Analysis of Wheat Bread-Making Gene (wbm) Evolution and Occurrence in Triticale Collection Reveal Origin via Interspecific Introgression into Chromosome 7AL
Abstract
1. Introduction
2. Material and Methods
2.1. Identification of wbm-Like Gene
2.2. Multiple Alignment of wbm and Related Protein and Nucleotide Sequences
2.3. Phylogenetic Tree Construction
2.4. wbm-Like Expression Analysis
2.5. Plant Material
2.6. DNA Isolation and PCR Analysis
3. Results
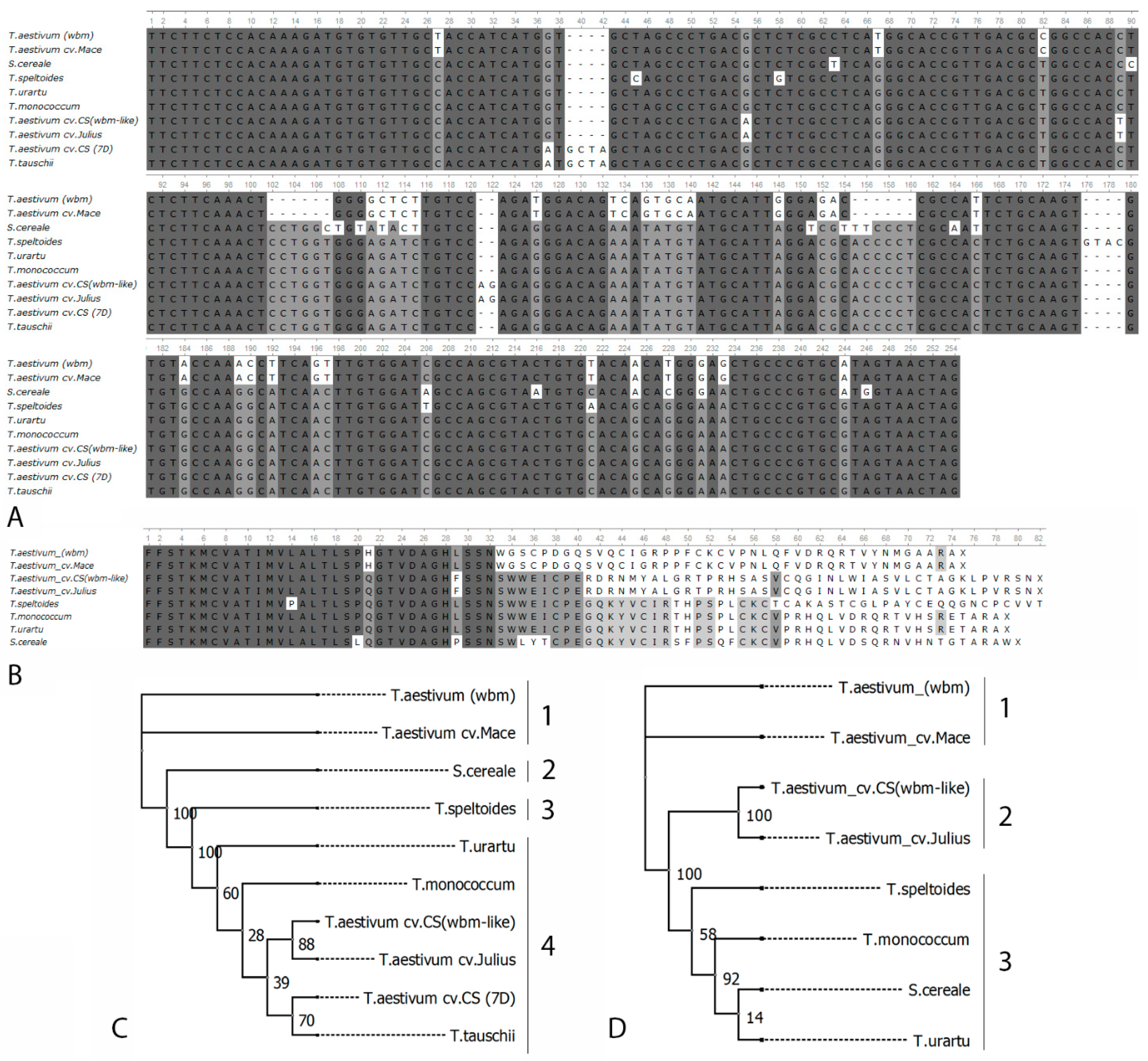
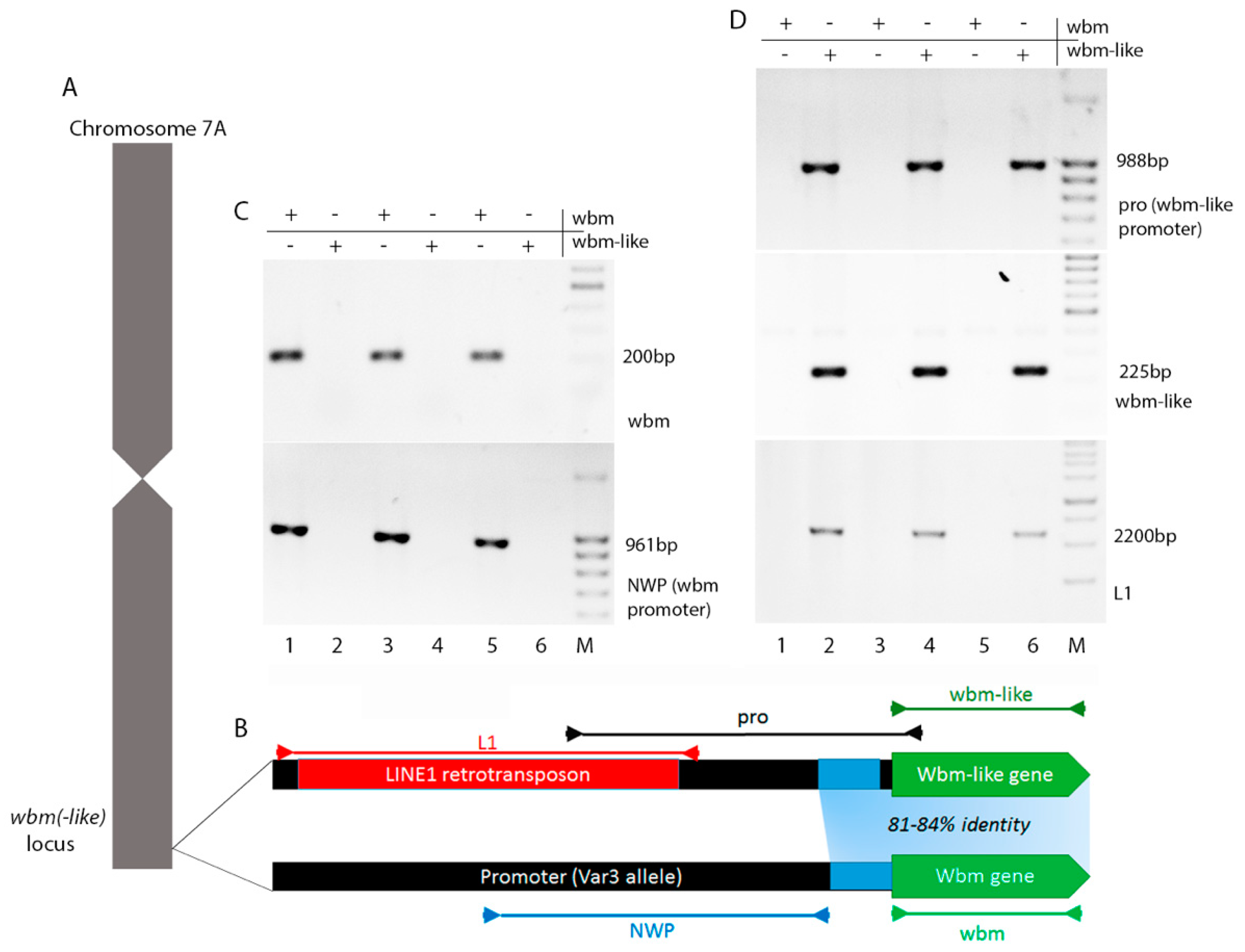
3.1. wbm Located on Chromosome 7A
3.2. Comparative Analysis of wbm and wbm-Like
3.3. Screening of Triticale Collection in the Presence of wbm
3.4. Genomic Region of wbm-Like Lacks Conservation with wbm and Surrounded by Transposon Insertions
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guzmán, C.; Xiao, Y.; Crossa, J.; González-Santoyo, H.; Huerta, J.; Singh, R.; Dreisigacker, S. Sources of the highly expressed wheat bread making (wbm) gene in CIMMYT spring wheat germplasm and its effect on processing and bread-making quality. Euphytica 2016, 209, 689–692. [Google Scholar] [CrossRef]
- Payne, P.I.; Nightingale, M.A.; Krattiger, A.F.; Holt, L.M. The relationship between HMW glutenin subunit composition and the bread-making quality of British-grown wheat varieties. J. Sci. Food Agric. 1987, 40, 51–65. [Google Scholar] [CrossRef]
- Morris, C.F. Puroindolines: The molecular genetic basis of wheat grain hardness. Plant Mol. Biol. 2002, 48, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, C.; Alvarez, J.B. Wheat waxy proteins: Polymorphism, molecular characterization and effects on starch properties. Theor. Appl. Genet. 2016, 129, 1–16. [Google Scholar] [CrossRef] [PubMed]
- D’Ovidio, R.; Masci, S.; Porceddu, E. Development of a set of oligonucleotide primers specific for genes at the Glu-1 complex loci of wheat. Theor. Appl. Genet. 1995, 91, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Lafiandra, D.; Tucci, G.; Pavoni, A.; Turchetta, T.; Margiotta, B. PCR analysis of x-and y-type genes present at the complex Glu-A1 locus in durum and bread wheat. Theor. Appl. Genet. 1997, 94, 235–240. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, W.; Gale, K. Multiplex-PCR typing of high molecular weight glutenin alleles in wheat. Euphytica 2003, 134, 51–60. [Google Scholar] [CrossRef]
- Liang, X.; Zhen, S.; Han, C.; Wang, C.; Li, X.; Ma, W.; Yan, Y. Molecular characterization and marker development for hexaploid wheat-specific HMW glutenin subunit 1By18 gene. Mol. Breed. 2015, 35, 221. [Google Scholar] [CrossRef]
- Kiszonas, A.M.; Morris, C.F. Wheat breeding for quality: A historical review. Cereal Chem. 2018, 95, 17–34. [Google Scholar] [CrossRef]
- Rasheed, A.; Jin, H.; Xiao, Y.; Zhang, Y.; Hao, Y.; Zhang, Y.; Hickey, L.T.; Morgounov, A.I.; Xia, X.; He, Z. Allelic effects and variations for key bread-making quality genes in bread wheat using high-throughput molecular markers. J. Cereal Sci. 2019, 85, 305–309. [Google Scholar] [CrossRef]
- Furtado, A.; Bundock, P.C.; Banks, P.; Fox, G.; Yin, X.; Henry, R. A novel highly differentially expressed gene in wheat endosperm associated with bread quality. Sci. Rep. 2015, 5, 10446. [Google Scholar] [CrossRef] [PubMed]
- Jessop, R.S. Stress tolerance in newer triticales compared to other cereals. In Triticale: Today and Tomorrow; Springer: Berlin/Heidelberg, Germany, 1996; pp. 419–427. [Google Scholar]
- Furman, B.J.; Qualset, C.O.; Skovmand, B.; Heaton, J.H.; Corke, H.; Wesenberg, D.M. Characterization and analysis of North American triticale genetic resources. Crop Sci. 1997, 37, 1951–1959. [Google Scholar] [CrossRef]
- Dennett, A.L.; Cooper, K.V.; Trethowan, R.M. The genotypic and phenotypic interaction of wheat and rye storage proteins in primary triticale. Euphytica 2013, 194, 235–242. [Google Scholar] [CrossRef]
- Blum, A. The abiotic stress response and adaptation of triticale—A review. Cereal Res. Commun. 2014, 42, 359–375. [Google Scholar] [CrossRef]
- Randhawa, H.; Bona, L.; Graf, R. Triticale breeding—Progress and prospect. In Triticale; Springer: Berlin/Heidelberg, Germany, 2015; pp. 15–32. [Google Scholar]
- Liu, W.; Maurer, H.P.; Leiser, W.L.; Tucker, M.R.; Weissmann, S.; Hahn, V.; Würschum, T. Potential for marker-assisted simultaneous improvement of grain and biomass yield in triticale. Bioenergy Res. 2017, 10, 449–455. [Google Scholar] [CrossRef]
- Ayalew, H.; Kumssa, T.T.; Butler, T.J.; Ma, X.-F. Triticale Improvement for Forage and Cover Crop Uses in the Southern Great Plains of the United States. Front. Plant Sci. 2018, 9, 1130. [Google Scholar] [CrossRef]
- Woś, H.; Brzeziński, W. Triticale for food—The quality driver. In Triticale; Springer: Berlin/Heidelberg, Germany, 2015; pp. 213–232. [Google Scholar]
- McGoverin, C.M.; Snyders, F.; Muller, N.; Botes, W.; Fox, G.; Manley, M. A review of triticale uses and the effect of growth environment on grain quality. J. Sci. Food Agric. 2011, 91, 1155–1165. [Google Scholar] [CrossRef]
- Consortium, I.W.G.S. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar]
- Alaux, M.; Rogers, J.; Letellier, T.; Flores, R.; Alfama, F.; Pommier, C.; Mohellibi, N.; Durand, S.; Kimmel, E.; Michotey, C.; et al. Linking the International Wheat Genome Sequencing Consortium bread wheat reference genome sequence to wheat genetic and phenomic data. Genome Biol. 2018, 19, 111. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Bian, X.; Tyrrell, S.; Davey, R.P. The Grassroots Life Science Data Infrastructure (2017). Available online: https://grassroots.tools (accessed on 1 September 2019).
- Howe, K.L.; Contreras-Moreira, B.; De Silva, N.; Maslen, G.; Akanni, W.; Allen, J.; Alvarez-Jarreta, J.; Barba, M.; Bolser, D.M.; Cambell, L. Ensembl Genomes 2020—Enabling non-vertebrate genomic research. Nucleic Acids Res. 2019. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; Team, U. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-González, R.; Borrill, P.; Lang, D.; Harrington, S.; Brinton, J.; Venturini, L.; Davey, M.; Jacobs, J.; Van Ex, F.; Pasha, A. The transcriptional landscape of polyploid wheat. Science 2018, 361, eaar6089. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- He, F.; Pasam, R.; Shi, F.; Kant, S.; Keeble-Gagnere, G.; Kay, P.; Forrest, K.; Fritz, A.; Hucl, P.; Wiebe, K. Exome sequencing highlights the role of wild-relative introgression in shaping the adaptive landscape of the wheat genome. Nat. Genet. 2019, 51, 896. [Google Scholar] [CrossRef]
- Fesenko, I.; Kirov, I.; Kniazev, A.; Khazigaleeva, R.; Lazarev, V.; Kharlampieva, D.; Grafskaia, E.; Zgoda, V.; Butenko, I.; Arapidi, G. Distinct types of short open reading frames are translated in plant cells. Genome Res. 2019, 29, 1464–1477. [Google Scholar] [CrossRef]
- Salmanowicz, B.P.; Dylewicz, M. Identification and characterization of high-molecular-weight glutenin genes in Polish triticale cultivars by PCR-based DNA markers. J. Appl. Genet. 2007, 48, 347–357. [Google Scholar] [CrossRef]
- Amiour, N.; Bouguennec, A.; Marcoz, C.; Sourdille, P.; Bourgoin, M.; Khelifi, D.; Branlard, G. Diversity of seven glutenin and secalin loci within triticale cultivars grown in Europe. Euphytica 2002, 123, 295–305. [Google Scholar] [CrossRef]
| Primer ID | Primers 5′-3′ | Expected Length of PCR Product (bp) |
|---|---|---|
| pro | F: TTGAAGAGAAGTGGCCAGCG R: GTTCATGCGATGCAGAGAGC | 988 |
| L1 | F: ACAACATCACAAGTGGAAAGGC R: AGGTTGGTAGTAATTCCAAATTCAAGT | 2200 |
| wbm | F: TGTGTGTTGCTACCATCATGG R: GGCAGCTCCCATGTTGTACA | 219 |
| wbm-like | F: TTACTACGCACGGGCAGTTT R: GTGTGTTGCCACCATCATGG | 225 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirov, I.; Pirsikov, A.; Milyukova, N.; Dudnikov, M.; Kolenkov, M.; Gruzdev, I.; Siksin, S.; Khrustaleva, L.; Karlov, G.; Soloviev, A. Analysis of Wheat Bread-Making Gene (wbm) Evolution and Occurrence in Triticale Collection Reveal Origin via Interspecific Introgression into Chromosome 7AL. Agronomy 2019, 9, 854. https://doi.org/10.3390/agronomy9120854
Kirov I, Pirsikov A, Milyukova N, Dudnikov M, Kolenkov M, Gruzdev I, Siksin S, Khrustaleva L, Karlov G, Soloviev A. Analysis of Wheat Bread-Making Gene (wbm) Evolution and Occurrence in Triticale Collection Reveal Origin via Interspecific Introgression into Chromosome 7AL. Agronomy. 2019; 9(12):854. https://doi.org/10.3390/agronomy9120854
Chicago/Turabian StyleKirov, Ilya, Andrey Pirsikov, Natalia Milyukova, Maxim Dudnikov, Maxim Kolenkov, Ivan Gruzdev, Stanislav Siksin, Ludmila Khrustaleva, Gennady Karlov, and Alexander Soloviev. 2019. "Analysis of Wheat Bread-Making Gene (wbm) Evolution and Occurrence in Triticale Collection Reveal Origin via Interspecific Introgression into Chromosome 7AL" Agronomy 9, no. 12: 854. https://doi.org/10.3390/agronomy9120854
APA StyleKirov, I., Pirsikov, A., Milyukova, N., Dudnikov, M., Kolenkov, M., Gruzdev, I., Siksin, S., Khrustaleva, L., Karlov, G., & Soloviev, A. (2019). Analysis of Wheat Bread-Making Gene (wbm) Evolution and Occurrence in Triticale Collection Reveal Origin via Interspecific Introgression into Chromosome 7AL. Agronomy, 9(12), 854. https://doi.org/10.3390/agronomy9120854






