 aA |
| (0.59) | (0.17) | (0.19) | (0.25) | (3.92) | (0.95) | (4.77) | |
| Entisols | 2.06 bcB | 1.41 cB | 1.09 dA | 0.87 cB | 2.26 cB | 1.20 cB | 3.45 cdB |
| (0.08) | (0.18) | (0.05) | (0.06) | (0.11) | (0.12) | (0.18) | |
| Enti-Fer | 3.20 bB | 1.53 bcB | 2.44 cB | 2.29 bA | 7.83 cB | 3.48 bB | 11.31 cB |
| (0.19) | (0.05) | (0.20) | (0.12) | (0.85) | (0.17) | (0.93) | |
| Groundnut | |||||||
| Anidsols | 5.40 dA | 2.88bcdA | 0.49 dB | 0.70 cA | 2.64 cA | 2.00 cA | 4.64 cA |
| (0.31) | (0.17) | (0.01) | (0.04) | (0.18) | (0.14) | (0.29) | |
| Andi-Fer | 18.34 aA | 5.46 aA | 2.28 bB | 2.50 bA | 41.75 aA | 13.61 aA | 55.36 aA |
| (0.68) | (0.25) | (0.09) | (0.02) | (1.69) | (0.53) | (1.91) | |
| Inceptisols | 6.25 dA | 2.50 cdA | 0.49 cdB | 0.46 cB | 3.04 cA | 1.14 cA | 4.18 cA |
| (0.34) | (0.15) | (0.01) | (0.03) | (0.18) | (0.07) | (0.22) | |
| Incepti-Fer | 9.76 cA | 3.88 bA | 2.65 abB | 3.58 aA | 25.42 bB | 14.23 aA | 39.65 bA |
| (0.56) | (0.35) | (0.23) | (0.31) | (1.12) | (2.22) | (3.07) | |
| Entisols | 6.62 dA | 2.52 dA | 0.98 cA | 1.20 cA | 6.38 cA | 3.00 cA | 9.38 cA |
| (0.35) | (0.16) | (0.08) | (0.07) | (0.22) | (0.16) | (0.25) | |
| Enti-Fer | 13.00 bA | 3.60 bcA | 2.95 aA | 2.18 bA | 38.20 aA | 7.77 bA | 45.97 bA |
| (0.58) | (0.26) | (0.10) | (0.12) | (1.65) | (0.43) | (1.53) | |
| Plant P Uptake | ||
|---|---|---|
| Source | White lupin | Groundnut |
| F value | F value | |
| Soil types (S) | 34.0 *** | 13.2 *** |
| Land management (LM) | 263.6 *** | 965.0 *** |
| S × LM | 44.5 *** | 14.0 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, K.; Sugihara, S.; Wasaki, J.; Tanaka, H. Effects of White Lupin and Groundnut on Fractionated Rhizosphere Soil P of Different P-Limited Soil Types in Japan. Agronomy 2019, 9, 68. https://doi.org/10.3390/agronomy9020068
Imai K, Sugihara S, Wasaki J, Tanaka H. Effects of White Lupin and Groundnut on Fractionated Rhizosphere Soil P of Different P-Limited Soil Types in Japan. Agronomy. 2019; 9(2):68. https://doi.org/10.3390/agronomy9020068
Chicago/Turabian StyleImai, Kaoru, Soh Sugihara, Jun Wasaki, and Haruo Tanaka. 2019. "Effects of White Lupin and Groundnut on Fractionated Rhizosphere Soil P of Different P-Limited Soil Types in Japan" Agronomy 9, no. 2: 68. https://doi.org/10.3390/agronomy9020068
APA StyleImai, K., Sugihara, S., Wasaki, J., & Tanaka, H. (2019). Effects of White Lupin and Groundnut on Fractionated Rhizosphere Soil P of Different P-Limited Soil Types in Japan. Agronomy, 9(2), 68. https://doi.org/10.3390/agronomy9020068





