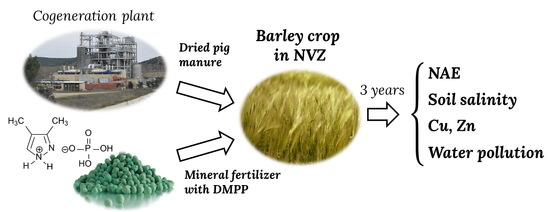
Dried Pig Manure from a Cogeneration Plant as a Fertilizer for Nitrate Vulnerable Zones
Abstract
1. Introduction
2. Materials and Methods
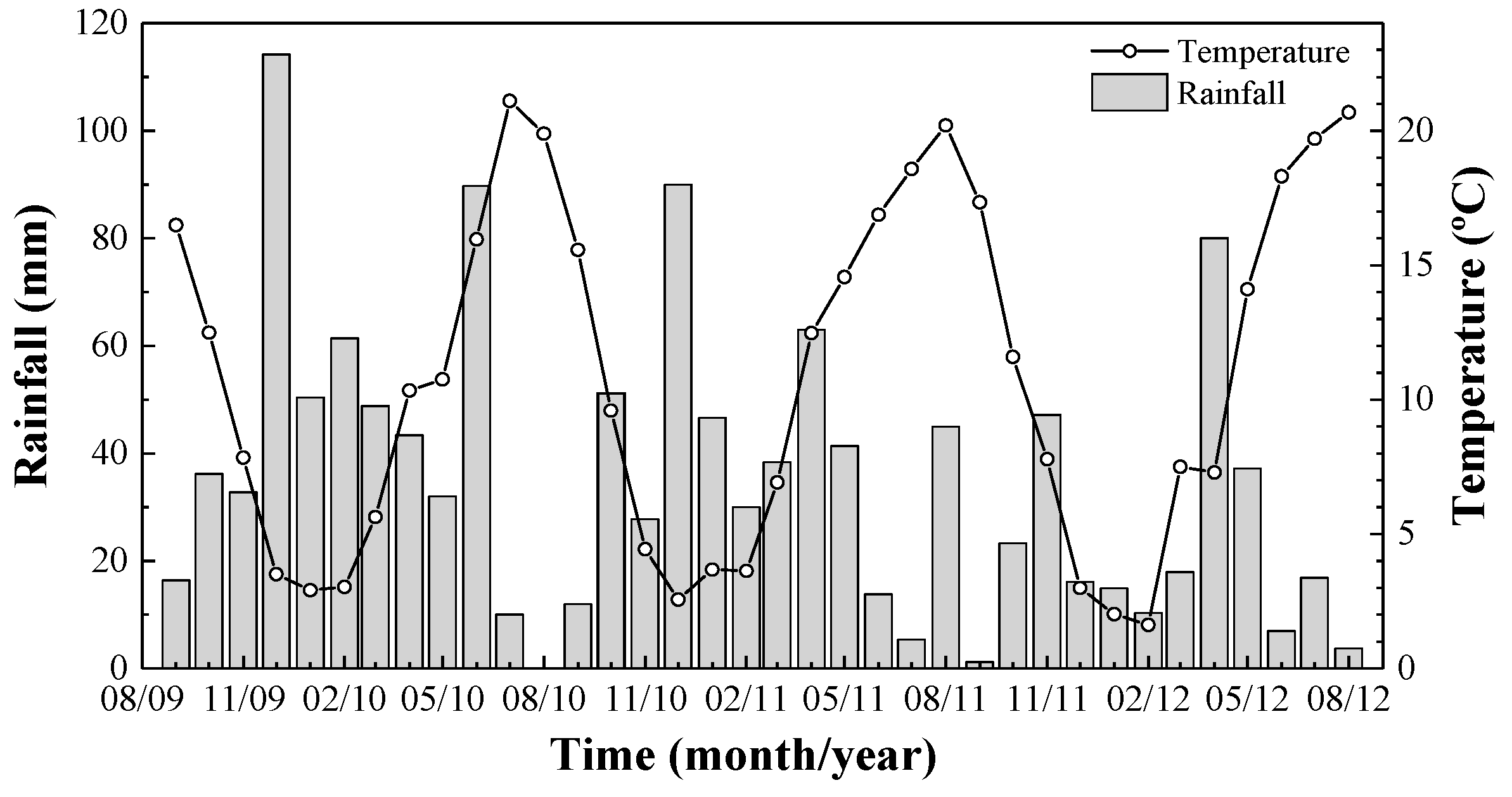
2.1. Location
2.2. Soil Samples
2.3. Organic and Mineral Fertilizers
2.4. Experimental Design
2.5. Methodology for Soil, Dried Pig Manure and Grain Analyses
2.6. Nitrogen Agronomic Efficiency Parameter (NAE)
2.7. Statistical Analyses
3. Results and Discussion
3.1. Grain Yield, N Content and NAE
3.2. Physicochemical Properties of Soils
3.3. Metal Pollution
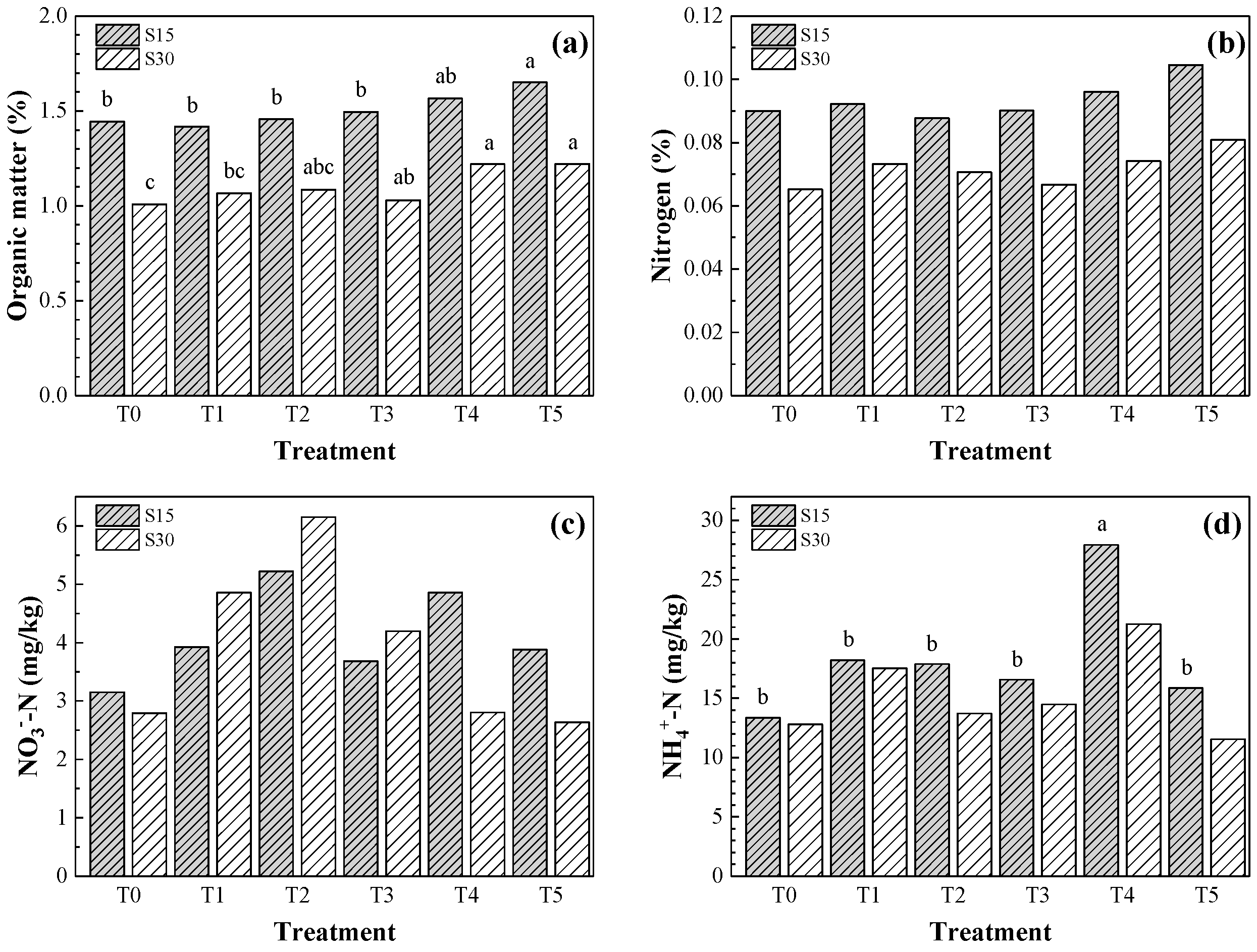
3.4. Organic Matter, Nitrogen, Nitrates and Ammonium Contents in the Soil
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter * | Soil |
|---|---|
| Location | Fompedraza (41°32′10″ N 4°8′45″ W) |
| Sand (%) | 46.0 ± 2.2 |
| Silt (%) | 25.6 ± 1.4 |
| Clay (%) | 28.4 ± 3.6 |
| Texture (USDA) | Sandy Clay Loam |
| pH | 7.9 ± 0.1 |
| EC (μS·cm−1) | 145 ± 24 |
| Organic matter (%) | 1.42 ± 0.05 |
| Total carbon (%) | 4.07 ± 0.45 |
| Total nitrogen (%) | 0.10 ± 0.01 |
| Phosphorus (mg·kg−1) | 22.1 ± 1.3 |
| CaCO3 (%) | 28.7 ± 1.4 |
| K (meq·100 g−1) | 0.30 ± 0.04 |
| Na (meq·100 g−1) | 0.05 ± 0.02 |
| Ca (meq·100 g−1) | 17.7 ± 2.1 |
| Mg (meq·100 g−1) | 0.85 ± 0.01 |
| Fe (%) | 1.27 ± 0.05 |
| Mn (mg·kg−1) | 252 ± 21 |
| Zn (mg·kg−1) | 21.1 ± 3.3 |
| Cu (mg·kg−1) | 7.55 ± 0.6 |
| Cr (mg·kg−1) | 26.1 ± 1.7 |
| Ni (mg·kg−1) | 27.6 ± 7.2 |
| Pb (mg·kg−1) | 22.2 ± 5.8 |
| Cd (μg·kg−1) | 6.86 ± 4.3 |
| Parameter * | Dried Pig Manure |
|---|---|
| Dry matter (%) | 29.9 ± 4.4 |
| pH | 7.8 ± 0.6 |
| EC (mS·cm−1) | 4.5 ± 2.2 |
| Organic matter (%) | 52.4 ± 15.3 |
| Total carbon (%) | 39.0 ± 3.3 |
| Total nitrogen (%) | 2.5 ± 0.1 |
| C:N ratio | 16.0 ± 2.1 |
| P (%) | 1.6 ± 0.7 |
| K (%) | 1.4 ± 0.2 |
| Na (%) | 0.6 ± 0.2 |
| Ca (%) | 1.7 ± 1.6 |
| Mg (%) | 1.9 ± 1.5 |
| Fe (mg·kg−1) | 226 ± 39 |
| Mn (mg·kg−1) | 493 ± 125 |
| Cu (mg·kg−1) | 176 ± 40 |
| Zn (mg·kg−1) | 745 ± 301 |
| Ni (mg·kg−1) | 12.0 ± 3.2 |
| Cr (mg·kg−1) | 16.8 ± 3.2 |
| Pb (mg·kg−1) | 11.9 ± 4.8 |
| Cd (mg·kg−1) | 0.08 ± 0.03 |
| N-NH4 (%) | 0.13 ± 0.02 |
References
- Sánchez, M.; González, J.L. The fertilizer value of pig slurry. I. Values depending on the type of operation. Bioresour. Technol. 2005, 96, 1117–1123. [Google Scholar] [PubMed]
- Jensen, J.; Larsen, M.M.; Bak, J. National monitoring study in Denmark finds increased and critical levels of copper and zinc in arable soils fertilized with pig slurry. Environ. Pollut. 2016, 214, 334–340. [Google Scholar] [PubMed]
- Pirlo, G.; Carè, S.; Casa, G.D.; Marchetti, R.; Ponzoni, G.; Faeti, V.; Fantin, V.; Masoni, P.; Buttol, P.; Zerbinatti, L.; et al. Environmental impact of heavy pig production in a sample of Italian farms. A cradle to farm-gate analysis. Sci. Total Environ. 2016, 565, 576–585. [Google Scholar] [PubMed]
- Van Grinsven, H.J.M.; Tiktak, A.; Rougoor, C.W. Evaluation of the Dutch implementation of the nitrates directive, the water framework directive and the national emission ceilings directive. Wageningen J. Life Sci. 2016, 78, 69–84. [Google Scholar]
- USDA. Spain: Record Year for Spanish Swine and Cattle Production. Available online: https://www.fas.usda.gov/data/spain-record-year-spanish-swine-and-cattle-production (accessed on 24 September 2018).
- European Commission. Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters Against Pollution Caused by Nitrates from Agricultural Sources. 91/676/EEC. 1991. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX:31991L0676 (accessed on 23 January 2019).
- Sutton, M.A.; Howard, C.M.; Erisman, J.W.; Billen, G.; Bleeker, A.; Grennfelt, P.; Van Grinsven, H.; Grizzetti, B. The European Nitrogen Assessment: Sources, Effects and Policy Perspectives; Cambridge University Press: Cambridge, UK, 2011; p. 664. [Google Scholar]
- Bernal, M.P.; Bescós, B.; Burgos, L.; Bustamante, M.; Clemente, R.; Fabbri, C.; Flotats Ripoll, X.; García-González, M.C.; Herrero Mallén, E.; Mattachini, G. Evaluation of Manure Management Systems in Europe; UPCommons: Valencia, Spain, 2015; p. 180. [Google Scholar]
- Sørensen, P.; Amato, M. Remineralisation and residual effects of N after application of pig slurry to soil. Eur. J. Agron. 2002, 16, 81–95. [Google Scholar]
- Li, L.L.; Li, S.T. Nitrogen mineralization from animal manures and its relation to organic N fractions. J. Integr. Agric. 2014, 13, 2040–2048. [Google Scholar]
- Fangueiro, D.; Surgy, S.; Fraga, I.; Monteiro, F.G.; Cabral, F.; Coutinho, J. Acidification of animal slurry affects the nitrogen dynamics after soil application. Geoderma 2016, 281, 30–38. [Google Scholar]
- L’Herroux, L.; Roux, S.L.; Appriou, P.; Martinez, J. Behaviour of metals following intensive pig slurry applications to a natural field treatment process in Brittany (France). Environ. Pollut. 1997, 97, 119–130. [Google Scholar]
- Formentini, T.A.; Mallmann, F.J.K.; Pinheiro, A.; Fernandes, C.V.S.; Bender, M.A.; da Veiga, M.; dos Santos, D.R.; Doelsch, E. Copper and zinc accumulation and fractionation in a clayey Hapludox soil subject to long-term pig slurry application. Sci. Total Environ. 2015, 536, 831–839. [Google Scholar]
- Biau, A.; Santiveri, F.; Mijangos, I.; Lloveras, J. The impact of organic and mineral fertilizers on soil quality parameters and the productivity of irrigated maize crops in semiarid regions. Eur. J. Soil Biol. 2012, 53, 56–61. [Google Scholar]
- Serna, M.D.; Bañuls, J.; Quiñones, A.; Primo-Millo, E.; Legaz, F. Evaluation of 3,4-dimethylpyrazole phosphate as a nitrification inhibitor in a Citrus-cultivated soil. Biol. Fertil. Soils 2000, 32, 41–46. [Google Scholar] [CrossRef]
- Yu, Q.G.; Chen, Y.X.; Ye, X.Z.; Tian, G.M.; Zhang, Z.J. Influence of the DMPP (3,4-dimethyl pyrazole phosphate) on nitrogen transformation and leaching in multi-layer soil columns. Chemosphere 2007, 69, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Ayuso, M.; Gabriel, J.L.; Quemada, M. Nitrogen use efficiency and residual effect of fertilizers with nitrification inhibitors. Eur. J. Agron. 2016, 80, 1–8. [Google Scholar] [CrossRef]
- Long, G.Q.; Sun, B. Nitrogen leaching under corn cultivation stabilized after four years application of pig manure to red soil in subtropical China. Agric. Ecosyst. Environ. 2012, 146, 73–80. [Google Scholar] [CrossRef]
- MAPAMA. Sistema de Información Agroclimática para el Regadío (SiAR). Available online: http://eportal.mapama.gob.es/websiar/Inicio.aspx (accessed on 24 September 2018).
- Xiong, X.; Yanxia, L.; Wei, L.; Chunye, L.; Wei, H.; Ming, Y. Copper content in animal manures and potential risk of soil copper pollution with animal manure use in agriculture. Resour. Conserv. Recycl. 2010, 54, 985–990. [Google Scholar] [CrossRef]
- Lipoth, S.L.; Schoenau, J.J. Copper, zinc, and cadmium accumulation in two prairie soils and crops as influenced by repeated applications of manure. J. Plant Nutr. Soil Sci. 2007, 170, 378–386. [Google Scholar] [CrossRef]
- Shi, J.; Yu, X.; Zhang, M.; Lu, S.; Wu, W.; Wu, J.; Xu, J. Potential risks of copper, zinc, and cadmium pollution due to pig manure application in a soil-rice system under intensive farming: A case study of Nanhu, China. J. Environ. Qual. 2011, 40, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Spanish Ministry of the Presidency. Real Decreto 261/1996, de 16 Febrero, Sobre Protección de las Aguas Contra la Contaminación Producida por los Nitratos Procedentes de Fuentes Agrarias; 261/1996; Spanish Ministry of the Presidency: Madrid, Spain, 1996; p. 4.
- López Bellido, L.; Betrán Aso, J.; Ramos Monreal, Á.; López Córcoles, H.; López Fuster, P.; Bermejo Corrales, J.L.; Urbano Terrón, P.; Piñeiro Andión, J.; Castro Insua, J.; Blázquez Rodríguez, R. Abonado de los Principales Cultivos en España, 2nd ed.; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2009; Volume 2, p. 144.
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; Klute, A., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Walkey, A.J.; Black, I.A. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954; pp. 1–19.
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic forms. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Norman, R.J.; Edberg, J.C.; Stucki, J.W. Determination of nitrate in soil extracts by dual-wavelength ultraviolet spectrophotometry. Soil Sci. Soc. Am. J. 1985, 49, 1182–1185. [Google Scholar] [CrossRef]
- Delogu, G.; Cattivelli, L.; Pecchioni, N.; De Falcis, D.; Maggiore, T.; Stanca, A.M. Uptake and agronomic efficiency of nitrogen in winter barley and winter wheat. Eur. J. Agron. 1998, 9, 11–20. [Google Scholar] [CrossRef]
- Duan, Y.; Xu, M.; Gao, S.; Yang, X.; Huang, S.; Liu, H.; Wang, B. Nitrogen use efficiency in a wheat–corn cropping system from 15 years of manure and fertilizer applications. Field Crops Res. 2014, 157, 47–56. [Google Scholar] [CrossRef]
- Zhengchao, Z.; Zhuoting, G.; Zhouping, S.; Fuping, Z. Effects of long-term repeated mineral and organic fertilizer applications on soil organic carbon and total nitrogen in a semi-arid cropland. Eur. J. Agron. 2013, 45, 20–26. [Google Scholar] [CrossRef]
- Azeez, J.O.; Van Averbeke, W.; Okorogbona, A.O.M. Differential responses in yield of pumpkin (Cucurbita maxima L.) and nightshade (Solanum retroflexum Dun.) to the application of three animal manures. Bioresour. Technol. 2010, 101, 2499–2505. [Google Scholar] [CrossRef] [PubMed]
- Gentile, R.; Vanlauwe, B.; Chivenge, P.; Six, J. Trade-offs between the short- and long-term effects of residue quality on soil C and N dynamics. Plant Soil 2010, 338, 159–169. [Google Scholar] [CrossRef]
- Villar, J.M.; Guillaumes, E. Use of nitrification inhibitor DMPP to improve nitrogen recovery in irrigated wheat on a calcareous soil. Span. J. Agric. Res. 2010, 8, 1218. [Google Scholar] [CrossRef]
- Ball-Coelho, B.R.; Roy, R.C. Enhanced ammonium sources to reduce nitrate leaching. Nutr. Cycl. Agroecosyst. 1999, 54, 73–80. [Google Scholar] [CrossRef]
- Riley, H. Soil mineral-N and N-fertilizer requirements of spring cereals in two long-term tillage trials on loam soil in southeast Norway. Soil Tillage Res. 1998, 48, 265–274. [Google Scholar] [CrossRef]
- Baethgen, W.E.; Christianson, C.B.; Lamothe, A.G. Nitrogen fertilizer effects on growth, grain yield, and yield components of malting barley. Field Crops Res. 1995, 43, 87–99. [Google Scholar] [CrossRef]
- Dobermann, A.R. Nitrogen use efficiency–state of the art. In IFA International Workshop on Enhanced-Efficiency Fertilizers; International Fertilizer Association: Frankfurt, Germany, 2005; pp. 1–16. [Google Scholar]
- Hooper, P. Strategic Applications of Nitrogen Fertiliser to Increase the Yield and Nitrogen Use Efficiency of Wheat. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2010. [Google Scholar]
- Medina, E.; Paredes, C.; Bustamante, M.A.; Moral, R.; Moreno-Caselles, J. Relationships between soil physico-chemical, chemical and biological properties in a soil amended with spent mushroom substrate. Geoderma 2012, 173–174, 152–161. [Google Scholar] [CrossRef]
- Díez, J.A.; Hernaiz, P.; Muñoz, M.J.; Torre, A.; Vallejo, A. Impact of pig slurry on soil properties, water salinization, nitrate leaching and crop yield in a four-year experiment in Central Spain. Soil Use Manag. 2004, 20, 444–450. [Google Scholar] [CrossRef]
- Morlat, R.; Chaussod, R. Long-term additions of organic amendments in a Loire Valley vineyard. I. Effects on properties of a calcareous sandy soil. Am. J. Enol. Vit. 2008, 59, 353–363. [Google Scholar]
- Bernal, M.P.; Roig, A.; Lax, A.; Navarro, A.F. Effects of the application of pig slurry on some physico-chemical and physical properties of calcareous soils. Bioresour. Technol. 1992, 42, 233–239. [Google Scholar] [CrossRef]
- Tarrasón, D.; Ojeda, G.; Ortiz, O.; Alcañiz, J.M. Differences on nitrogen availability in a soil amended with fresh, composted and thermally-dried sewage sludge. Bioresour. Technol. 2008, 99, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, W.; Jia, J.; Kong, P.; Tong, X.; Lu, Y.; Xie, L.; Ma, F.; Giesy, J.P. Effects of pig manure containing copper and zinc on microbial community assessed via phospholipids in soils. Environ. Monit. Assess. 2014, 186, 5297–5306. [Google Scholar] [CrossRef] [PubMed]
- Maltas, A.; Charles, R.; Jeangros, B.; Sinaj, S. Effect of organic fertilizers and reduced-tillage on soil properties, crop nitrogen response and crop yield: Results of a 12-year experiment in Changins, Switzerland. Soil Tillage Res. 2013, 126, 11–18. [Google Scholar] [CrossRef]
- Meng, L.; Ding, W.; Cai, Z. Long-term application of organic manure and nitrogen fertilizer on N2O emissions, soil quality and crop production in a sandy loam soil. Soil Biol. Biochem. 2005, 37, 2037–2045. [Google Scholar] [CrossRef]
- Berenguer, P.; Santiveri, F.; Boixadera, J.; Lloveras, J. Fertilisation of irrigated maize with pig slurry combined with mineral nitrogen. Eur. J. Agron. 2008, 28, 635–645. [Google Scholar] [CrossRef]
- Guillaumes, E.; Carrasco, I.; Villar, J.M. Response of wheat to additional nitrogen fertilizer application after pig slurry on over-fertilized soils. Agron. Sustain. Dev. 2006, 26, 127–133. [Google Scholar] [CrossRef]
- Yu, Q.G.; Ye, X.Z.; Chen, Y.X.; Zhang, Z.J.; Tian, G.M. Influences of nitrification inhibitor 3,4-dimethyl pyrazole phosphate on nitrogen and soil salt-ion leaching. J. Environ. Sci. 2008, 20, 304–308. [Google Scholar] [CrossRef]
- Zhu, T.; Zeng, S.; Qin, H.; Zhou, K.; Yang, H.; Lan, F.; Huang, F.; Cao, J.; Müller, C. Low nitrate retention capacity in calcareous soil under woodland in the karst region of southwestern China. Soil Biol. Biochem. 2016, 97, 99–101. [Google Scholar] [CrossRef]
- Díez, J.A.; Arauzo, M.; Hernaiz, P.; Sanz, A. The side effects of nitrification inhibitors on leaching water and soil salinization in a field experiment. Span. J. Agric. Res. 2010, 8, 218. [Google Scholar] [CrossRef]
- Morrill, L.G.; Dawson, J.E. Patterns observed for the oxidation of ammonium to nitrate by soil organisms. Soil Sci. Soc. Am. J. 1967, 31, 757–760. [Google Scholar] [CrossRef]
- Khalil, M.I.; Hossain, M.B.; Schmidhalter, U. Carbon and nitrogen mineralization in different upland soils of the subtropics treated with organic materials. Soil Biol. Biochem. 2005, 37, 1507–1518. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, T.; Cai, Z.; Müller, C. Nitrogen cycling in forest soils across climate gradients in Eastern China. Plant Soil 2011, 342, 419–432. [Google Scholar]
- Guerrero, C.; Moral, R.; Gómez, I.; Zornoza, R.; Arcenegui, V. Microbial biomass and activity of an agricultural soil amended with the solid phase of pig slurries. Bioresour. Technol. 2007, 98, 3259–3264. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Scherer, H.W. Fixation and defixation of ammonium in soils: A review. Biol. Fertil. Soils 2010, 47, 1–14. [Google Scholar] [CrossRef]

| Fertilizer | Treatment | Fertilizer Application Rate (t·ha−1·year−1) | Nitrogen Rate (kg N·ha−1·year−1) | KCl Application Rate (t·ha−1·year−1) |
|---|---|---|---|---|
| Control | T0 | |||
| Mineral (ENTEC®) | T1 | 0.45 | 108 | |
| T2 | 0.38 | 90 | 0.08 | |
| Organic (dried pig manure) | T3 | 9.5 | 85 | |
| T4 | 14.2 | 133 | ||
| T5 | 19.0 | 170 |
| Year | Treatment | Grain Yield (kg·ha−1) | N Concentration (%N) | N Content (kg N·ha−1) | NAE (kg grain·kg−1 N) |
|---|---|---|---|---|---|
| 2010 | T0 | 1414 ± 313 c | 1.32 ± 0.08 b | 18.7 ± 4.4 b | |
| T1 | 3340 ± 595 a | 1.79 ± 0.18 a | 59.7 ± 17.1 a | 18 ± 7 ab | |
| T2 | 3215 ± 423 ab | 1.73 ± 0.17 a | 55.6 ± 13.4 a | 20 ± 6 a | |
| T3 | 2013 ± 225 bc | 1.37 ± 0.05 b | 27.6 ± 4.2 b | 7 ± 2 ab | |
| T4 | 1957 ± 345 bc | 1.35 ± 0.05 b | 26.4 ± 6.1 b | 4 ± 2 b | |
| T5 | 2043 ± 356 bc | 1.29 ± 0.08 b | 26.4 ± 4.7 b | 4 ± 3 b | |
| 2011 | T0 | 1227 ± 490 b | 1.37 ± 0.08 b | 16.8 ± 5.7 b | |
| T1 | 2403 ± 544 a | 1.87 ± 0.10 a | 45.0 ± 11.1 a | 11 ± 6 a | |
| T2 | 2457 ± 535 a | 1.98 ± 0.25 a | 48.6 ± 12.0 a | 14 ± 7 a | |
| T3 | 1599 ± 388 ab | 1.37 ± 0.05 b | 21.8 ± 3.8 b | 4 ± 3 a | |
| T4 | 1538 ± 320 ab | 1.52 ± 0.10 b | 23.3 ± 5.4 b | 2 ± 1 a | |
| T5 | 1367 ± 371 ab | 1.50 ± 0.12 b | 20.4 ± 5.9 b | 1 ± 0 a | |
| 2012 | T0 | 637 ± 182 b | 1.34 ± 0.08 b | 8.50 ± 2.4 b | |
| T1 | 1609 ± 442 a | 1.88 ± 0.13 a | 30.3 ± 14.8 a | 9 ± 6 a | |
| T2 | 1667 ± 469 a | 2.03 ± 0.17 a | 33.8 ± 13.6 a | 11 ± 6 a | |
| T3 | 1800 ± 403 a | 1.39 ± 0.04 b | 24.9 ± 6.8ab | 14 ± 8 a | |
| T4 | 1532 ± 282 a | 1.54 ± 0.08 b | 23.6 ± 4.6 ab | 7 ± 3 a | |
| T5 | 1545 ± 298 a | 1.51 ± 0.08 b | 23.3 ± 4.5 ab | 5 ± 3 a |
| Treatment | pH | EC (µS·cm−1) | Assim. P (mg·kg−1) | K (meq·100 g−1) | Na (meq·100 g−1) | Ca (meq·100 g−1) | Mg (meq·100 g−1) |
|---|---|---|---|---|---|---|---|
| T0 | 7.90 ± 0.05 | 144.2 ± 3.5 b | 22.6 ± 6.7 b | 0.45 ± 0.07 b | 0.04 ± 0.02 | 17.5 ± 2.0 | 0.85 ± 0.16 c |
| T1 | 7.91 ± 0.08 | 144.5 ± 10.7 b | 22.2 ± 7.4 b | 0.42 ± 0.04 b | 0.08 ± 0.03 | 18.2 ± 2.1 | 0.85 ± 0.12 bc |
| T2 | 7.86 ± 0.05 | 162.7 ± 17.2 a | 27.2 ± 10.3 b | 0.56 ± 0.03 ab | 0.07 ± 0.04 | 18.6 ± 2.1 | 0.93 ± 0.14 ab |
| T3 | 7.92 ± 0.09 | 165.2 ± 28.3 a | 25.4 ± 3.2 b | 0.54 ± 0.07 ab | 0.11 ± 0.06 | 18.2 ± 1.4 | 0.97 ± 0.11 a |
| T4 | 7.85 ± 0.10 | 173.7 ± 30.3 a | 28.8 ± 11.1 b | 0.59 ± 0.12 a | 0.14 ± 0.03 | 19.2 ± 2.5 | 1.13 ± 0.21 a |
| T5 | 7.91 ± 0.06 | 157.7 ± 5.7 a | 34.7 ± 4.6 a | 0.64 ± 0.09 a | 0.18 ± 0.14 | 18.3 ± 2.4 | 1.10 ± 0.23 a |
| Treatment | Fe (%) | Cu (mg·kg−1) | Mn (mg·kg−1) | Zn (mg·kg−1) | Ni (mg·kg−1) | Cr (mg·kg−1) | Pb (mg·kg−1) |
|---|---|---|---|---|---|---|---|
| T0 | 1.16 ± 0.18 | 7.39 ± 0.57 | 247.7 ± 28.5 | 21.4 ± 3.4 b | 28.1 ± 7.6 | 25.3 ± 5.8 | 22.7 ± 7.1 |
| T1 | 1.25 ± 0.24 | 7.47 ± 1.14 | 267.5 ± 49.5 | 25.4 ± 5.1 ab | 33.5 ± 6.8 | 28.3 ± 6.1 | 28.7 ± 3.9 |
| T2 | 1.34 ± 0.25 | 7.81 ± 1.25 | 281.8 ± 54.6 | 28.8 ± 5.0 a | 32.6 ± 8.8 | 30.4 ± 10.3 | 26.7 ± 7.2 |
| T3 | 1.31 ± 0.22 | 9.18 ± 2.23 | 280.2 ± 45.9 | 26.6 ± 4.2 ab | 30.4 ± 12.8 | 28.4 ± 11.3 | 22.7 ± 11.1 |
| T4 | 1.07 ± 0.51 | 8.58 ± 0.60 | 282.6 ± 30.0 | 27.4 ± 3.9 ab | 32.3 ± 11.1 | 31.0 ± 10.7 | 26.1 ± 10.0 |
| T5 | 1.29 ± 0.25 | 7.89 ± 1.22 | 292.7 ± 41.9 | 26.9 ± 4.3 ab | 31.2 ± 10.1 | 28.5 ± 9.0 | 23.8 ± 6.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Bravo-Sánchez, C.T.; Martín-Gil, J.; Martín-Ramos, P. Dried Pig Manure from a Cogeneration Plant as a Fertilizer for Nitrate Vulnerable Zones. Agronomy 2019, 9, 46. https://doi.org/10.3390/agronomy9020046
Sánchez-Báscones M, Antolín-Rodríguez JM, Bravo-Sánchez CT, Martín-Gil J, Martín-Ramos P. Dried Pig Manure from a Cogeneration Plant as a Fertilizer for Nitrate Vulnerable Zones. Agronomy. 2019; 9(2):46. https://doi.org/10.3390/agronomy9020046
Chicago/Turabian StyleSánchez-Báscones, Mercedes, Juan M. Antolín-Rodríguez, Carmen T. Bravo-Sánchez, Jesús Martín-Gil, and Pablo Martín-Ramos. 2019. "Dried Pig Manure from a Cogeneration Plant as a Fertilizer for Nitrate Vulnerable Zones" Agronomy 9, no. 2: 46. https://doi.org/10.3390/agronomy9020046
APA StyleSánchez-Báscones, M., Antolín-Rodríguez, J. M., Bravo-Sánchez, C. T., Martín-Gil, J., & Martín-Ramos, P. (2019). Dried Pig Manure from a Cogeneration Plant as a Fertilizer for Nitrate Vulnerable Zones. Agronomy, 9(2), 46. https://doi.org/10.3390/agronomy9020046