Spatial and Temporal Distribution of Ecballium elaterium in Almond Orchards
Abstract
1. Introduction
2. Material and Methods
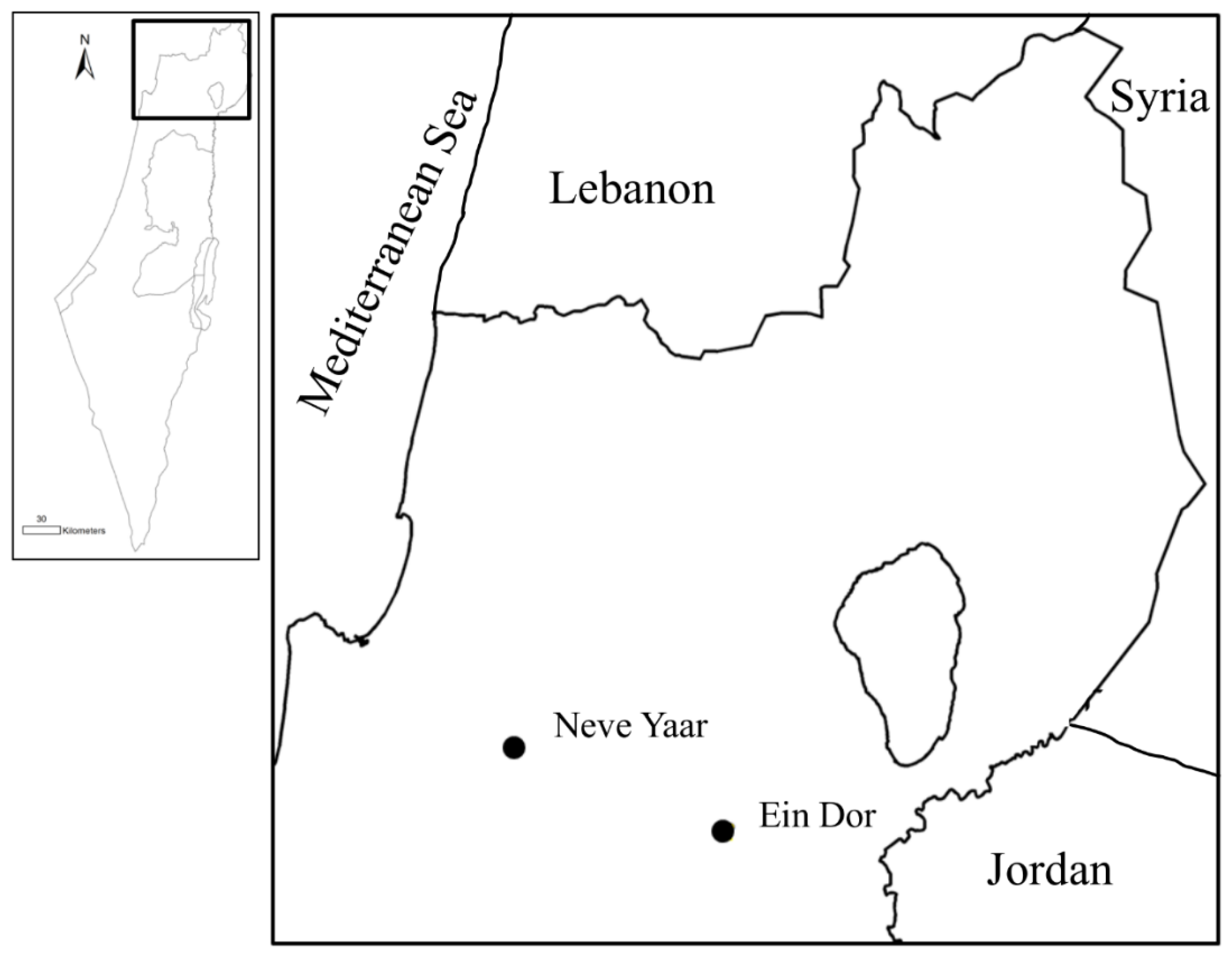
2.1. Study Sites and Data Collection
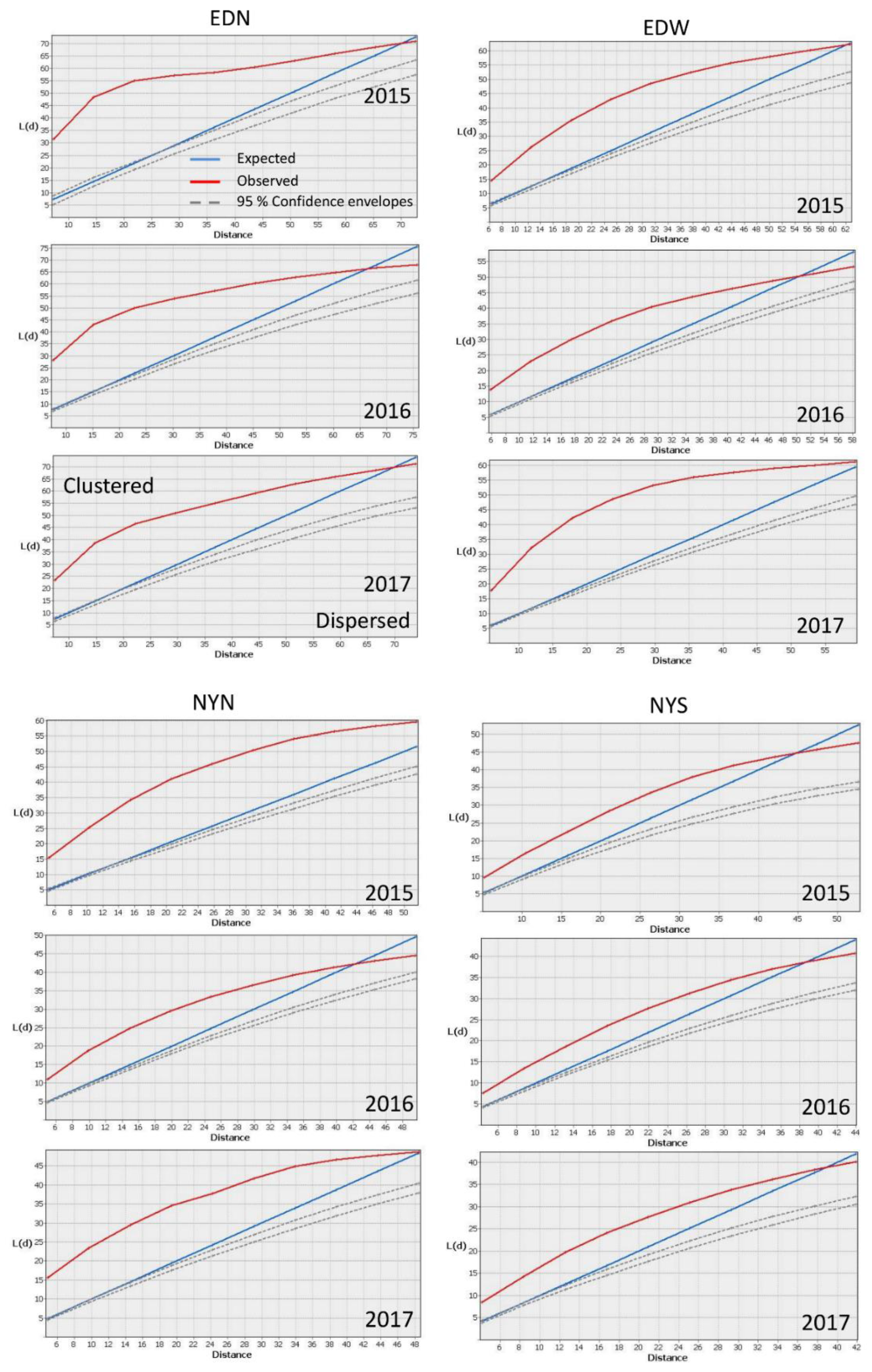
2.2. Spatially Analyzing Patterns
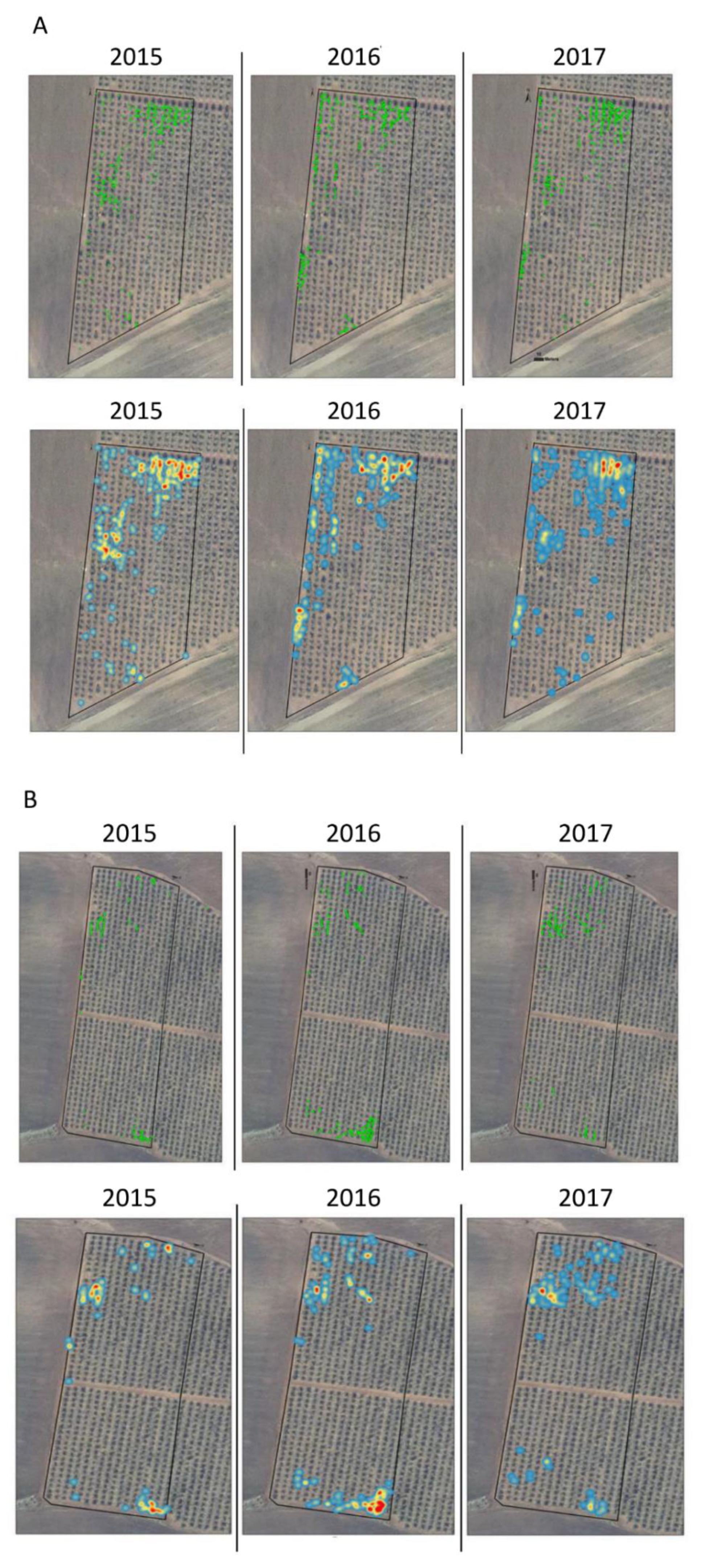
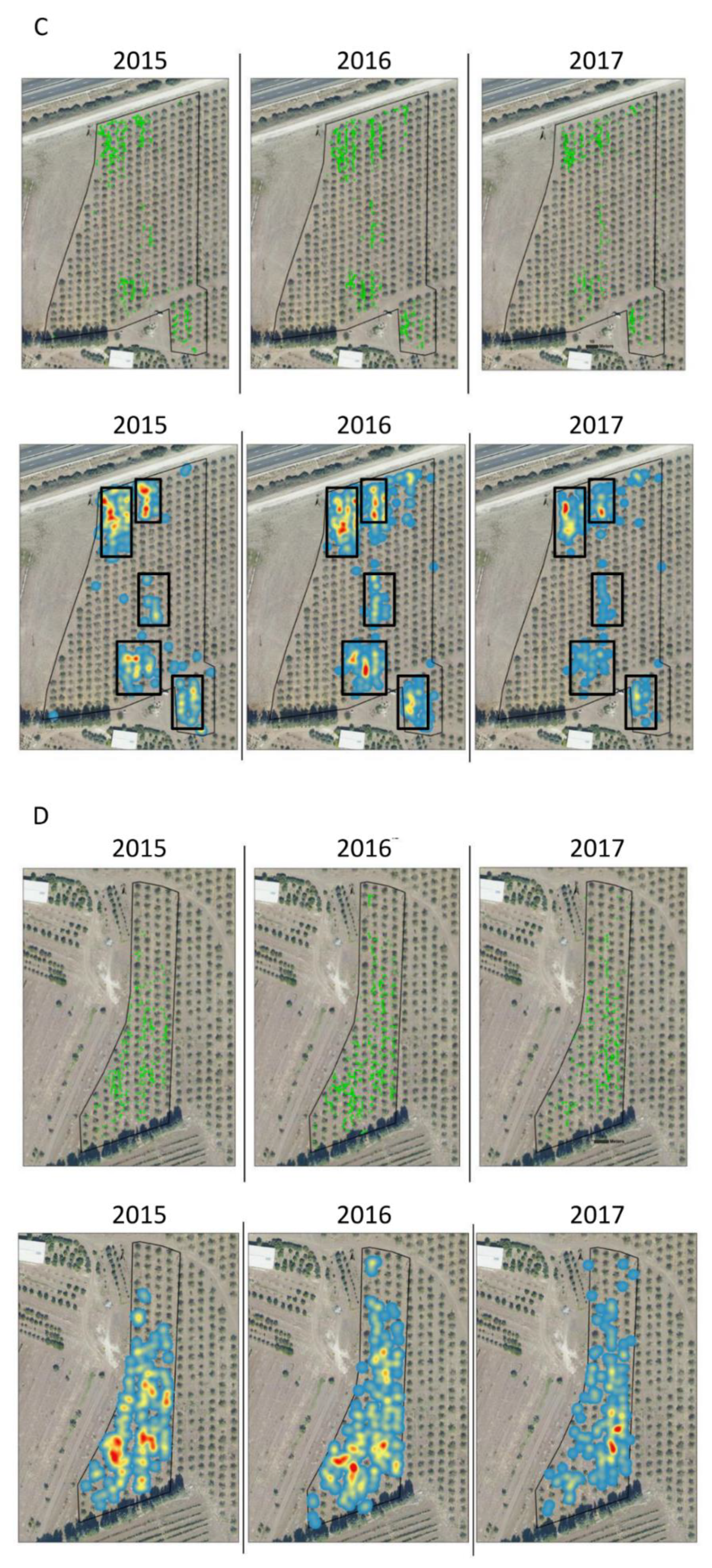
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steward, B.L.; Tian, L.F. Real-time machine vision weed-sensing. ASAE Pap. 1998. Paper number 983033. [Google Scholar]
- Lopez-Granados, F. Weed detection for site-specific weed management: Mapping and real-time approaches. Weed Res. 2011, 51, 1–11. [Google Scholar] [CrossRef]
- Dieleman, J.A.; Mortensen, D.A.; Cooper, E.M.; Sims, J.T.; Cunningham, S.D.; Huang, J.W.; Berti, W.R. Characterizing the spatial pattern of Abutilon theophrasti seedling patches. Weed Res. 1999, 39, 455–467. [Google Scholar] [CrossRef]
- Heijting, S.; Van Der Werf, W.; Stein, A.; Kropff, M.J. Are weed patches stable in location? Application of an explicitly two-dimensional methodology. Weed Res. 2007, 47, 381–395. [Google Scholar] [CrossRef]
- Andújar, D.; Barroso, J.; Fernández-Quintanilla, C.; Dorado, J. Spatial and temporal dynamics of Sorghum halepense patches in maize crops. Weed Res. 2012, 52, 411–420. [Google Scholar] [CrossRef]
- Calha, I.M.; Sousa, E.; González-Andújar, J.L. Infestation maps and spatial stability of main weed species in maize culture. Planta Daninha 2014, 32, 275–282. [Google Scholar] [CrossRef]
- San Martín, C.; Andújar, D.; Fernández-Quintanilla, C.; Dorado, J. Spatial distribution patterns of weed communities in corn fields of central Spain. Weed Sci. 2015, 63, 936–945. [Google Scholar] [CrossRef]
- Metcalfe, H.; Milne, A.E.; Webster, R.; Lark, R.M.; Murdoch, A.J.; Kanelo, L.; Storkey, J. Defining the habitat niche of Alopecurus myosuroides at the field scale. Weed Res. 2018, 58, 165–176. [Google Scholar] [CrossRef]
- Walter, A.M.; Christensen, S.; Simmelsgaard, S.E. Spatial correlation between weed species densities and soil properties. Weed Res. 2002, 42, 26–38. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Scopel, A.L.; Ghersa, C.M.; Sánchez, R.A. The population ecology of Datura ferox in soybean crops. A simulation approach incorporating seed dispersal. Agric. Ecosyst. Environ. 1987, 19, 177–188. [Google Scholar] [CrossRef]
- Blank, L.; Carmel, Y. Woody vegetation patch types affect herbaceous species richness and composition in a Mediterranean ecosystem. Community Ecol. 2012, 13, 72–81. [Google Scholar] [CrossRef]
- Cardina, J.; Johnson, G.A.; Sparrow, D.H. The nature and consequence of weed spatial distribution. Weed Sci. 1997, 45, 364–373. [Google Scholar] [CrossRef]
- Thill, D.C.; Mallory-Smith, C.A. The nature and consequence of weed spread in cropping systems. Weed Sci. 1997, 45, 337–342. [Google Scholar] [CrossRef]
- Blanco-Moreno, J.M.; Chamorro, L.; Masalles, R.M.; Recasens, J.; Sans, F.X. Spatial distribution of Lolium rigidum seedlings following seed dispersal by combine harvesters. Weed Res. 2004, 44, 375–387. [Google Scholar] [CrossRef]
- Garibay, S.V.; Richner, W.; Stamp, P.; Nakamoto, T.; Yamagishi, J.; Abivardi, C.; Edwards, P.J. Extent and implications of weed spatial variability in arable crop fields. Plant Prod. Sci. 2001, 4, 259–269. [Google Scholar] [CrossRef]
- Gerhards, R.; Christensen, S. Real-time weed detection, decision making and patch spraying in maize, sugarbeet, winter wheat and winter barley. Weed Res. 2003, 43, 385–392. [Google Scholar] [CrossRef]
- Gerhards, R.; Wyse-Pester, D.Y.; Johnson, G.A. Characterizing spatial stability of weed populations using interpolated maps. Weed Sci. 1997, 45, 108–119. [Google Scholar] [CrossRef]
- Wilson, B.J.; Brain, P. Long-term stability of distribution of Alopecurus myosuroides Huds. within cereal fields. Weed Res. 1991, 31, 367–373. [Google Scholar] [CrossRef]
- Wyse-Pester, D.Y.; Mortensen, D.A.; Gotway, C.A. Statistical methods to quantify spatial stability of weed population. Proc. North Cent. Weed Control Conf. 1995, 50, 512. [Google Scholar]
- Johnson, G.A.; Mortensen, D.A.; Gotway, C.A. Spatial and temporal analysis of weed seedling populations using geostatistics. Weed Sci. 1996, 44, 704–710. [Google Scholar] [CrossRef]
- Myers, M.W.; Curran, W.S.; Vangessel, M.J.; Majek, B.A.; Scott, B.A.; Mortensen, D.A.; Calvin, D.D.; Karsten, H.D.; Roth, G.W. The effect of weed density and application timing on weed control and corn grain yield. Weed Technol. 2005, 19, 102–107. [Google Scholar] [CrossRef]
- Lati, R.N.; Filin, S.; Eizenberg, H. Effect of tuber density and trifloxysulfuron application timing on purple nutsedge (Cyperus rotundus) control. Weed Sci. 2012, 60, 494–500. [Google Scholar] [CrossRef]
- Cousens, R. A simple model relating yield loss to weed density. Ann. Appl. Biol. 1985, 107, 239–252. [Google Scholar] [CrossRef]
- Longchamps, L.; Panneton, B.; Reich, R.; Simard, M.-J.; Leroux, G.D. Spatial pattern of weeds based on multispecies infestation maps created by imagery. Weed Sci. 2016, 64, 474–485. [Google Scholar] [CrossRef]
- Colbach, N.; Forcella, F.; Johnson, G.A. Spatial and temporal stability of weed populations over five years. Weed Sci. 2000, 48, 366–377. [Google Scholar] [CrossRef]
- Mueller-Warrant, G.W.; Whittaker, G.W.; Young, W.C. GIS analysis of spatial clustering and temporal change in weeds of grass seed crops. Weed Sci. 2008, 56, 647–669. [Google Scholar] [CrossRef]
- Mitchel, A. The ESRI Guide to GIS Analysis, Volume 2: Spatial Measurements and Statistics; ESRI Press: Redlands, CA, USA, 2005. [Google Scholar]
- Dafni, A.; Benítez, G.C.; Blanché, C.; Rammón-Laca, L.; Petanidou, T.; Aytaç, B.; Horvat, M.; Lucchese, F.; Geva-Kleinberger, A. The etymological, ecological, historical and ethnobotanical roots of the vernacular names of Ecballium elaterium (L.) Rich. (Squirting cucumber). J. Ethnobiol. Tradit. Med. Photon 2013, 119, 515–537. [Google Scholar]
- Lewes, D. Observations on the internal pressure of the ripening fruit of Ecballium elaterium. Kew Bull. 1951, 6, 443–444. [Google Scholar] [CrossRef]
- Beckie, H.J.; Reboud, X. Selecting for weed resistance: Herbicide rotation and mixture. Weed Technol. 2009, 23, 363–370. [Google Scholar] [CrossRef]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef]
- CRDC Integrated weed management Hub. Section 3: Herbicide Resistance. Grains Research and Development Corporation, Australia Government; CRDC Integrated weed management Hub: Canberra, Australia, 2014. [Google Scholar]
- Sezen, U.U.; Chazdon, R.L.; Holsinger, K.E. Proximity is not a proxy for parentage in an animal-dispersed Neotropical canopy palm. Proc. R. Soc. B 2009, 276, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Wehenkel, C.; Brazão-Protázio, J.M.; Carrillo-Parra, A.; Martínez-Guerrero, J.H.; Crecente-Campo, F. Spatial distribution patterns in the very rare and species-rich Picea chihuahuana tree community (Mexico). PLoS ONE 2015, 10, e0140442. [Google Scholar]
- Ripley, B.D. Spatial Statistics; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 575. [Google Scholar]
- Lynch, H.J.; Moorcroft, P.R. A spatiotemporal Ripley’s K-function to analyze interactions between spruce budworm and fire in British Columbia, Canada. Can. J. For. Res. 2008, 38, 3112–3119. [Google Scholar] [CrossRef]
- Birger, N. Environmental Friendly Management of Ecballium elaterium in Almond Orchards Based on Understanding the Relationship Between Spatial Distribution, Weed Biology and Chemical Control. Master’s Thesis November 2018. [Google Scholar]
- Roham, R.; Pirdashti, H.; Yaghubi, M.; Nematzadeh, G. Spatial distribution of nutsedge (Cyperus spp. L.) seed bank in rice growth cycle using geostatistics. Crop Prot. 2014, 55, 133–141. [Google Scholar] [CrossRef]
- Andújar, D.; Ruiz, D.; Ribeiro, Á.; Fernández-Quintanilla, C.; Dorado, J. Spatial distribution patterns of johnsongrass (Sorghum halepense) in commercial corn fields in Spain. Weed Sci. 2011, 59, 82–89. [Google Scholar] [CrossRef]
- Mitskas, M.B.; Tsolis, C.E.; Eleftherohorinos, I.G.; Damalas, C.A. Interference between corn and johnsongrass (Sorghum halepense) from seed or rhizomes. Weed Sci. 2003, 51, 540–545. [Google Scholar] [CrossRef]
- Barroso, J.; Andújar, D.; San Martín, C.; Fernández-Quintanilla, C.; Dorado, J. Johnsongrass (Sorghum halepense) seed dispersal in corn crops under Mediterranean conditions. Weed Sci. 2012, 60, 34–41. [Google Scholar] [CrossRef]
- Hubbell, S.P.; Foster, R.B.; O’Brien, S.T.; Harms, K.E.; Condit, R.; Wechsler, B.; Wright, S.J.; De Lao, S.L. Light-gap disturbances, recruitment limitation, and tree diversity in a neotropical forest. Science 1999, 283, 554–557. [Google Scholar] [CrossRef]
- Plotkin, J.B.; Chave, J.; Ashton, P.S. Cluster analysis of spatial patterns in Malaysian tree species. Am. Nat. 2002, 160, 629–644. [Google Scholar] [CrossRef]
- Gunatilleke, C.V.S.; Gunatilleke, I.; Esufali, S.; Harms, K.E.; Ashton, P.M.S.; Burslem, D.; Ashton, P.S. Species–habitat associations in a Sri Lankan dipterocarp forest. J. Trop. Ecol. 2006, 22, 371–384. [Google Scholar] [CrossRef]
- Mendelsohn, O.; Dayan, T.; Aidlin-Harari, S.; Silberstein, M.; Orlov, V.; Blank, L. Mediterranean fruit fly subplot hot spots prediction by experts’ experience. J. Appl. Entomol. 2018, 142, 371–379. [Google Scholar] [CrossRef]
- Bar-Massada, A.; Belmaker, J. Non-stationarity in the co-occurrence patterns of species across environmental gradients. J. Ecol. 2017, 105, 391–399. [Google Scholar] [CrossRef]
- Wiegand, T.; Gunatilleke, S.; Gunatilleke, N. Species associations in a heterogeneous Sri Lankan dipterocarp forest. Am. Nat. 2007, 170, E77–E95. [Google Scholar] [CrossRef] [PubMed]
- Nordmeyer, H.; Dunker, M.; Stafford, J.V. Variable weed densities and soil properties in a weed mapping concept for patchy weed control. In Proceedings of the Precision agriculture ’99, Part 1; Sheffield Academic Press: Odense Denmark, 1999; pp. 453–462. [Google Scholar]
- Andújar, D.; Rueda-Ayala, V.; Dorado, J.; Gerhards, R.; Fernández-Quintanilla, C. Multivariate Analysis of the Agricultural Management Presence of Sorghum halepense (L.) Pers. Relationships in Maize Crops. Gesunde Pflanz. 2014, 66, 17–22. [Google Scholar]
- Timmermann, C.; Gerhards, R.; Kühbauch, W. The economic impact of site-specific weed control. Precis. Agric. 2003, 4, 249–260. [Google Scholar] [CrossRef]

| Plot Name | Abbreviations | Plantation Year | Survey Area (m2) |
|---|---|---|---|
| Ein Dor West | EDW | 2006 | 24,800 |
| Ein Dor North | EDN | 2007 | 32,800 |
| Newe Yaar North | NYN | 2007 | 18,900 |
| Newe Yaar South | NYS | 2007 | 6600 |
| 2015 | 2016 | 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Oct-Dec (2014) | Jan–Mar | Apr–Jun | Oct–Dec (2015) | Jan–Mar | Apr–Jun | Oct–Dec (2016) | Jan–Mar | Apr–Jun | |
| Newe Yaar | |||||||||
| Average Temperature (°C) | 18.2 ± 5.2 | 13.4 ± 5 | 21.5 ± 5.9 | 18.4 ± 6.2 | 14.3 ± 5.2 | 23.0 ± 5.9 | 17.8 ± 6.5 | 12.4 ± 4.7 | 22.0 ± 5.7 |
| Precipitation (mm) | 186.8 | 379.9 | 78.1 | 141.2 | 334.6 | 17 | 312.1 | 179.6 | 23.9 |
| Ein Dor | |||||||||
| Average Temperature (°C) | 18.1 ± 5.4 | 13.3 ± 5.2 | 21.9 ± 6.3 | 18.5 ± 6.3 | 14.2 ± 5.2 | 23.0 ± 5.9 | 18.0 ± 6.9 | 12.4 ± 4.8 | 22.9 ± 6.1 |
| Precipitation (mm) | 178.6 | 297.7 | 68.5 | 122 | 308.8 | 13.3 | 242.1 | 158.8 | 20.5 |
| Plot Name | Number of Plants | Average Distance between Plants (M) | ANN Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | |
| EDW | 297 | 505 | 526 | 2.89 | 1.09 | 1.37 | 0.63 | 0.33 | 0.41 |
| EDN | 155 | 294 | 191 | 2.42 | 1.36 | 1.87 | 0.29 | 0.27 | 0.33 |
| NYN | 443 | 768 | 467 | 1.47 | 0.72 | 1.04 | 0.4 | 0.34 | 0.37 |
| NYS | 280 | 515 | 452 | 1.62 | 0.87 | 1.22 | 0.56 | 0.42 | 0.49 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blank, L.; Birger, N.; Eizenberg, H. Spatial and Temporal Distribution of Ecballium elaterium in Almond Orchards. Agronomy 2019, 9, 751. https://doi.org/10.3390/agronomy9110751
Blank L, Birger N, Eizenberg H. Spatial and Temporal Distribution of Ecballium elaterium in Almond Orchards. Agronomy. 2019; 9(11):751. https://doi.org/10.3390/agronomy9110751
Chicago/Turabian StyleBlank, Lior, Nitzan Birger, and Hanan Eizenberg. 2019. "Spatial and Temporal Distribution of Ecballium elaterium in Almond Orchards" Agronomy 9, no. 11: 751. https://doi.org/10.3390/agronomy9110751
APA StyleBlank, L., Birger, N., & Eizenberg, H. (2019). Spatial and Temporal Distribution of Ecballium elaterium in Almond Orchards. Agronomy, 9(11), 751. https://doi.org/10.3390/agronomy9110751





