Development of Sakon Nakhon Rice Variety for Blast Resistance through Marker Assisted Backcross Breeding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population Development and Masker-Assisted Selection
2.2. Greenhouse Validation
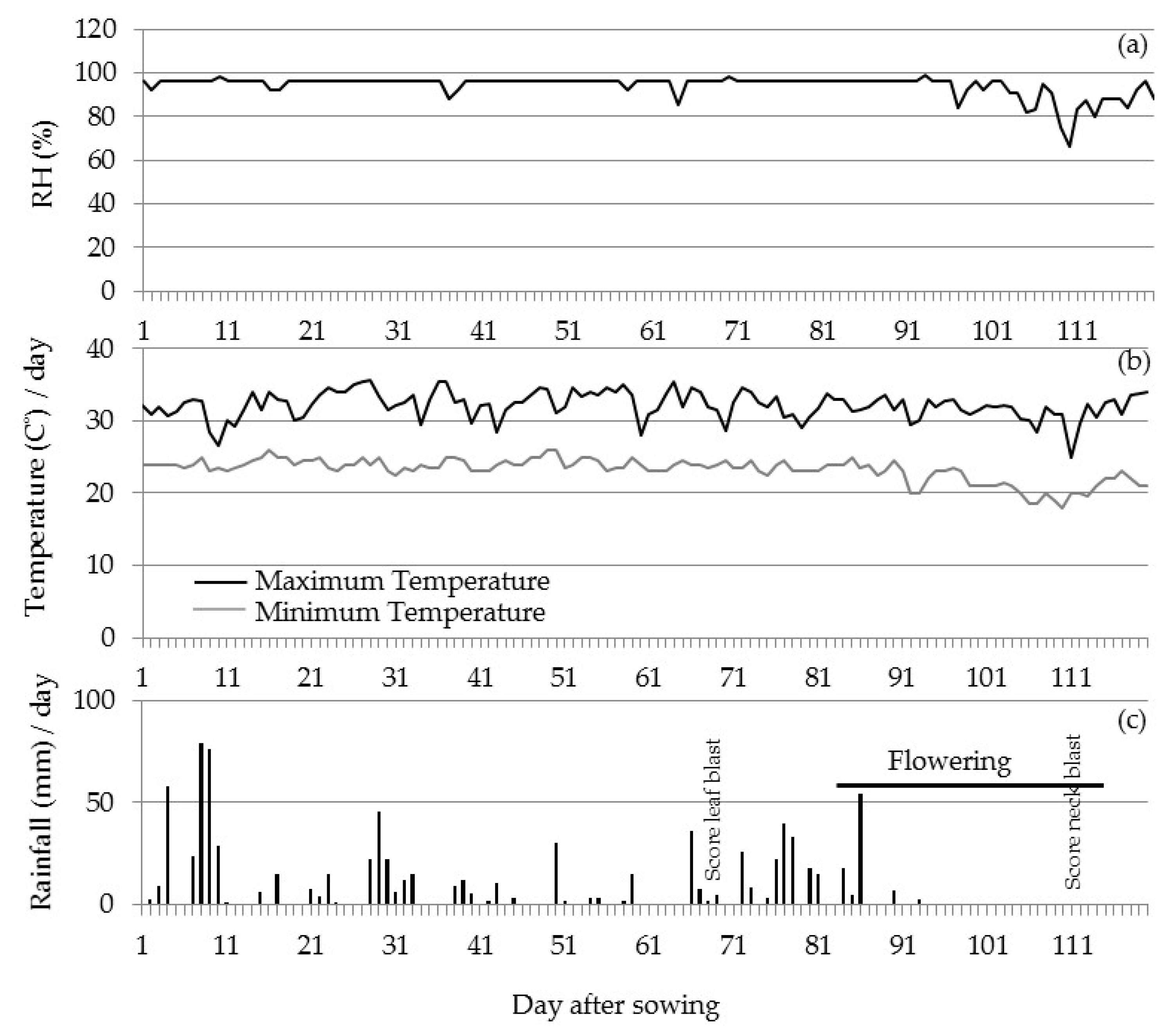
2.3. Field Validation
2.4. Evaluation of Seed Quality
2.5. Data Analysis
3. Results
3.1. Development of Backcross Introgression Lines (ILs) via MAS
3.2. Blast Resistance Validation
3.2.1. Greenhouse Validation
3.2.2. Field Validation
3.3. Agronomic and Yield Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Office of Agriculture Economics. Agricultural Statistics of THAILAND 2015; Annual report of 2015; Ministry of Agriculture and Cooperatives: Bangkok, Thailand, 2015; p. 240.
- Kharkwal, M.C.; Shu, Q.Y. The Role of Induced Mutations in World Food Security. In Induced Plant Mutations in the Genomics Era; Shu, G.Y., Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- Lanceras, J.C.; Huang, Z.L.; Naivikul, O.; Vanavichit, A.; Ruanjaichon, V.; Tragoonrung, S. Mapping of genes for cooking and eating qualities in Thai Jasmine rice (KDML 105). DNA Res. 2000, 7, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.P.; Pathak, P.; Sahrawat, K.L. Community Watershed Management for Sustainable Intensification in Northeast Thailand; International Crops Research Institute for the Semi-Arid Tropics: Andhra Pradesh, India, 2012; p. 152. [Google Scholar]
- Jaruchai, W.; Monkham, T.; Chankaew, S.; Suriharn, B.; Sanitchon, J. Evaluation of stability and yield potential of upland rice genotypes in north and northeast Thailand. J. Integr. Agric. 2017, 16, 1–9. [Google Scholar] [CrossRef]
- Prasunluk, N.; Athipanyakul, T. Production and marketing management of Sakon Nakhon rice and purple rice varieties in Khon Kaen province. KKU J. Health Sci. 2014, 2, 51–59. [Google Scholar]
- Voraphab, I.; Hanboonsong, Y. The population of insect vector (Matsumuratettix hiroglyphicus (Matsumura)) and the incidence of sugarcane white leaf disease from upland rice rotation with sugarcane and sugarcane mono cropping system. Khon Kaen Agric. J. 2012, 3, 287–292. [Google Scholar]
- Scardaci, S.C.; Webster, R.K.; Greer, C.A.; Hill, J.E.; William, J.F.; Mutters, R.G.; Brandon, D.M.; McKenzie, K.S.; Oster, J.J. Rice Blast: A New Disease in California; Agronomy Fact Sheet Series; Department of Agronomy and Range Science, University of California: Davis, CA, USA, 1997. [Google Scholar]
- Kiyosawa, S. Genetics and epidemiological modeling of breakdown of plant disease resistance. Phytopathology 1982, 20, 93–117. [Google Scholar] [CrossRef]
- Hu, H.-Y.; Zhuang, J.-Y.; Chai, R.-Y.; Wu, J.-L.; Fan, Y.-Y.; Zheng, K.-L. Isolation and characterization of defense response genes involved in neck blast resistance of rice. Acta Genet. Sin. 2006, 33, 251–261. [Google Scholar] [CrossRef]
- Talbot, N.J. On the trail of a serial Killer: Exploring the biology of Magnaporthe grisea. Microbiology 2003, 57, 177–202. [Google Scholar] [CrossRef]
- Katsube, T.; Koshimizu, Y. Influence of blast disease on harvest of rice plant. 1. Effect of panicle infection on yield components and quality. Bull. Tohoku Agric. Exp. Stn. 1970, 39, 55–96. [Google Scholar]
- Skamnioti, P.; Gurr, S.J. Against the grain: Safeguarding rice from rice blast disease. Trends Biotechnol. 2009, 27, 141–150. [Google Scholar] [CrossRef]
- Eamchit, S.; Mew, T.W. Comparison of virulence of Xanthomonas campestris pv. oryzae in Thailand and the Philippines. Plant Dis. 1982, 66, 556–559. [Google Scholar]
- Jongdee, B.; Pantuwan, G.; Fukai, S.; Fischer, K. Improving drought tolerance in rainfed lowland rice: An example from Thailand. “New directions for a diverse planet”. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004; pp. 1–14. [Google Scholar]
- Michiaki, I. Probenazole—A plant defense activator. R. Soc. Chem. 2001, 12, 28–31. [Google Scholar]
- Ashkani, S.; Rafii, M.; Shabanimofrad, M.; Miah, G.; Sahebi, M.; Azizi, P.; Tanweer, F.A.; Akhtar, M.S.; Nasehi, A. Molecular breeding strategy and challenges towards improvement of blast disease resistance in rice crop. Front. Plant Sci. 2015, 6, 886. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.R.; Rai, A.K.; Gupta, S.K.; Vijayan, J.; Devenna, B.N.; Ray, S. Rice blast management through host-plant resistance: Retrospect and prospect. Agric. Res. 2012, 1, 37–52. [Google Scholar] [CrossRef]
- Chen, J.; Shi, Y.; Liu, W.; Chai, R.; Fu, Y.; Zhuang, J.; Wu, J. A Pid3 allele from rice cultivar Gumei2 confers resistance to Magnaporthe oryzae. J. Genet. Genom. 2011, 38, 209–216. [Google Scholar] [CrossRef]
- Ellur, R.K.; Khanna, A.; Yadav, A.; Pathaniaa, S.; Rajashekarab, H.; Singha, V.K.; Krishnana, S.G.; Bhowmicka, P.K.; Nagarajand, M.; Vinodd, K.K.; et al. Improvement of Basmati rice varieties for resistance to blast and bacterial blight diseases using marker assisted backcross breeding. Plant Sci. 2016, 242, 330–341. [Google Scholar] [CrossRef]
- Sharma, B.; Pandey, M.P. Identification of rice germplasm with resistance to bacterial blight (Xanthomonas oryzae pv. oryzae). Bangladesh J. Agric. Res. 2012, 37, 349–353. [Google Scholar] [CrossRef]
- Basavaraj, S.H.; Singh, V.K.; Singh, A.; Singh, A.; Singh, A.; Yadav, S.; Ellur, R.K.; Singh, D.; Gopalakrishnan, S.; Nagarajan, M.; et al. Marker-assisted improve of bacterial blight resistance in parental lines of Pusa RH10, a superfine grain aromatic rice hybrid. Mol. Breed. 2010, 26, 293–305. [Google Scholar] [CrossRef]
- Sivolap, Y.M. Molecular markers and plant breeding. Cytol. Genet. 2013, 47, 188–195. [Google Scholar] [CrossRef]
- Koide, Y.; Kobayashi, N.; Xu, D.; Fukuta, Y. Resistance gene and selection DNA marker for blast disease in rice (Oryza sativa L.). Jpn. Agric. Res. Q. 2009, 43, 255–280. [Google Scholar] [CrossRef]
- Fjellstrom, R.; Conaway-Bormans, C.A.; McClung, A.M.; Marchetti, M.A.; Shank, A.R.; Park, W.D. Development of DNA markers suitable for marker-assisted selection of three Pi genes conferring resistance to multiple Pyricularia grisea pathotypes. Crop Sci. 2004, 44, 1790–1798. [Google Scholar] [CrossRef]
- Silprakhon, S. Identification and mapping genes controlling leaf blast resistance in double haploid lines IR64× Azucena population. In Proceedings of the 1st International Conference on Rice for Future, Bangkok, Thailand, 31 August–3 September 2004. [Google Scholar]
- Noenplab, A.; Vanavichit, A.; Toojinda, T.; Sirithunya, P.; Tragoonrung, S.; Sriprakhon, S.; Vongsaprom, C. QTL mapping for leaf and neck blast resistance in Khao Dawk Mali105 and Jao Hom Nin recombinant inbred lines. Sci. Asia 2006, 33, 133–142. [Google Scholar] [CrossRef]
- Suwannual, T.; Chankaew, S.; Monkham, T.; Saksirirat, W.; Sanitchon, J. Pyramiding of four blast resistance QTLs into Thai rice cultivar RD6 through marker-assisted selection. Plant Breed. 2017, 53, 1–8. [Google Scholar] [CrossRef]
- Manivong, P.; Korinsak, S.; Korinsak, S.; Siangliw, J.L.; Vanavichit, A.; Toojinda, T. Marker-assisted selection to improve submergence tolerance, blast resistance and strong fragrance in glutinous rice. Thai J. Genet. 2014, 7, 110–122. [Google Scholar]
- Singh, A.K.; Gopalakrishnan, S.; Singh, V.P.; Prabhu, K.V.; Mohapatra, T.; Singh, N.K.; Sharma, T.; Nagarajan, M.; Vinod, K.K. Marker assisted selection: A paradigm shifts in Basmati breeding. Indian J. Genet. 2011, 71, 1–9. [Google Scholar]
- Sreewongchai, T.; Toojinda, T.; Thanintorn, N.; Kosawang, C.; Vanavichit, A.; Tharreau, D.; Sirithunya, P. Development of elite indica rice lines with wild spectrum of resistance to Thai blast isolates by pyramiding multiple resistance QTLs. Plant Breed. 2010, 129, 176–180. [Google Scholar] [CrossRef]
- Wongsaprom, C.; Sirithunya, P.; Vanavichit, A.; Pantuwan, G.; Jongdee, B.; Sidhiwong, N.; Siangliw, J.L.; Toojinda, T. Two introgressed quantitative trait loci confer a broad-spectrum resistance to blast disease in the genetic background of the cultivar RD6, a Thai glutinous jasmine rice. Field Crop Res. 2010, 199, 245–251. [Google Scholar] [CrossRef]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant Mol. Biol. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- International Rice Research Institute. Standard Evaluation System for Rice (SES); International Rice Research Institute: Manila, Philippines, 2002. [Google Scholar]
- Yi, M.; New, K.; Vanavichit, A.; Chai-arree, W.; Toojinda, T. Marker assisted backcross breeding to improve cooking quality traits in Myanmar rice cultivar Manawthukha. Field Crop Res. 2007, 113, 178–186. [Google Scholar] [CrossRef]
- Sirithanya, P. Mapping Gene Controlling Blast Resistance in Rice (Oryza sativa L.). Ph.D. Thesis, Kasetsart University, Bangkok, Thailand, 1998. [Google Scholar]
- Ahn, S.W. International collaboration on breeding for resistance to rice blast. In Rice Blast Disease; Zeigler, R.S., Leong, S.A., Teng, P.S., Eds.; Centre for Agriculture and Bioscience International (CABI): Wallingford, UK, 1994; pp. 137–153. [Google Scholar]
- Witcombe, J.R.; Virk, D.S. Number of crosses and population size for participatory and classical plant breeding. Euphytica 2001, 122, 451–462. [Google Scholar] [CrossRef]
- Hospital, F. Selection in backcross programmes. Philos. Trans. R. Soc. B 2005, 360, 1503–1511. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rafii, M.Y.; Ismail, M.R.; Mahmood, M.; Rahim, H.A.; Alam, M.A.; Ashkani, S.; Malek, M.A.; Latif, M.A. Marker-assisted backcrossing: A useful method for rice improvement. Taylor Francis J. 2015, 29, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Waiyalert, A.; Sreewongchai, T.; Chaisan, T.; Sripichit, P. Mapping of blast disease resistance genes in BC2F6 population of the cross KDML105 × IR64. Kasetsart J. 2015, 49, 327–334. [Google Scholar]
- Ballini, E.; Nguyen, T.T.T.; Morel, J.B. Diversity and genetics of nitrogen induced susceptibility to the blast fungus in rice and wheat. Rice 2013, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N.K. Nutrient management for improving upland rice productivity and sustainability. Commun. Soil Sci. Plant Anal. 2007, 32, 2603–2629. [Google Scholar] [CrossRef]
- Pooja, K.; Katoch, A. Past, present and future of rice blast management. Plant Sci. 2014, 3, 165–173. [Google Scholar] [CrossRef]



| Lines and Varieties | QTLs | Blast Isolate | BSR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NKI11397 | KKN191082 | SKN205327 | KKU2016 | |||||||
| SKN 39-10-19-51 | - | 9.00 a | VS | 9.00 a | VS | 9.00 a | VS | 8.50 ab | VS | 0 |
| SKN 39-10-19-53 | - | 8.50 ab | VS | 9.00 a | VS | 8.83 a | VS | 8.83 a | VS | 0 |
| SKN 39-10-19-130 | - | 8.11 ab | VS | 9.00 a | VS | 5.83 bcd | S | 8.16 ab | VS | 0 |
| SKN 39-10-19-45 | 11 | 0.33 e | R | 0.83 c | R | 1.16 e | R | 0.66 e | R | 1 |
| SKN 39-10-19-57 | 11 | 0.33 e | R | 1.00 c | R | 0.41 e | R | 1.50 e | R | 1 |
| SKN 39-10-19-81 | 12 | 9.00 a | VS | 9.00 a | VS | 9.00 a | VS | 8.33 ab | VS | 0 |
| SKN 39-10-19-115 | 12 | 9.00 a | VS | 8.16 a | VS | 9.00 a | VS | 5.33 cd | MS | 0 |
| SKN 39-10-19-187 | 12 | 7.50 b | VS | 9.00 a | VS | 4.91 cd | MS | 6.83 abc | S | 0 |
| SKN 39-10-19-245 | 11, 12 | 0.50 e | R | 1.77 c | R | 0.83 e | R | 1.16 e | R | 1 |
| SKN 39-10-19-247 | 11, 12 | 0.58 e | R | 1.16 c | R | 0.83 e | R | 1.00 e | R | 1 |
| SKN 39-10-19-212 | 11, 12 | 0.16 e | R | 0.72 c | R | 0.58 e | R | 1.00 e | R | 1 |
| SKN 39-10-19-137 | 11, 12 | 0.08 e | R | 0.83 c | R | 0.33 e | R | 1.41 e | R | 1 |
| SKN 39-10-19-29 | 11, 12 | 0.41 e | R | 0.66 c | R | 0.50 e | R | 0.83 e | R | 1 |
| SKN (recurrent parent) | - | 9.00 a | VS | 9.00 a | VS | 9.00 a | VS | 7.50 abc | VS | 0 |
| RD6 NIL (donor parent) | - | 0.66 e | R | 1.00 c | R | 0.91 e | R | 1.16 e | R | 1 |
| JHN (resistance check) | - | 4.66 c | MS | 9.00 a | VS | 4.25 d | MS | 6.16 bc | S | 0 |
| IR64 (resistance check) | - | 0.00 e | VR | 0.91 c | R | 0.25 e | R | 1.33 e | R | 1 |
| Azucena (resistance check) | - | 8.50 ab | VS | 7.66 a | VS | 8.00 ab | VS | 3.00 de | MR | 0 |
| P0489 (resistance check) | - | 2.83 d | MR | 6.00 b | S | 4.83 cd | MS | 1.16 e | R | 0.25 |
| KDML105 (susceptibility check) | 8.50 ab | VS | 9.00 a | VS | 9.00 a | VS | 7.16 abc | S | 0 | |
| RD6 (susceptibility check) | 8.83 a | VS | 9.00 a | VS | 7.50 abc | VS | 8.83 a | VS | 0 | |
| F-TEST | ** | ** | ** | ** | ||||||
| C.V.% | 29.69 | 25.05 | 50.97 | 40.05 | ||||||
| Lines & Varieties | QTLs | Leaf Blast | Neck Blast | Day to Flowering | |||
|---|---|---|---|---|---|---|---|
| Ban Haed | KKU | Ban Haed | KKU | Ban Haed | KKU | ||
| SKN 39-10-19-130-6 | - | 1.67 d | 5.00 c | 9 a | 9 a | 89 hi | 87 d |
| SKN 39-10-19-57-6 | 11 | 0.00 f | 0.00 d | 1 c | 1 d | 92 g | 88 c |
| SKN 39-10-19-187-9 | 12 | 1.67 d | 5.84 bc | 7 b | 8 b | 92 g | 84 e |
| SKN 39-10-19-247-7 | 11, 12 | 0.33 ef | 0.00 d | 1 c | 1 d | 98 d | 86 d |
| SKN 39-10-19-137-1 | 11, 12 | 0.00 f | 0.33 d | 1 c | 1 d | 96 de | 88 c |
| SKN 39-10-19-29-12 | 11, 12 | 0.00 f | 0.00 d | 1 c | 1 d | 95 ef | 90 b |
| SKN 39-10-19-29-13 | 11, 12 | 0.00 f | 0.00 d | 1 c | 1 d | 96 de | 90 b |
| SKN recurrent parent | - | 1.00 de | 4.78 c | 7 ab | 7 c | 93 fg | 89 b |
| RD6 NIL donor parent | 1, 2, 11, 12 | 0.00 f | 4.78 c | 1 c | 1 d | 122 ab | 106 a |
| JHN resistance check | 0.00 f | 0.00 d | 1 c | 1 d | 105 c | 106 a | |
| P0489 resistance check | 0.00 f | 0.00 d | 1 c | 1 d | 87 i | 106 a | |
| Thanyasirin resistance check | 3.00 c | 5.00 c | 1 c | 1 d | 124 a | 106 a | |
| ULR081 resistance check | 0.00 f | 0.00 d | 1 c | 1 d | 91 gh | 80 f | |
| ULR082 susceptibility check | 4.33 b | 8.78 a | 1 c | ND | 120 b | ND | |
| RD6 susceptibility check | 5.93 a | 6.56 b | 1 c | 1 d | 121 ab | 106 a | |
| KDML 105 susceptibility check | - | 9.00 a | - | ND | - | ND | |
| Grand Mean | 1.30 | 3.25 | 2.31 | 2.49 | 101.5 | 93.7 | |
| F-test | ** | ** | ** | ** | ** | ** | |
| C.V.% | 38.95 | 19.74 | 42.3 | 15.12 | 1.26 | 0.62 | |
| Line/Variety | QTLs | PH | NETP | PL | NGP | TGW | HI | GY |
|---|---|---|---|---|---|---|---|---|
| SKN 39-10-19-130-6 | - | 130 ab | 15 efg | 26 ab | 104 d | 31.2 a | 0.21 a | 3233 abc |
| SKN 39-10-19-57-6 | 11 | 135 a | 18 bcd | 26 ab | 109 cd | 29.8 ab | 0.17 ab | 2677 abc |
| SKN 39-10-19-187-9 | 12 | 137 a | 17 cdef | 26 ab | 118 bcd | 29.9 ab | 0.19 a | 3236 abc |
| SKN 39-10-19-247-7 | 11, 12 | 130 ab | 19 bc | 25 abc | 121 bcd | 31.1 a | 0.20 a | 3724 a |
| SKN 39-10-19-137-1 | 11, 12 | 140 a | 16 def | 25 bc | 116 cd | 31.6 a | 0.17 ab | 2618 abc |
| SKN 39-10-19-29-12 | 11, 12 | 132 ab | 17 cde | 27 ab | 121 bcd | 29.2 ab | 0.21 a | 3493 ab |
| SKN 39-10-19-29-13 | 11, 12 | 127 abc | 17 cdef | 26 ab | 119 bcd | 28.8 ab | 0.21 a | 3123 abc |
| SKN | - | 132. ab | 16 cdef | 27 a | 115 cd | 30.8 a | 0.19 a | 3489 ab |
| RD6 NIL | 1, 2, 11, 12 | 120 bc | 19 bc | 27 ab | 154 ab | 27.2 bc | 0.13 bc | 3022 abc |
| JHN | 84 e | 23 a | 25 bc | 126 bcd | 22.5 d | 0.13 bc | 2163 abc | |
| Thanyasirin | 116 cd | 21 ab | 25 abc | 127 bcd | 26.9 bc | 0.12 bcd | 2145 abc | |
| P0489 | 105 d | 12 g | 25 abc | 172 a | 22.6 d | 0.17 ab | 2307 abc | |
| ULR081 | 121 bc | 19 bc | 23 c | 125 bcd | 29.9 ab | 0.20 a | 3298 abc | |
| ULR082 | 133 ab | 14 fg | 26 ab | 132 bcd | 28.6 ab | 0.08 d | 1568 c | |
| RD 6 | 106 d | 17 cdef | 27 ab | 143 abc | 24.4 cd | 0.08 cd | 1734 bc | |
| Mean | 123 | 17.2 | 25.7 | 127 | 28.3 | 0.17 | 2789 | |
| F-test | ** | ** | ** | ** | ** | ** | ** | |
| C.V.% | 6.64 | 10.44 | 4.51 | 9.86 | 6.59 | 18.24 | 21.54 |
| Line/Variety | QTLs | Fragrance | Whole Grain Size | Brown Rice Size | ||||
|---|---|---|---|---|---|---|---|---|
| Length | Breadth | L/B Ratio | Length | Breadth | L/B Ratio | |||
| SKN 39-10-19-130-6 | - | 1 | 10.81 b | 2.58 | 4.20 ab | 7.64 c | 2.10 c | 3.64 a |
| SKN 39-10-19-57-6 | 11 | 2 | 11.10 ab | 2.57 | 4.32 ab | 8.14 ab | 2.13 bc | 3.82 a |
| SKN 39-10-19-187-9 | 12 | 1 | 11.63 a | 2.68 | 4.34 ab | 7.98 abc | 2.18 ab | 3.65 a |
| SKN 39-10-19-247-7 | 11, 12 | 2 | 11.30 ab | 2.55 | 4.44 ab | 7.94 abc | 2.19 ab | 3.63 a |
| SKN 39-10-19-137-1 | 11, 12 | 1 | 11.02 ab | 2.71 | 4.07 b | 7.87 bc | 2.17 abc | 3.64 a |
| SKN 39-10-19-29-12 | 11, 12 | 2 | 11.12 ab | 2.69 | 4.12 ab | 8.28 a | 2.19 ab | 3.79 a |
| SKN 39-10-19-29-13 | 11, 12 | 2 | 11.39 ab | 2.66 | 4.28 ab | 7.94 abc | 2.11 c | 3.78 a |
| SKN | - | 2 | 11.64 a | 2.60 | 4.51 a | 7.96 abc | 2.14 bc | 3.73 a |
| RD6 NIL | 1, 2, 11, 12 | 2 | 9.91 c | 2.81 | 3.53 c | 7.24 d | 2.22 a | 3.26 b |
| Mean | 11.1 | 2.65 | 4.2 | 7.89 | 2.16 | 3.66 | ||
| F-test | ** | ns | ** | ** | * | ** | ||
| C.V. (%) | 3.8 | 3.95 | 5.74 | 2.87 | 1.92 | 3.46 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srichant, N.; Chankaew, S.; Monkham, T.; Thammabenjapone, P.; Sanitchon, J. Development of Sakon Nakhon Rice Variety for Blast Resistance through Marker Assisted Backcross Breeding. Agronomy 2019, 9, 67. https://doi.org/10.3390/agronomy9020067
Srichant N, Chankaew S, Monkham T, Thammabenjapone P, Sanitchon J. Development of Sakon Nakhon Rice Variety for Blast Resistance through Marker Assisted Backcross Breeding. Agronomy. 2019; 9(2):67. https://doi.org/10.3390/agronomy9020067
Chicago/Turabian StyleSrichant, Nawaporn, Sompong Chankaew, Tidarat Monkham, Petcharat Thammabenjapone, and Jirawat Sanitchon. 2019. "Development of Sakon Nakhon Rice Variety for Blast Resistance through Marker Assisted Backcross Breeding" Agronomy 9, no. 2: 67. https://doi.org/10.3390/agronomy9020067
APA StyleSrichant, N., Chankaew, S., Monkham, T., Thammabenjapone, P., & Sanitchon, J. (2019). Development of Sakon Nakhon Rice Variety for Blast Resistance through Marker Assisted Backcross Breeding. Agronomy, 9(2), 67. https://doi.org/10.3390/agronomy9020067





