PGR and Its Application Method Affect Number and Length of Runners Produced in ‘Maehyang’ and ‘Sulhyang’ Strawberries
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture Conditions
2.2. Plant Growth Regulators Tested
2.3. Methods of Supplying PGR Solution
2.3.1. Injection
2.3.2. Drench
2.3.3. Spray
2.4. Measurements of Growth and Morphological Parameters
2.5. Measurements of Contents of Starch, Soluble Sugar, and Protein
2.5.1. Soluble Sugar and Starch
2.5.2. Protein
2.6. Measurements of Antioxidant Enzymes Activities
2.6.1. Superoxidase Dismutase (SOD)
2.6.2. Peroxidase (POD)
2.6.3. Catalase (CAT)
2.7. Statistical Analysis
3. Results
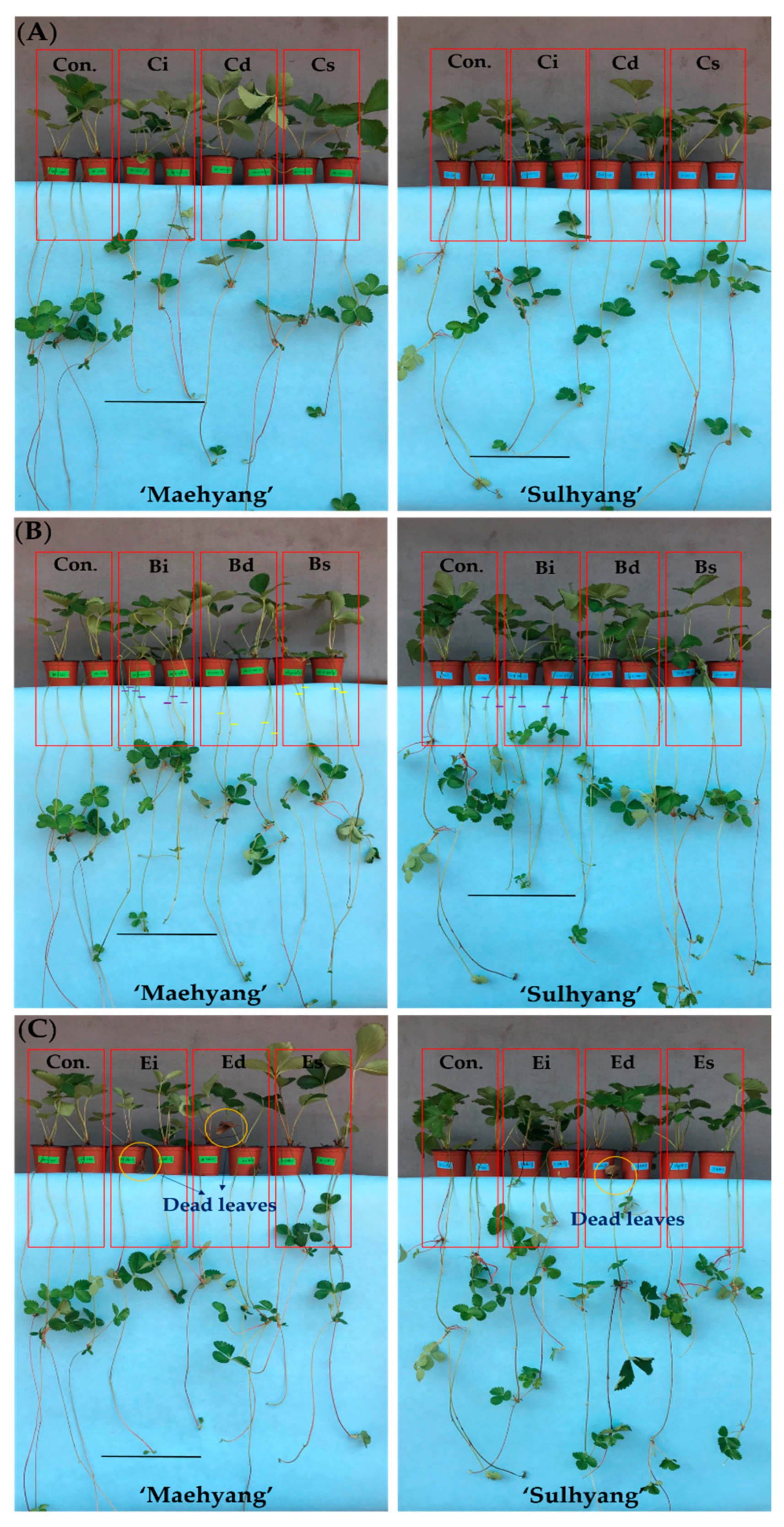
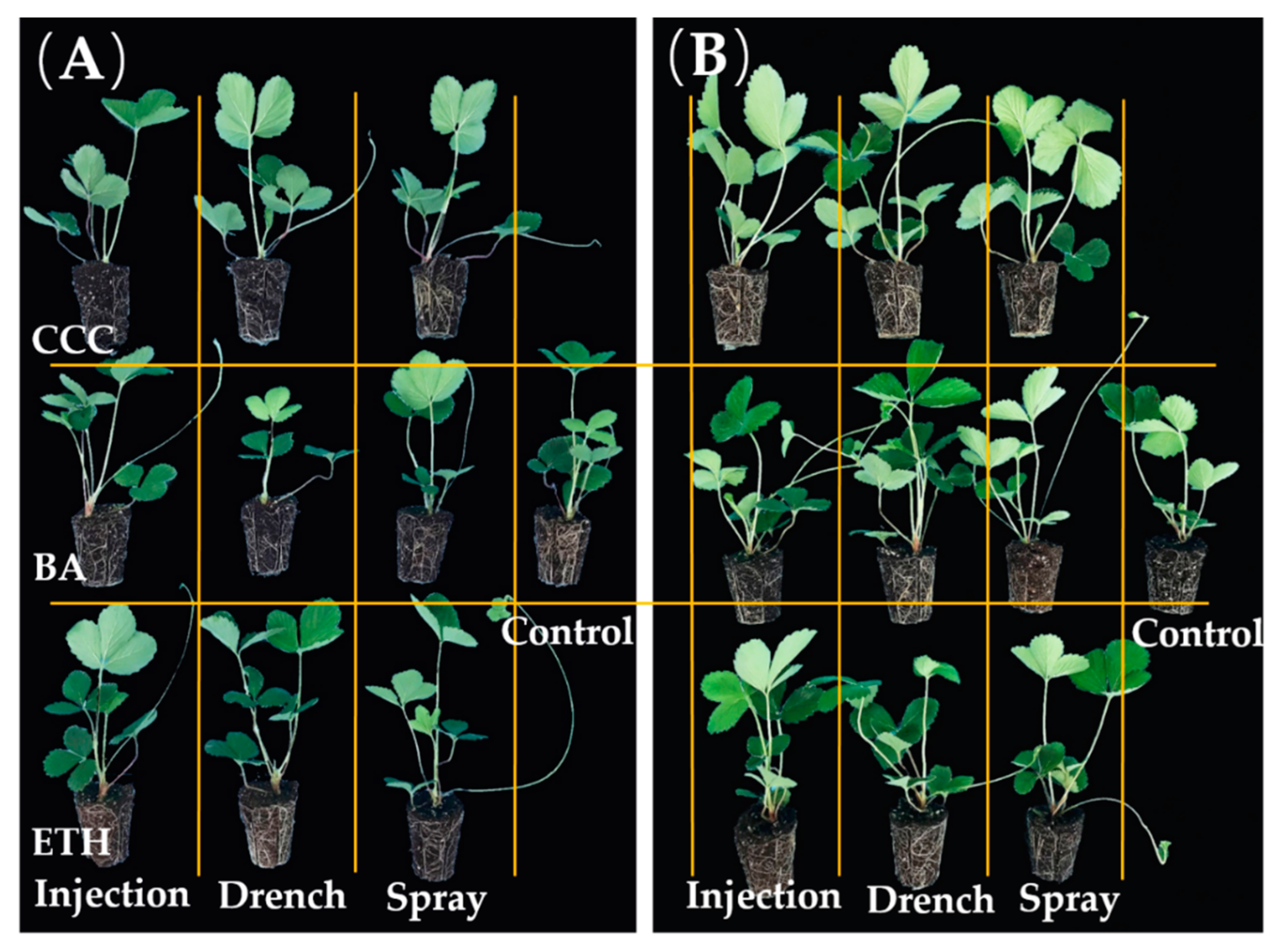
3.1. Effects of PGR and Application Method on Runners and Mother Plants
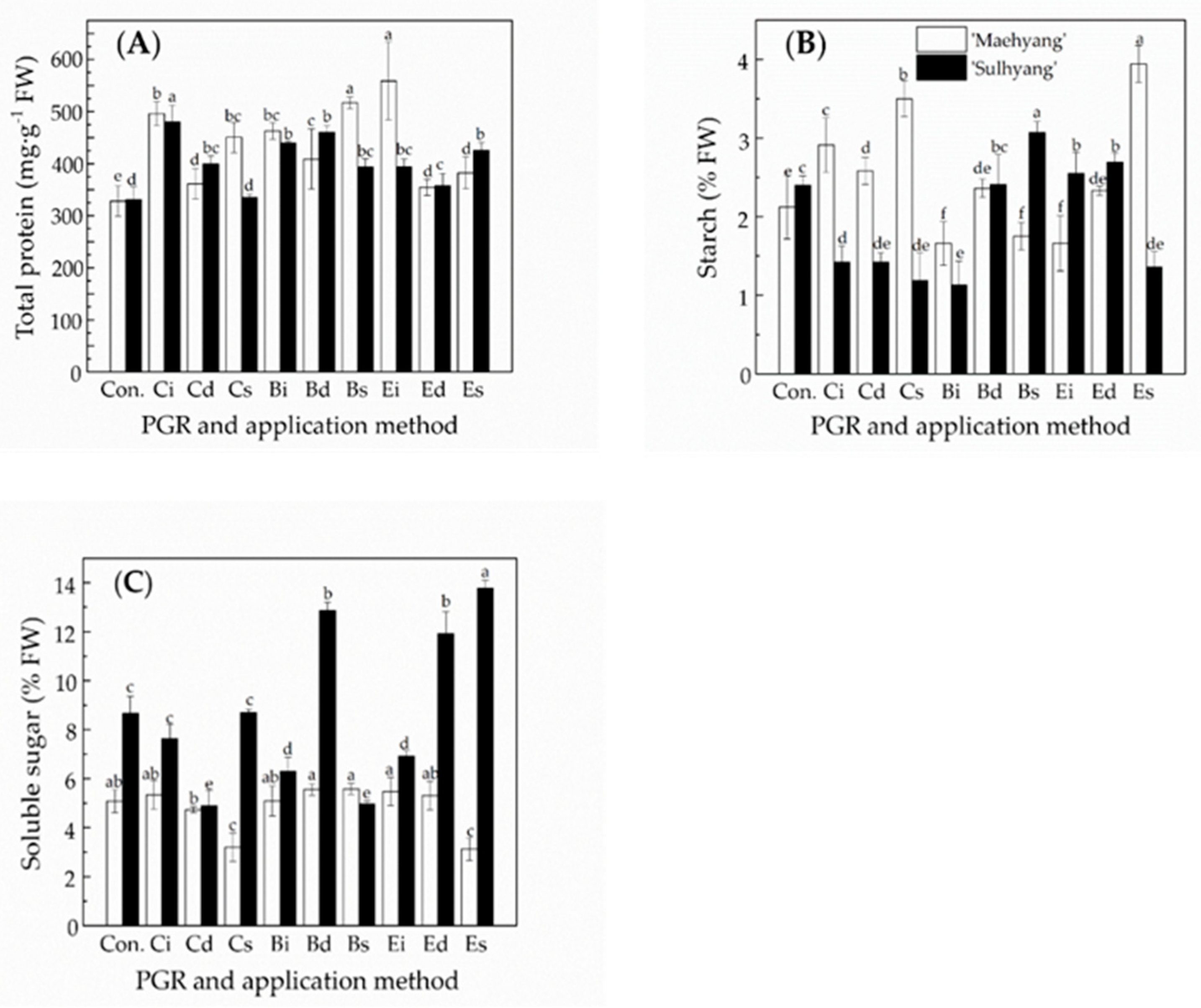
3.2. The Effects of the PGR Solution and the Application Method on Endogenous Compounds
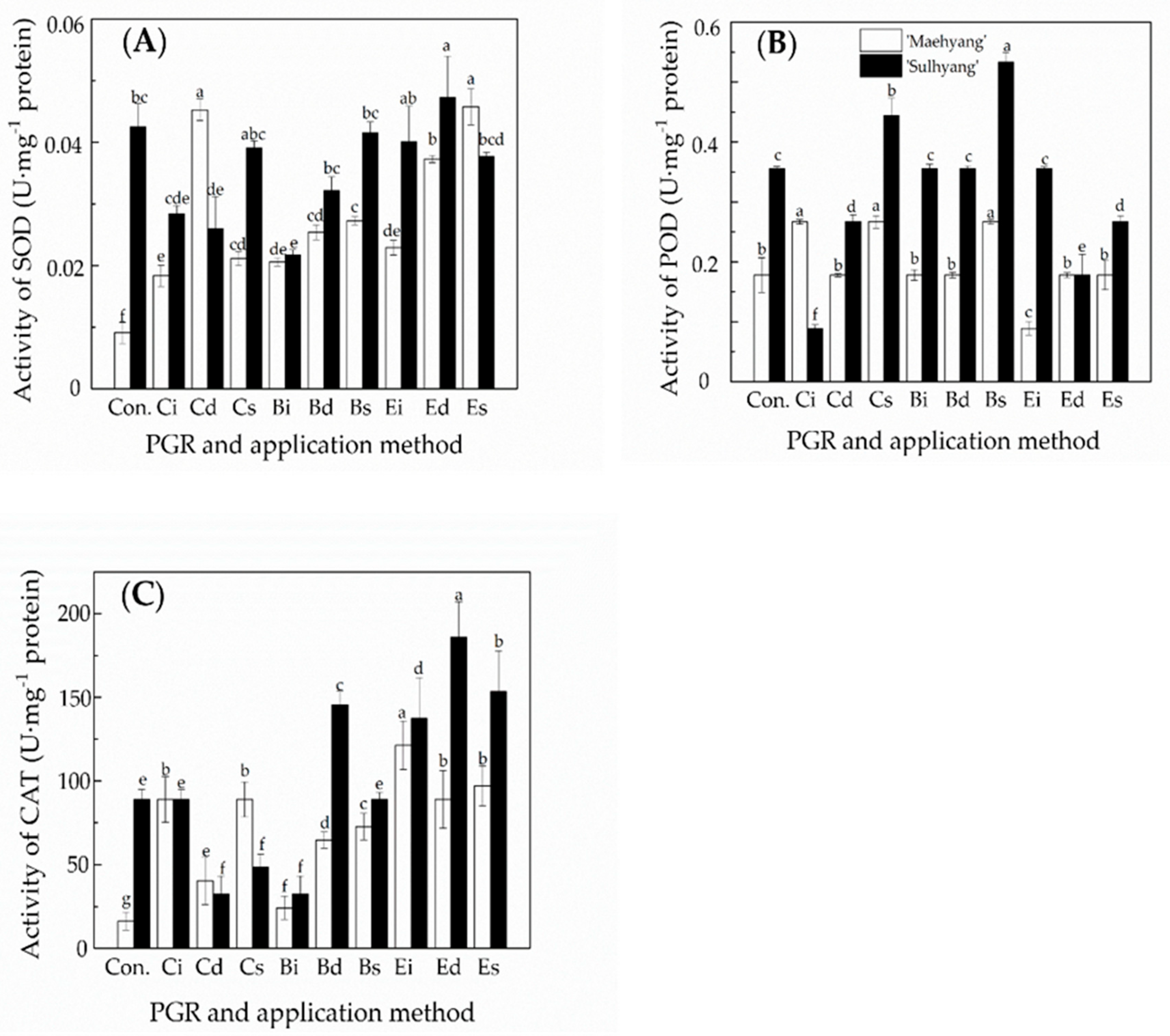
3.3. The Effects of the PGR Solution and the Application Method on the Activities of Antioxidant Enzymes
3.4. The Effects of the PGR Solution and the Application Method on the Runner Plants
4. Discussion and Conclusions
4.1. Discussion
4.2. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Torrico, A.; Salazar, S.; Kirschbaum, D.; Conci, V. Yield losses of asymptomatic strawberry plants infected with strawberry mild yellow edge virus. Eur. J. Plant Pathol. 2018, 150, 983–990. [Google Scholar] [CrossRef]
- Simpson, D. The economic importance of strawberry crops. In The Genomes of Rosaceous Berries and Their Wild Relatives; Springer: Cham, Switzerland, 2018; pp. 1–7. [Google Scholar]
- Xu, Q.; Fan, N.; Zhuang, L.; Yu, J.; Huang, B. Enhanced stolon growth and metabolic adjustment in creeping bentgrass with elevated CO2 concentration. Environ. Exp. Bot. 2018, 155, 87–97. [Google Scholar] [CrossRef]
- Park, S.W.; Kwack, Y.; Chun, C. Growth and propagation rate of strawberry transplants produced in a plant factory with artificial lighting as affected by separation time from stock plants. Hortic. Environ. Biotechnol. 2018, 59, 199–204. [Google Scholar] [CrossRef]
- Barrett, S.C. Influences of clonality on plant sexual reproduction. Proc. Natl. Acad. Sci. USA 2015, 112, 8859–8866. [Google Scholar] [CrossRef] [PubMed]
- Dolgun, O. Field performance of organically propagated and grown strawberry plugs and fresh plants. J. Sci. Food Agric. 2007, 87, 1364–1367. [Google Scholar] [CrossRef]
- Kumar, D.; Wareing, P. Factors controlling stolon development in the potato plant. New Phytol. 1972, 71, 639–648. [Google Scholar] [CrossRef]
- Nickell, L.G. Plant growth regulators. Chem. Eng. News 1978, 56, 18–34. [Google Scholar] [CrossRef]
- Rajala, A.; Peltonen-Sainio, P. Plant growth regulator effects on spring cereal root and shoot growth. Agron. J. 2001, 93, 936–943. [Google Scholar] [CrossRef]
- Lindstrom, R.; Tolbert, N. (2-Chloroethyl) trimethylammonium chloride and related compounds as plant growth substances. IV. Effect on chrysanthemums and poinsettias. Q. Bull. Mich. State Univ. Agric. Exp. Stn. 1960, 42, 917–928. [Google Scholar]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kaur, N.; Gupta, A.K. Effect of chlorocholine chloride sprays on the carbohydrate composition and activities of sucrose metabolising enzymes in potato (Solanum tuberosum L.). Plant Growth Regul. 1998, 26, 97–103. [Google Scholar] [CrossRef]
- Farooq, U.; Bano, A. Effect of abscisic acid and chlorocholine chloride on nodulation and biochemical content of Vigna radiata L. under water stress. Pak. J. Bot. 2006, 38, 1511–1518. [Google Scholar]
- Strydhorst, S.; Hall, L.; Perrott, L. Plant growth regulators: What agronomists need to know. Am. Soc. Agron. 2018, 51, 22–26. [Google Scholar] [CrossRef]
- Gianessi, L.P.; Marcelli, M.B. Pesticide Use in US Crop Production: 1997; National Center for Food and Agricultural Policy: Washington, DC, USA, 2000; p. 32. [Google Scholar]
- Wertheim, S.; Webster, A. Manipulation of growth and development by plant bioregulators. In Fundamentals of Temperate Zone Tree Fruit Production; Backhuys Publishers: Leiden, The Netherlands, 2005; pp. 267–294. [Google Scholar]
- Fishel, F.M. Plant Growth Regulators; Document PI-139; Pesticide Information Office, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2006; p. 3. [Google Scholar]
- Zhu, X.; Zeng, Y.; Zhang, Z.; Yang, Y.; Zhai, Y.; Wang, H.; Liu, L.; Hu, J.; Li, L. A new composite of graphene and molecularly imprinted polymer based on ionic liquids as functional monomer and cross-linker for electrochemical sensing 6-benzylaminopurine. Biosens. Bioelectron. 2018, 108, 38–45. [Google Scholar] [CrossRef]
- Chen, B.; Yang, H. 6-Benzylaminopurine alleviates chilling injury of postharvest cucumber fruit through modulating antioxidant system and energy status. J. Sci. Food Agric. 2013, 93, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; He, J.; Wang, Y.; Wang, C.; Ma, S.; Sun, X. Colorimetric recognition of 6-benzylaminopurine in environmental samples by using thioglycolic acid functionalized silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 27–33. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Sadasivam, S. Biochemical Methods; New Age International: Seborga, Italy, 1996; pp. 108–109. [Google Scholar]
- Ahmad, P.; Jhon, R. Effect of salt stress on growth and biochemical parameters of Pisum sativum L. Arch. Agron. Soil Sci. 2005, 51, 665–672. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C. Quantification of starch in plant tissues. Nat. Protoc. 2006, 1, 1342. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, Z. The regulation of soluble sugars in the growth and development of plants. Bot. Res. 2014, 3, 71–76. [Google Scholar]
- Jong, J.G.T.D.; Veldstra, H. Investigations on cytokinins. I. Effect of 6-benzylaminopurine on growth and starch content of Lemna minor. Physiol. Plant. 1971, 24, 235–238. [Google Scholar] [CrossRef]
- Wang, Z.; Dilley, D.R. Aminoethoxyvinylglycine, combined with ethephon, can enhance red color development without over-ripening apples. HortScience 2001, 36, 328–331. [Google Scholar]
- Emam, Y.; Karimi, H. Influence of chlormequat chloride on five winter barley cultivars. Iran Agric. Res. 1996, 15, 101–114. [Google Scholar]
- Lord, K.; Wheeler, A. Uptake and movement of 14C-chlormequat chloride applied to leaves of barley and wheat. J. Exp. Bot. 1981, 32, 599–603. [Google Scholar] [CrossRef]
- Truernit, E.; Siemering, K.R.; Hodge, S.; Grbic, V.; Haseloff, J. A map of KNAT gene expression in the Arabidopsis root. Plant Mol. Biol. 2006, 60, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A.; Schaewen, A.; Leegood, R.C.; Lea, P.J.; Quick, W.P. Regulation of leaf senescence by cytokinin, sugars, and light: Effects on NADH-dependent hydroxypyruvate reductase. Plant Physiol. 1998, 116, 329–335. [Google Scholar] [CrossRef]
- Saltveit, M.E. Postharvest Biology and Handling of Tomateos. In Tomatoes; Wageningen University and Research: Wageningen, The Netherland, 2018; pp. 309–323. [Google Scholar]
- Foster, K.R.; Reid, D.M.; Pharis, R.P. Ethylene biosynthesis and ethephon metabolism and transport in barley. Crop Sci. 1992, 32, 1345–1352. [Google Scholar] [CrossRef]
- Jo, E.H.; Soundararajan, P.; Park, Y.G.; Jeong, B.R. Effect of silicon on growth and tolerance of Torenia fournieri in vitro to NaCl stress. Flower Res. J. 2018, 26, 68–76. [Google Scholar] [CrossRef]
- Yazar, S.; Baydan, E. The subchronic toxic effects of plant growth promoters in mice. Ankara Univ. Vet. Fak. Derg. 2008, 55, 17–21. [Google Scholar]
- Sørensen, M.T.; Danielsen, V. Effects of the plant growth regulator, chlormequat, on mammalian fertility. Int. J. Androl. 2006, 29, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Kaczperski, M.P.; Armitage, A.M.; Lewis, P.M. Accelerating growth of plug-grown pansies with carbon dioxide and light. HortScience 1994, 29, 442. [Google Scholar]
- Boldt, J.L. Whole Plant Response of Chrysanthemum to Paclobutrazol, Chlormequat Chloride, and (s)-Abscisic Acid as a Function of Exposure Time Using a Split-Root System. Ph.D. Dissertation, University of Florida, Gainesville, FL, USA, 2008; pp. 15–17. [Google Scholar]
| Cultivar (C) | PGR (P) | Treatment Method (M) | Runner | |||||
|---|---|---|---|---|---|---|---|---|
| Number | Length of the Longest Runner (cm) | Length of the 1st & 2nd Internode (cm) | Diameter (mm) | Fresh Weight (g) | Dry Weight (g) | |||
| ‘Maehyang’ | CCC | Injection | 1.7 ± 0.2 b z | 74.1 ± 3.1 b | 30.9 ± 2.0 b | 2.40 ± 0.04 cd | 5.17 ± 0.35 bc | 0.27 ± 0.02 a |
| Drench | 1.5 ± 0.2 b | 85.0 ± 5.8 ab | 37.6 ± 4.0 ab | 2.54 ± 0.10 bc | 6.36 ± 0.52 a–c | 0.32 ± 0.05 a | ||
| Spray | 2.0 ± 0.1 b | 87.8 ± 2.4 a | 38.5 ± 1.6 ab | 2.42 ± 0.12 cd | 5.56 ± 0.57 a–c | 0.29 ± 0.07 a | ||
| BA | Injection | 3.5 ± 0.4 a | 74.9 ± 2.7 b | 32.1 ± 1.5 b | 2.75 ± 0.24 a | 6.80 ± 0.86 ab | 0.39 ± 0.04 a | |
| Drench | 1.7 ± 0.2 b | 83.4 ± 2.1 ab | 37.8 ± 1.3 ab | 2.49 ± 0.06 bc | 7.23 ± 0.83 a–c | 0.35 ± 0.06 a | ||
| Spray | 1.8 ± 0.2 b | 86.9 ± 4.0 a | 40.0 ± 1.7 a | 2.43 ± 0.08 cd | 5.48 ± 0.30 a–c | 0.25 ± 0.04 a | ||
| ETH | Injection | 1.7 ± 0.2 b | 73.9 ± 2.1 b | 31.3 ± 1.9 ab | 2.41 ± 0.07 cd | 5.29 ± 0.44 bc | 0.26 ± 0.01 a | |
| Drench | 1.3 ± 0.2 b | 83.5 ± 6.5 ab | 33.8 ± 3.1 ab | 2.70 ± 0.08 ab | 7.26 ± 0.80 a | 0.32 ± 0.05 a | ||
| Spray | 1.5 ± 0.2 b | 81.8 ± 3.6 ab | 36.3 ± 1.3 ab | 2.34 ± 0.10 cd | 4.83 ± 0.57 c | 0.27 ± 0.05 a | ||
| ‘Sulhyang’ | CCC | Injection | 2.0 ± 0.4 bc | 72.8 ± 1.5 a–c | 28.1 ± 1.8 ab | 2.11 ± 0.13 c | 6.10 ± 0.62 a–c | 0.28 ± 0.02 bc |
| Drench | 2.2 ± 0.3 bc | 81.3 ± 3.5 a | 31.1 ± 2.8 ab | 2.24 ± 0.09 a–c | 3.27 ± 0.96 c | 0.29 ± 0.02 a–c | ||
| Spray | 1.7 ± 0.3 bc | 77.5 ± 4.8 ab | 30.8 ± 2.2 ab | 2.32 ± 0.09 a–c | 7.06 ± 0.71 a | 0.35 ± 0.03 ab | ||
| BA | Injection | 3.5 ± 0.2 a | 64.2 ± 4.5 c | 27.9 ± 1.3 ab | 2.40 ± 0.10 a–c | 3.45 ± 0.15 a–c | 0.37 ± 0.03 a | |
| Drench | 2.2 ± 0.2 bc | 72.6 ± 2.2 a–c | 34.0 ± 2.7 a | 2.23 ± 0.09 a–c | 5.41 ± 0.78 a–c | 0.23 ± 0.04 c | ||
| Spray | 2.3 ± 0.4 b | 79.8 ± 2.6 a | 33.4 ± 1.2 a | 2.18 ± 0.08 bc | 4.53 ± 0.54 c | 0.27 ± 0.04 bc | ||
| ETH | Injection | 1.3 ± 0.2 c | 66.1 ± 3.6 c | 25.5 ± 2.4 b | 2.22 ± 0.13 a–c | 4.94 ± 0.71 a–c | 0.23 ± 0.03 c | |
| Drench | 2.0 ± 0.3 bc | 67.9 ± 3.5 bc | 28.8 ± 2.0 ab | 2.65 ± 0.13 a | 6.69 ± 0.85 ab | 0.27 ± 0.04 bc | ||
| Spray | 1.8 ± 0.3 bc | 68.1 ± 2.9 bc | 30.7 ± 1.3 ab | 2.60 ± 0.09 ab | 6.12 ± 0.60 a–c | 0.31 ± 0.03 a–c | ||
| F-test y | C | * | *** | *** | ** | NS | NS | |
| P | *** | * | NS | * | NS | NS | ||
| M | ** | *** | *** | NS | ** | NS | ||
| C × P | NS | NS | NS | * | *** | NS | ||
| C × M | NS | NS | NS | NS | NS | NS | ||
| P × M | *** | * | NS | * | NS | * | ||
| C × P × M | NS | NS | NS | NS | NS | NS | ||
| Cultivar (C) | PGR (P) | Application Method (M) | Shoot | Root | |||
|---|---|---|---|---|---|---|---|
| Length (cm) | Crown Diameter (mm) | Fresh Weight (g) | Length of Longest Root (cm) | Fresh Weight (g) | |||
| ‘Maehyang’ | CCC | Injection | 24.2 ± 1.0 a z | 7.20 ± 0.21 a | 6.31 ± 0.32 a–c | 18.2 ± 1.3 | 1.76 ± 0.14 |
| Drench | 24.6 ± 1.2 a | 7.13 ± 0.10 a | 7.01 ± 0.24 ab | 17.2 ± 0.7 | 1.84 ± 0.26 | ||
| Spray | 24.4 ± 1.6 a | 7.60 ± 0.37 a | 7.28 ± 0.75 a | 16.0 ± 1.2 | 1.75 ± 0.16 | ||
| BA | Injection | 24.9 ± 1.0 a | 7.36 ± 0.53 a | 6.17 ± 0.39 a–c | 18.3 ± 0.1 | 1.78 ± 0.20 | |
| Drench | 17.9 ± 0.6 d | 7.02 ± 0.13 a | 3.54 ± 0.15 d | 16.9 ± 1.6 | 1.60 ± 0.14 | ||
| Spray | 19.5 ± 2.2 cd | 6.74 ± 0.24 b | 5.01 ± 0.39 c | 16.6 ± 0.3 | 1.86 ± 0.26 | ||
| ETH | Injection | 24.0 ± 1.6 a | 7.71 ± 0.36 a | 6.83 ± 0.22 ab | 17.7 ± 1.1 | 1.94 ± 0.16 | |
| Drench | 22.7 ± 1.8 ab | 7.54 ± 0.41 a | 7.08 ± 0.76 ab | 19.1 ± 1.0 | 2.15 ± 0.27 | ||
| Spray | 24.8 ± 1.0 a | 7.19 ± 0.30 a | 5.93 ± 0.34 a–c | 18.0 ± 0.4 | 1.72 ± 0.21 | ||
| ‘Sulhyang’ | CCC | Injection | 26.1 ± 1.3 ab | 6.91 ± 0.13 ab | 5.97 ± 0.92 ab | 15.5 ± 2.0 | 1.70 ± 0.33 |
| Drench | 26.1 ± 0.7 ab | 6.61 ± 0.30 ab | 5.46 ± 1.20 ab | 17.8 ± 1.0 | 1.89 ± 0.30 | ||
| Spray | 27.1 ± 1.7 a | 7.20 ± 0.36 a | 6.13 ± 0.70 ab | 17.5 ± 0.6 | 2.11 ± 0.15 | ||
| BA | Injection | 23.1 ± 0.1 b–d | 6.82 ± 0.12 ab | 4.21 ± 0.30 b | 15.8 ± 0.8 | 1.93 ± 0.15 | |
| Drench | 27.3 ± 0.5 a | 7.20 ± 0.39 a | 7.14 ± 0.45 a | 17.6 ± 0.1 | 1.94 ± 0.37 | ||
| Spray | 22.1 ± 0.5 cd | 6.85 ± 0.13 ab | 3.88 ± 0.32 b | 15.8 ± 0.4 | 1.96 ± 0.17 | ||
| ETH | Injection | 24.4 ± 0.8 a–c | 6.46 ± 0.24 ab | 4.19 ± 1.79 b | 16.2 ± 0.4 | 1.73 ± 0.30 | |
| Drench | 24.3 ± 0.4 a–c | 5.43 ± 0.12 c | 5.64 ± 0.47 ab | 17.1 ± 0.6 | 1.91 ± 0.31 | ||
| Spray | 21.1 ± 1.7 d | 6.22 ± 0.33 b | 4.24 ± 0.46 b | 15.3 ± 0.7 | 1.59 ± 0.22 | ||
| F-test y | C | * | *** | ** | NS | NS | |
| P | *** | NS | ** | NS | NS | ||
| M | NS | NS | NS | NS | NS | ||
| C × P | * | *** | * | NS | NS | ||
| C × M | * | NS | NS | NS | NS | ||
| P × M | NS | NS | * | NS | NS | ||
| C × P × M | * | NS | * | NS | NS | ||
| Cultivar (C) | PGR (P) | Method (M) | The Largest Leaf | |||||
|---|---|---|---|---|---|---|---|---|
| Number | Length (cm) | Width (cm) | Thickness (mm) | Petiole Diameter (mm) | Chlorophyll (SPAD) | |||
| ‘Maehyang’ | CCC | Injection | 5.0 ± 0.0 az | 7.2 ± 0.2 ab | 4.8 ± 0.1 ab | 0.55 ± 0.05 | 2.43 ± 0.08 ab | 35.7 ± 2.1 b |
| Drench | 5.0 ± 0.0 a | 7.5 ± 0.2 ab | 5.4 ± 0.1 a | 0.55 ± 0.04 | 2.44 ± 0.06 ab | 38.8 ± 0.3 ab | ||
| Spray | 5.0 ± 0.0 a | 7.5 ± 0.5 ab | 5.2 ± 0.4 ab | 0.53 ± 0.05 | 2.46 ± 0.12 ab | 38.2 ± 1.2 ab | ||
| BA | Injection | 5.0 ± 0.0 a | 7.5 ± 0.5 ab | 5.5 ± 0.5 a | 0.60 ± 0.04 | 2.44 ± 0.10 ab | 39.5 ± 1.5 ab | |
| Drench | 5.0 ± 0.0 a | 5.2 ± 0.2 d | 3.7 ± 0.1 c | 0.51 ± 0.05 | 2.04 ± 0.14 c | 38.5 ± 0.9 ab | ||
| Spray | 3.0 ± 0.0 c | 5.8 ± 0.2 cd | 4.2 ± 0.1 bc | 0.51 ± 0.04 | 2.28 ± 0.05 bc | 39.3 ± 0.6 ab | ||
| ETH | Injection | 4.0 ± 0.0 b | 7.7 ± 0.7 a | 5.3 ± 0.5 ab | 0.54 ± 0.04 | 2.43 ± 0.11 ab | 39.8 ± 0.8 a | |
| Drench | 4.7 ± 0.3 a | 6.9 ± 0.7 a–c | 4.9 ± 0.6 ab | 0.57 ± 0.04 | 2.66 ± 0.15 a | 38.1 ± 1.5 ab | ||
| Spray | 4.7 ± 0.3 a | 6.8 ± 0.7 a–c | 4.9 ± 0.1 ab | 0.53 ± 0.02 | 2.33 ± 0.07 a–c | 39.6 ± 1.0 ab | ||
| ‘Sulhyang’ | CCC | Injection | 5.3 ± 0.3 ab | 8.0 ± 0.2 a–d | 5.6 ± 0.2 ab | 0.34 ± 0.01 | 2.51 ± 0.18 a | 32.6 ± 4.4 b |
| Drench | 5.0 ± 0.6 ab | 7.7 ± 0.7 a–d | 5.6 ± 0.6 ab | 0.32 ± 0.01 | 2.22 ± 0.10 a–c | 35.4 ± 1.7 ab | ||
| Spray | 5.7 ± 0.3 a | 8.3 ± 0.5 a–c | 5.8 ± 0.3 ab | 0.33 ± 0.02 | 2.50 ± 0.06 a | 37.2 ± 2.0 ab | ||
| BA | Injection | 5.3 ± 0.3 ab | 6.9 ± 0.4 b–d | 4.9 ± 0.2 b | 0.35 ± 0.01 | 2.03 ± 0.01 c | 36.2 ± 2.3 ab | |
| Drench | 5.3 ± 0.3 ab | 8.7 ± 0.6 a | 6.2 ± 0.4 a | 0.33 ± 0.02 | 2.49 ± 0.09 a | 34.5 ± 0.7 ab | ||
| Spray | 5.3 ± 0.3 ab | 6.7 ± 0.5 cd | 4.9 ± 0.3 b | 0.31 ± 0.03 | 1.96 ± 0.06 c | 35.3 ± 1.5 ab | ||
| ETH | Injection | 5.7 ± 0.3 a | 8.3 ± 0.4 ab | 5.6 ± 0.3 ab | 0.34 ± 0.02 | 2.37 ± 0.11 ab | 40.2 ± 1.2 a | |
| Drench | 5.0 ± 0.6 ab | 7.8 ± 0.3 a–d | 5.2 ± 0.2 ab | 0.33 ± 0.02 | 2.38 ± 0.01 ab | 37.9 ± 0.1 ab | ||
| Spray | 4.3 ± 0.3 b | 6.6 ± 0.6 d | 4.8 ± 0.5 b | 0.31 ± 0.03 | 2.10 ± 0.16 bc | 37.1 ± 2.4 ab | ||
| F-testy | C | *** | ** | ** | *** | * | ** | |
| P | * | ** | * | NS | ** | NS | ||
| M | ** | NS | NS | NS | NS | NS | ||
| C × P | ** | NS | NS | NS | NS | NS | ||
| C × M | NS | * | NS | NS | NS | NS | ||
| P × M | NS | NS | NS | NS | NS | NS | ||
| C × P × M | * | * | ** | NS | *** | NS | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Guo, Z.; Park, Y.G.; Wei, H.; Jeong, B.R. PGR and Its Application Method Affect Number and Length of Runners Produced in ‘Maehyang’ and ‘Sulhyang’ Strawberries. Agronomy 2019, 9, 59. https://doi.org/10.3390/agronomy9020059
Liu C, Guo Z, Park YG, Wei H, Jeong BR. PGR and Its Application Method Affect Number and Length of Runners Produced in ‘Maehyang’ and ‘Sulhyang’ Strawberries. Agronomy. 2019; 9(2):59. https://doi.org/10.3390/agronomy9020059
Chicago/Turabian StyleLiu, Chen, Ziwei Guo, Yoo Gyeong Park, Hao Wei, and Byoung Ryong Jeong. 2019. "PGR and Its Application Method Affect Number and Length of Runners Produced in ‘Maehyang’ and ‘Sulhyang’ Strawberries" Agronomy 9, no. 2: 59. https://doi.org/10.3390/agronomy9020059
APA StyleLiu, C., Guo, Z., Park, Y. G., Wei, H., & Jeong, B. R. (2019). PGR and Its Application Method Affect Number and Length of Runners Produced in ‘Maehyang’ and ‘Sulhyang’ Strawberries. Agronomy, 9(2), 59. https://doi.org/10.3390/agronomy9020059






