Pre-Harvest Foliar Application of Mineral Nutrients to Retard Chlorophyll Degradation and Preserve Bio-Active Compounds in Broccoli
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Determination of Plant Growth
2.3. Determination of Postharvest Quality
2.4. Chemicals and Bioactive Compounds
2.4.1. Nitrogen, Crude Protein, Phosphorus, Potassium, and Vitamin C
2.4.2. Calcium, Iron, Manganese, and Zinc Content
2.4.3. Total Chlorophylls, Phenolic Contents, Total Flavonoids
2.4.4. Sulforaphane Extraction
2.4.5. Extraction of Glucosinolates
2.4.6. Peroxidase Activity
2.5. Statistical Analysis
3. Results and Discussion
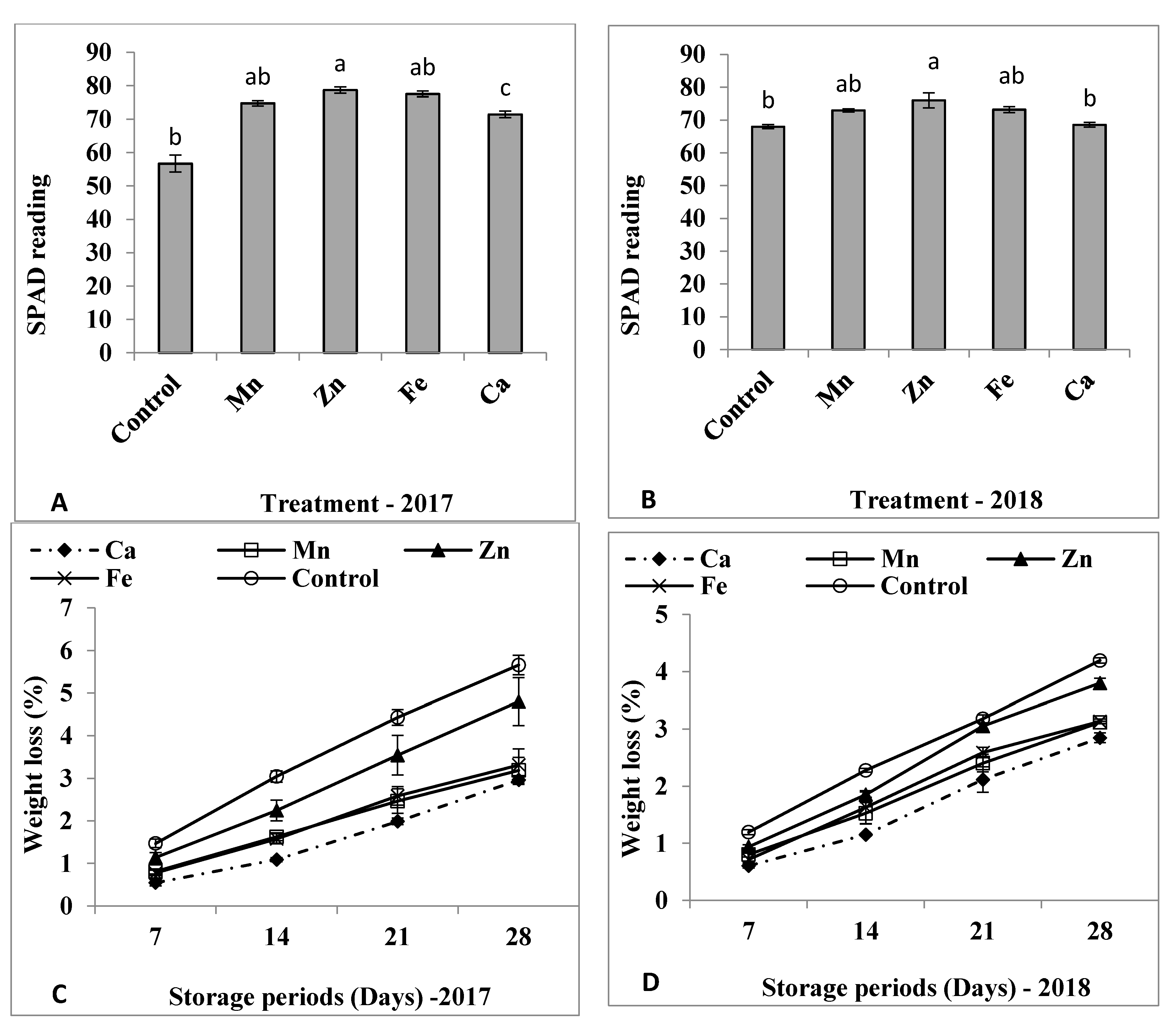
3.1. Plant Growth and SPAD
3.2. Weight Loss
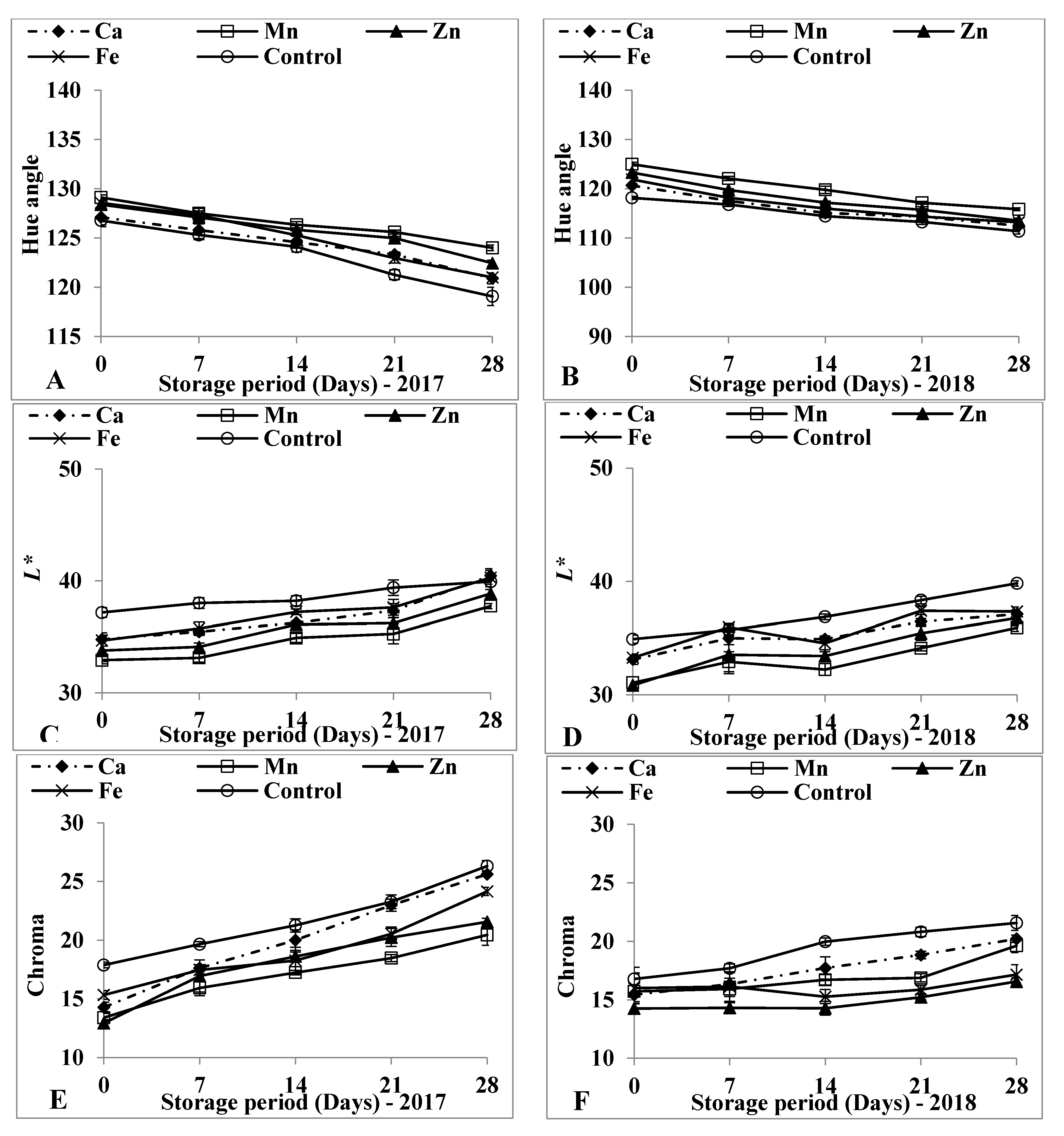
3.3. Surface Colour
3.4. Mineral Elements
3.5. Bioactive Compounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, L.; Zhu, Y.; Wang, F. Calcium sulfate treatment enhances bioactive compounds and antioxidant capacity in broccoli sprouts during growth and storage. Postharvest Biol. Technol. 2018, 139, 12–19. [Google Scholar] [CrossRef]
- Ain, Q.; Ayub, G.; Ilyas, M.; Ahmad, M.; Begum, F.; Saeed, A.; Shah, K. Response of broccoli to foliar application of zinc and boron concentrations. Pure Appl. Biol. 2016, 5, 1. [Google Scholar] [CrossRef]
- Shi, J.; Gao, L.; Zuo, J.; Wang, Q.; Wang, Q.; Fan, L. Exogenous sodium nitroprusside treatment of broccoli heads extends shelf life, enhances antioxidant enzyme activity, and inhibits chlorophyll-degradation. Postharvest Biol. Technol. 2016, 116, 98–104. [Google Scholar] [CrossRef]
- Ben-Fadhel, Y.; Saltaji, S.; Khlifi, M.A.; Salmieri, S.; Dang, K.; Lacroix, M. Active edible coating and γ-irradiation as cold combined treatments to assure the safety of broccoli heads (Brassica oleracea L.). Int. J. Food Microbiol. 2017, 241, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, E.; Barrios, S.; Baenas, N.; Moreno, D.A.; Heinzen, H.; Lema, P. Effect of temperature on glucosinolate content and shelf life of ready-to-eat broccoli heads packaged in passive modified atmosphere. Postharvest Biol. Technol. 2018, 138, 125–133. [Google Scholar] [CrossRef]
- Perini, M.A.; Sin, I.N.; Reyes, J.A.M.; Gómez Lobato, M.E.; Civello, P.M.; Martínez, G.A. Hot water treatments performed in the base of the broccoli stem reduce postharvest senescence of broccoli (Brassica oleracea L. Var italic) heads stored at 20 °C. LWT 2017, 77, 314–322. [Google Scholar] [CrossRef]
- Xu, F.; Tang, Y.; Dong, S.; Shao, X.; Wang, H.; Zheng, Y.; Yang, Z. Reducing yellowing and enhancing antioxidant capacity of broccoli in storage by sucrose treatment. Postharvest Biol. Technol. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- Fernández, V.; Sotiropoulos, T.; Brown, P. Foliar Fertilization. Scientific Principles and Field Practices; International Fertilizer Industry Association: Paris, France, 2013. [Google Scholar]
- Aghdam, M.S.; Dokhanieh, A.Y.; Hassanpour, H.; Rezapour, F.J. Enhancement of antioxidant capacity of cornelian cherry (Cornus mas) fruit by postharvest calcium treatment. Sci. Hortic. 2013, 161, 160–164. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Piskin, A. Effect of Zinc applied together with compound fertilizer on yield and quality of sugar beet (Beta vulgaris L.). J. Plant Nutr. 2017, 40, 2521–2531. [Google Scholar] [CrossRef]
- Burnell, J.N. The biochemistry of manganese in plants. In Manganese in Soils and Plants; Graham, R.D., Hannam, R.J., Uren, N.C., Eds.; The Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; pp. 125–137. [Google Scholar]
- Carrasco-Gil, S.; Rios, J.J.; Álvarez-Fernández, A.; Abadía, A.; García-Mina, J.M.; Abadía, J. Effects of individual and combined metal foliar fertilisers on iron- and manganese-deficient Solanum lycopersicum plants. Plant Soil 2016, 402, 27–45. [Google Scholar] [CrossRef]
- Ozbahce, A.; Zengin, M. Effects of foliar and soil applications of different manganese fertilizers on yield and net return of bean. J. Plant Nutr. 2014, 37, 161–171. [Google Scholar] [CrossRef]
- Hansen, N.C.; Schmitt, M.A.; Anderson, J.E.; Strock, J.S. Iron deficiency of soybean in the upper Midwest and associated soil properties. Agron. J. 2003, 95, 1595–1601. [Google Scholar] [CrossRef]
- Mengel, K. Iron availability in plant tissues—Iron chlorosis on calcareous soils. Iron Nutrition in Soils and Plants. In Proceedings of the Seventh International Symposium on Iron Nutrition and Interactions in Plants, Zaragoza, Spain, 27 June–2 July 1993; Abadía, J., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 389–397. [Google Scholar]
- Chohura, P.; Kołota, E.; Komosa, A. The effect of the kind of Fe chelate on yielding and quality of greenhouse tomato fruits. Folia Hortic. 2012, 24, 109–114. [Google Scholar] [CrossRef]
- Roosta, H.R.; Mohsenian, Y. Effects of foliar spray of different Fe sources on pepper (Capsicum annum L.) plants in aquaponic system. Sci. Hortic. 2012, 146, 182–191. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis, 15th ed.; Association of Official Agricultural Chemist: Arlington, VA, USA, 1990; Volume 1, p. 673. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Text book; Printice-Hall of India Privat Limited: New Delhi, Indian, 1973; Volume 381, pp. 144–197. [Google Scholar]
- Moran, R. Formulae for Determination of Chlorophyllous Pigments Extracted with N, N-Dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Gu, Z.X.; Guo, Q.H.; Gu, Y.J. Factors Influencing Glucoraphanin and Sulforaphane Formation in Brassica Plants: A Review. J. Integr. Agric. 2012, 11, 1804–1816. [Google Scholar] [CrossRef]
- Han, D.; Row, K.H. Separation and Purification of Sulforaphane from Broccoli by Solid Phase Extraction. Int. J. Mol. Sci. 2011, 12, 1854–1861. [Google Scholar] [CrossRef]
- Bjerg, B.; Olsen, O.; Rasmussen, K.W.; Sorensen, H. New Principles of Ion-Exchange Techniques Suitable to Sample Preparation and Group Separation of Natural Products Prior to Liquid Chromatography. J. Liq. Chromatogr. 1984, 7, 691–707. [Google Scholar] [CrossRef]
- Bjerg, B.; Sørensen, H. Quantitative Analysis of Glucosinolates in Oilseed Rape Based on HPLC of Desulfoglucosinolates and HPLC of Intact Glucosinolates. In Glucosinolates in Rapeseeds: Analytical Aspects, Proceedings of a Seminar in the CEC Programme of Research on Plant Productivity, Gembloux, Belgium, 1–3 October 1987; Wathelet, J.P., Ed.; Springer: Dordrecht, The Netherlands, 1987; pp. 125–150. [Google Scholar]
- In, B.C.; Motomura, S.; Inamoto, K.; Doi, M.; Mori, G. Multivariente analysis of relation between pre-harvest environmental factors, postharvest morphological and physiological factors and vase life of cut Asomi Red Roses. Jpn. Soc. Hortic. Sci. 2007, 76, 66–72. [Google Scholar] [CrossRef][Green Version]
- Mahmoud, A.; Abdelaziz, S.; El-Mogy, M.M.; Abdeldaym, E.A. Effect of foliar ZnO and FeO nanoparticles application on growth and nutritional quality of red radish and assessment of their accumulation on human health. Agriculture (Poľnohospodárstvo) 2019, 65, 16–29. [Google Scholar] [CrossRef]
- Hadi, M.R.; Taheri, R.; Balali, G.R. Effects of Iron and Zinc Fertilizers on the Accumulation of Fe and Zn Ions in Potato Tubers. J. Plant Nutr. 2015, 38, 202–211. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.I.C.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 191–248. [Google Scholar]
- Xu, C.; Li, X.; Zhang, L. The Effect of Calcium Chloride on Growth, Photosynthesis, and Antioxidant Responses of Zoysia japonica under Drought Conditions. PLoS ONE 2013, 8, e68214. [Google Scholar] [CrossRef]
- Nath, A.; Bagchi, B.; Misra, L.K.C.; Deka, B. Changes in post-harvest phytochemical qualities of broccoli heads during ambient and refrigerated storage. Food Chem. 2011, 127, 1510–1514. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Hassanpouraghdam, M.B.; Paliyath, G.; Farmani, B. The language of calcium in postharvest life of fruits, vegetables and flowers. Sci. Hortic. 2012, 144, 102–115. [Google Scholar] [CrossRef]
- Duffy, B. Zinc and plant disease. In Mineral Nutrition and Plant Disease; Datnoff, L.E., Elmer, W.H., Huber, D.M., Eds.; The American Phytopathological Society: Sao Paulo, MN, USA, 2007; pp. 155–175. [Google Scholar]
- Burnell, J.N. The Biochemistry of Manganese in Plants. In Manganese in Soils and Plants, Proceedings of the International Symposium on ‘Manganese in Soils and Plants’ held at the Waite Agricultural Research Institute, The University of Adelaide, Glen Osmond, South Australia, 22–26 August 1988; As an Australian Bicentennial Event; Graham, R.D., Hannam, R.J., Uren, N.C., Eds.; Springer: Dordrecht, The Netherlands, 1988; pp. 125–137. [Google Scholar]
- Fageria, N.K. The Use of Nutrients in Crop Plants; Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Liu, H.; Gan, W.; Rengel, Z.; Zhao, P. Effects of zinc fertilizer rate and application method on photosynthetic characteristics and grain yield of summer maize. J. Soil Sci. Plant Nutr. 2016, 16, 550–562. [Google Scholar] [CrossRef]
- Mohammadi, M.; Majnoun Hoseini, N.; Chaichi, M.R.; Alipour, H.; Dashtaki, M.; Safikhani, S. Influence of nano-iron oxide and zinc sulfate on physiological characteristics of peppermint. Commun. Soil Sci. Plant Anal. 2018, 49, 2315–2326. [Google Scholar] [CrossRef]
- Gu, X.H.; Sun, L.Q.; Gao, B.; Sun, Q.Z.; Liu, C.; Zhang, J.L.; Li, X.D. Effects of calcium fertilizer application on peanut growth, physiological characteristics, yield and quality under drought stress. Ying Yong Sheng Tai Xue Bao 2015, 26, 1433–1439. [Google Scholar] [PubMed]
- Adriano, D.C. Trace Elements in Terrestrial Environments—Biogeochemistry, Bioavailability, and Risks of Metals. J. Environ. Qual. 2003, 32, 374. [Google Scholar]
- Šlosár, M.; Mezeyová, I.; Hegedüsová, A.; Andrejiová, A.; Kováčik, P.; Lošák, T.; Kopta, T.; Keutgen, A.J. Effect of zinc fertilisation on yield and selected qualitative parameters of broccoli. Plant Soil Environ. 2017, 63, 282–287. [Google Scholar]
- Kou, L.; Yang, T.; Luo, Y.; Liu, X.; Huang, L.; Codling, E. Pre-harvest calcium application increases biomass and delays senescence of broccoli microgreens. Postharvest Biol. Technol. 2014, 87, 70–78. [Google Scholar] [CrossRef]
- Davarpanah, S.; Tehranifar, A.; Davarynejad, G.; Abadía, J.; Khorasani, R. Effects of foliar applications of zinc and boron nano-fertilizers on pomegranate (Punica granatum cv. Ardestani) fruit yield and quality. Sci. Hortic. 2016, 210, 57–64. [Google Scholar] [CrossRef]
- Taghipour, S.; Rahimi, A.; Zartoshti, M.R.; Arslan, Y. The Effect of Micronutrients on Antioxidant Properties of Thyme (Thymus vulgaris L.) under Humic Acid Using Condition. YYÜ TAR BİL DERG 2017, 27, 589–600. [Google Scholar]
- Serrano, M.; Martinez-Romero, D.; Guillén, F.; Castillo, S.; Valero, D. Maintenance of broccoli quality and functional properties during cold storage as affected by modified atmosphere packaging. Postharvest Biol. Technol. 2006, 39, 61–68. [Google Scholar] [CrossRef]
- Rasouli, M.; Saba, M.K. Pre-harvest zinc spray impact on enzymatic browning and fruit flesh color changes in two apple cultivars. Sci. Hortic. 2018, 240, 318–325. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Y.; Li, J. Effects of 6-Benzylaminopurine–Calcium Chloride–Salicylic Acid on Yellowing and Reactive Oxygen Metabolism of Broccoli. Trans. Tianjin Univ. 2018, 24, 318–325. [Google Scholar] [CrossRef]
- Havlin, J.L.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 7th ed.; Pearson Educational, Inc.: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Fahey, J.W.; Holtzclaw, W.D.; Wehage, S.L.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Sulforaphane Bioavailability from Glucoraphanin-Rich Broccoli: Control by Active Endogenous Myrosinase. PLoS ONE 2015, 10, e0140963. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Rosa, E.A.S. Effect of post-harvest treatments on the level of glucosinolates in broccoli. J. Sci. Food Agric. 1999, 79, 1028–1032. [Google Scholar] [CrossRef]
- Sun, J.; Kou, L.; Geng, P.; Huang, H.; Yang, T.; Luo, Y.; Chen, P. Metabolomic Assessment reveals an elevated level of glucosinolate Content in CaCl2-treated broccoli microgreens. J. Agric. Food Chem. 2015, 63, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Guo, L.; Zhou, Y.; Shen, C.; Gu, Z. Calcium mitigates the stress caused by ZnSO4 as a sulphur fertilizer and enhances the sulforaphane formation of broccoli sprouts. RSC Adv. 2015, 5, 12563–12570. [Google Scholar] [CrossRef]
- Yang, R.; Hui, Q.; Gu, Z.; Zhou, Y.; Guo, L.; Shen, C.; Zhang, W. Effects of CaCl2 on the metabolism of glucosinolates and the formation of isothiocyanates as well as the antioxidant capacity of broccoli sprouts. J. Funct. Foods 2016, 24, 156–163. [Google Scholar] [CrossRef]
- Aminizadeh, M.; Riahi-Madvar, A.; Mohammadi, M. Effects of iron and cupper ions on sulforaphane content and peroxidase activity in Lepidium draba seedlings. Ethno-Pharm. Prod. 2014, 1, 8–14. [Google Scholar]
- Khathutshelo, M.V.; Mpumelelo, N.; Wonder, N.; Fhatuwani, M.N. Effects of Foliar Spray Application of Selected Micronutrients on the Quality of Bush Tea. Hortscience 2016, 51, 873–879. [Google Scholar] [CrossRef]
| 2017 | Cont. | Mn | Zn | Fe | Ca |
|---|---|---|---|---|---|
| N (%) | 3.37 ± 0.03 z c y | 3.79 ± 0.02 ab | 3.76 ± 0.05 ab | 3.71 ± 0.03 b | 3.94 ± 0.03 a |
| P (%) | 0.45 ± 0.02 b | 0.66 ± 0.01 a | 0.63 ± 0.02 a | 0.61 ± 0.02 a | 0.61 ± 0.01 a |
| Fe (mg/kg) | 152.65 ± 0.90 b | 152.77 ± 0.66 b | 145.48 ± 0.30 c | 165.03 ± 0.24 a | 151.25 ± 0.40 b |
| Mn (mg/kg) | 60.36 ± 0.47 b | 63.69 ± 0.27 a | 60.06 ± 0.14 bc | 59.01 ± 0.58 bc | 58.22 ± 0.59 c |
| Zn (mg/kg) | 132.67 ± 1.48 b | 131.86 ± 1.12 b | 136.29 ± 0.40 a | 132.86 ± 1.16 b | 129.26 ± 0.90 b |
| Ca (%) | 1.86 ± 0.02 b | 1.88 ± 0.03 b | 1.86 ± 0.05 b | 1.85 ± 0.04 b | 2.11 ± 0.06 a |
| 2018 | Cont. | Mn | Zn | Fe | Ca |
| N (%) | 4.20 ± 0.04 c | 4.59 ± 0.02 b | 4.58 ± 0.02 b | 4.63 ± 0.05 b | 4.84 ± 0.06 a |
| P (%) | 0.52 ± 0.07 b | 0.75 ± 0.01 a | 0.71 ± 0.01 a | 0.69 ± 0.05 ab | 0.73 ± 0.02 a |
| Fe (mg/kg) | 162.65 ± 0.91 b | 161.11 ± 1.38 b | 163.15 ± 2.35 b | 171.37 ± 0.88 a | 159.25 ± 0.80 b |
| Mn (mg/kg) | 50.36 ± 0.47 b | 54.69 ± 0.95 a | 50.73 ± 0.52 b | 51.34 ± 0.62 ab | 48.88 ± 1.20 b |
| Zn (mg/kg) | 116.34 ± 0.55 b | 117.20 ± 0.60 b | 121.39 ± 0.77 a | 114.33 ± 0.62 b | 116.59 ± 0.50 b |
| Ca (%) | 2.76 ± 0.06 b | 2.74 ± 0.06 b | 2.76 ± 0.05 b | 2.72 ± 0.06 b | 3.21 ± 0.11 a |
| TPC Content (mg/GAE/g FW) | |||||
|---|---|---|---|---|---|
| 2017 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 90.16 ± 0.45 z B y e | 91.73 ± 0.30 AB d | 92.72 ± 0.40 A e | 91.16 ± 0.32 AB e | 90.49 ± 0.58 B e |
| 7 d | 97.923 ± 0.13 B d | 101.41 ± 0.67 A c | 96.99 ± 0.26 B d | 101.33 ± 0.72 A d | 99.00 ± 0.57 AB d |
| 14 d | 121.42 ± 0.46 A c | 120.18 ± 0.24 A b | 120.87 ± 0.34 A c | 119.42 ± 0.57 A c | 108.41 ± 1.17 B c |
| 21 d | 139.37 ± 0.74 A a | 137.22 ± 0.61 AB a | 132.30 ± 0.37 B a | 135.18 ± 0.30 AB b | 117.42 ± 3.13 C b |
| 28 d | 135.44 ± 0.63 A b | 135.61 ± 0.56 A a | 128.28 ± 0.46 B b | 131.70 ± 0.91 AB a | 122.98 ± 1.57 C a |
| 2018 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 95.18 ± 0.83 AB a | 97.40 ± 0.59 A e | 97.39 ± 0.69 A e | 96.83 ± 0.91 A e | 92.49 ± 0.60 B e |
| 7 d | 103.43 ± 1.18 AB b | 106.74 ± 0.41 A d | 104.05 ± 0.91 AB d | 102.71 ± 0.50 B d | 103.08 ± 0.63 B d |
| 14 d | 112.79 ± 0.93 BC c | 116.10 ± 0.69 A c | 116.54 ± 0.54 A c | 115.09 ± 0.41 AB c | 111.41 ± 0.69 C c |
| 21 d | 128.98 ± 0.35 A d | 128.21 ± 0.95 A b | 129.03 ± 0.28 A b | 125.85 ± 0.85 AB b | 124.09 ± 1.17 B b |
| 28 d | 145.44 ± 0.63 AB e | 144.43 ± 0.34 AB a | 147.81 ± 0.58 A a | 142.70 ± 0.62 B a | 143.16 ± 1.40 B a |
| AsA Content (mg/100g FW) | |||||
| 2017 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 127.27 ± 0.41 B a | 130.52 ± 0.56 A a | 132.62 ± 0.68 A a | 130.82 ± 0.18 A a | 125.65 ± 1.04 B a |
| 7 d | 123.33 ± 0.88 A b | 124.33 ± 0.33 A b | 123.54 ± 0.64 A b | 124.56 ± 0.62 A b | 121.78 ± 1.02 A b |
| 14 d | 114.59 ± 0.45 AB c | 118.79 ± 0.80 A c | 117.14 ± 0.89 A c | 117.20 ± 1.17 A c | 111.59 ± 1.82 B c |
| 21 d | 91.94 ± 1.04 BC d | 95.51 ± 0.63 AB d | 99.25 ± 0.43 A d | 97.34 ± 1.55 A d | 90.00 ± 0.92 C d |
| 28 d | 79.40 ± 0.59 C e | 95.30 ± 0.51 A d | 80.53 ± 0.48 BC e | 82.38 ± 0.62 B e | 69.92 ± 0.71 D e |
| 2018 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 147.96 ± 0.81 A a | 143.83 ± 1.26 AB a | 145.63 ± 1.25 AB a | 141.51 ± 0.60 B a | 143.83 ± 1.90 AB a |
| 7 d | 140.53 ± 0.54 A b | 137.03 ± 1.01 ABC b | 138.55 ± 0.63 AB b | 136.56 ± 0.65 BC a | 134.94 ± 0.84 C b |
| 14 d | 131.62 ± 0.45 A c | 128.13 ± 0.49 BC c | 131.48 ± 0.59 AB c | 126.35 ± 0.64 C b | 127.14 ± 1.27 C c |
| 21 d | 113.08 ± 1.23 A d | 100.37 ± 0.55 B d | 112.84 ± 0.55 A d | 96.81 ± 3.74 B c | 98.33 ± 0.58 B d |
| 28 d | 91.76 ± 0.87 A e | 89.36 ± 0.56 ABC e | 91.53 ± 0.71 AB e | 88.38 ± 0.56 C d | 88.59 ± 0.61 BC e |
| Crude Protein Content (%) | |||||
|---|---|---|---|---|---|
| 2017 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 20.79 ± 0.43 z C y a | 24.55 ± 0.49 AB a | 22.67 ± 0.15 BC a | 22.93 ± 0.46 B a | 25.49 ± 0.40 A a |
| 7 d | 20.54 ± 0.66 A a | 21.71 ± 0.43 A b | 23.35 ± 1.21 A a | 22.50 ± 0.69 A a | 21.86 ± 0.34 A b |
| 14 d | 12.36 ± 0.32 A b | 13.84 ± 0.49 A c | 13.33 ± 0.24 A b | 13.91 ± 0.33 A b | 13.93 ± 0.43 A c |
| 21 d | 11.68 ± 0.28 C bc | 13.02 ± 0.12 AB c | 13.16 ± 1.16 AB bc | 13.62 ± 0.23 A b | 12.33 ± 0.33 BC c |
| 28 d | 9.79 ± 0.36 A c | 10.50 ± 0.58 A d | 10.35 ± 0.53 A c | 10.20 ± 0.42 A c | 9.98 ± 0.25 A d |
| 2018 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 30.45 ± 0.58 C a | 35.89 ± 0.36 AB a | 32.67 ± 0.16 C a | 32.93 ± 0.46 BC a | 36.43 ± 1.27 A a |
| 7 d | 25.89 ± 0.76 B b | 30.04 ± 0.60 A b | 26.02 ± 0.47 B b | 26.67 ± 0.57 AB b | 27.93 ± 1.38 AB a |
| 14 d | 20.91 ± 0.80 B c | 25.51 ± 0.52 A c | 21.16 ± 1.05 B c | 22.24 ± 1.22 AB c | 20.60 ± 0.92 B b |
| 21 d | 19.45 ± 0.61 AB c | 21.55 ± 0.61 A d | 19.11 ± 0.94 AB c | 19.52 ± 0.62 AB c | 17.20 ± 0.92 B bc |
| 28 d | 9.79 ± 1.00 A d | 10.55 ± 0.65 A e | 10.98 ± 0.32 A d | 9.73 ± 0.38 A d | 19.36 ± 0.57 A c |
| Total chlorophyll content (mg/g FW) | |||||
| 2017 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 1.67 ± 0.07 B a | 1.79 ± 0.05 A a | 1.91 ± 0.03 A a | 1.91 ± 0.04 A a | 1.77 ± 0.02 A a |
| 7 d | 1.57 ± 0.05 A ab | 1.65 ± 0.10 A ab | 1.73 ± 0.08 A a | 1.70 ± 0.10 A bc | 1.65 ± 0.07 A ab |
| 14 d | 1.32 ± 0.05 A b | 1.32 ± 0.04 A c | 1.38 ± 0.02 A b | 1.46 ± 0.04 A cd | 1.29 ± 0.00 A c |
| 21 d | 1.00 ± 0.06 B c | 1.37 ± 0.09 A bc | 1.34 ± 0.07 AB b | 1.34 ± 0.05 A d | 1.34 ± 0.07 A bc |
| 28 d | 0.81 ± 0.04 B c | 0.99 ± 0.02 A d | 1.00 ± 0.03 A c | 1.00 ± 0.02 A e | 0.98 ± 0.05 A d |
| 2018 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 2.16 ± 0.09 C a | 2.74 ± 0.04 A a | 2.44 ± 0.05 AB a | 2.47 ± 0.04 AB a | 2.27 ± 0.07 BC a |
| 7 d | 1.96 ± 0.04 C b | 2.45 ± 0.06 A b | 2.20 ± 0.03 B b | 2.21 ± 0.02 B ab | 2.15 ± 0.03 B a |
| 14 d | 1.62 ± 0.05 C b | 2.13 ± 0.06 A c | 1.91 ± 0.03 B c | 1.89 ± 0.02 B bc | 1.85 ± 0.02 B b |
| 21 d | 1.51 ± 0.01 C bc | 1.90 ± 0.03 A d | 1.64 ± 0.05 BC d | 1.71 ± 0.04 B c | 1.60 ± 0.03 BC c |
| 28 d | 1.27 ± 0.06 A c | 1.49 ± 0.04 A e | 1.27 ± 0.04 A e | 1.44 ± 0.06 A d | 1.36 ± 0.05 A d |
| Glucoraphanin (µmol/g FW) | |||||
|---|---|---|---|---|---|
| 2017 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 10.48 ± 0.29 z B y a | 12.04 ± 0.15 A a | 12.19 ± 0.22 A a | 11.99 ± 0.17 A a | 12.17 ± 0.11 A a |
| 7 d | 9.15 ± 0.04 B b | 11.13 ± 0.10 A b | 11.43 ± 0.24 A b | 11.40 ± 0.03 A b | 11.06 ± 0.12 A b |
| 14 d | 8.36 ± 0.18 C b | 9.14 ± 0.05 B c | 9.166 ± 0.16 B c | 10.18 ± 0.12 A c | 9.11 ± 0.04 B c |
| 21 d | 5.74 ± 0.24 B c | 7.22 ± 0.07 A d | 7.13 ± 0.05 A d | 7.10 ± 0.05 A d | 7.27 ± 0.14 A d |
| 28 d | 4.45 ± 0.21 B d | 6.02 ± 0.05 A e | 6.07 ± 0.08 A e | 5.74 ± 0.15 A e | 5.92 ± 0.07 A e |
| 2018 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 13.43 ± 0.07 B a | 14.50 ± 0.52 AB a | 15.50 ± 0.58 A a | 14.21 ± 0.26 AB a | 15.08 ± 0.36 AB a |
| 7 d | 12.21 ± 0.09 D b | 13.38 ± 0.08 BC ab | 14.18 ± 0.11 AB ab | 13.01 ± 0.35 CD ab | 14.53 ± 0.09 A a |
| 14 d | 11.76 ± 0.20 C bc | 12.26 ± 0.14 BC b | 13.23 ± 0.00 B bc | 14.37 ± 0.37 A ab | 13.27 ± 0.23 B b |
| 21 d | 9.99 ± 0.21 C c | 10.75 ± 0.14 BC c | 11.84 ± 0.26 A c | 10.76 ± 0.30 ABC bc | 11.39 ± 0.22 AB c |
| 28 d | 6.95 ± 0.55 A d | 8.03 ± 0.32 A d | 8.37 ± 0.17 A d | 8.40 ± 0.37 A c | 8.41 ± 0.20 A d |
| Glucobrassicin (µmol/g FW) | |||||
| 2017 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 4.01 ± 0.01 B a | 5.20 ± 0.01 A a | 4.10 ± 0.05 B a | 5.23 ± 0.03 A a | 4.08 ± 0.04 B a |
| 7 d | 3.91 ± 0.14 A a | 4.11 ± 0.06 A b | 4.09 ± 0.06 A a | 4.10 ± 0.06 A b | 4.07 ± 0.04 A a |
| 14 d | 2.26 ± 0.10 C b | 3.11 ± 0.05 A c | 3.06 ± 0.05 A b | 2.99 ± 0.01 AB c | 2.77 ± 0.04 B b |
| 21 d | 1.40 ± 0.08 B c | 1.91 ± 0.01 A d | 2.00 ± 0.03 A c | 1.97 ± 0.01 A d | 1.85 ± 0.03 A c |
| 28 d | 1.06 ± 0.03 D c | 1.36 ± 0.04 BC e | 1.67 ± 0.05 A d | 1.58 ± 0.05 AB e | 1.23 ± 0.06 CD d |
| 2018 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 4.39 ± 0.08 C a | 4.94 ± 0.05 B a | 5.44 ± 0.13 A a | 4.99 ± 0.06 B a | 4.89 ± 0.09 C a |
| 7 d | 4.13 ± 0.05 C a | 4.24 ± 0.06 BC b | 4.79 ± 0.07 A ab | 4.546 ± 0.11 AB b | 4.39 ± 0.11 BC a |
| 14 d | 3.06 ± 0.04 B b | 3.11 ± 0.06 B c | 3.67 ± 0.15 A bc | 3.17 ± 0.07 B c | 3.72 ± 0.14 A b |
| 21 d | 2.09 ± 0.08 C c | 2.55 ± 0.11 AB d | 2.95 ± 0.06 A c | 2.35 ± 0.11 BC d | 2.77 ± 0.10 AB c |
| 28 d | 1.54 ± 0.07 B d | 1.83 ± 0.04 A e | 1.95 ± 0.04 A d | 1.77 ± 0.06 AB e | 2.00 ± 0.06 A d |
| Sulforaphane (μg/g FW) | |||||
|---|---|---|---|---|---|
| 2017 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 130.60 ± 0.35 z C y a | 131.92 ± 0.22 C a | 139.42 ± 0.60 A a | 132.78 ± 0.14 C a | 137.76 ± 1.29 A a |
| 7 d | 104.27 ± 0.65 D b | 108.68 ± 0.51 C b | 113.48 ± 0.80 B b | 106.35 ± 0.54 CD b | 118.89 ± 0.26 A b |
| 14 d | 98.67 ± 0.22 C c | 103.60 ± 0.67 B b | 106.45 ± 0.58 A c | 101.36 ± 0.68 BC c | 106.30 ± 0.42 A b |
| 21 d | 89.55 ± 0.47 A c | 91.00 ± 0.29 A c | 90.96 ± 0.26 A e | 90.77 ± 0.38 A e | 89.31 ± 0.50 A c |
| 28 d | 67.69 ± 0.70 B d | 71.63 ± 0.67 A d | 70.56 ± 0.55 A d | 71.39 ± 0.54 A d | 70.19 ± 0.31 A d |
| 2018 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 124.78 ± 0.56 AB a | 123.51 ± 0.55 B a | 127.84 ± 1.43 AB a | 125.36 ± 1.15 AB a | 129.38 ± 1.38 A a |
| 7 d | 116.81 ± 0.38 C b | 118.50 ± 0.51 BC b | 120.50 ± 0.58 AB b | 116.13 ± 0.71 C b | 121.45 ± 0.60 A b |
| 14 d | 111.58 ± 0.49 C c | 113.54 ± 0.54 BC c | 115.61 ± 0.57 AB c | 113.50 ± 0.63 BC b | 116.59 ± 0.59 A c |
| 21 d | 99.11 ± 1.75 B d | 101.70 ± 0.61 AB d | 105.40 ± 0.66 A d | 104.42 ± 1.04 A c | 105.25 ± 0.39 A d |
| 28 d | 92.39 ± 1.51 AB e | 90.86 ± 0.35 B e | 95.61 ± 0.43 A e | 94.35 ± 0.99 AB d | 93.43 ± 1.25 AB e |
| Peroxidase (units mg−1protein) | |||||
| 2017 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 0.83 ± 0.04 B d | 0.93 ± 0.02 A e | 0.95 ± 0.01 A e | 1.00 ± 0.01 A e | 0.88 ± 0.02 AB e |
| 7 d | 0.96 ± 0.03 C d | 1.74 ± 0.04 A d | 1.74 ± 0.03 A d | 1.79 ± 0.01 A d | 1.47 ± 0.08 B d |
| 14 d | 1.84 ± 0.07 B c | 2.65 ± 0.14 A c | 2.51 ± 0.03 A c | 2.48 ± 0.06 A c | 2.64 ± 0.16 A c |
| 21 d | 3.34 ± 0.06 D b | 5.15 ± 0.07 A b | 4.96 ± 0.09 AB b | 4.71 ± 0.06 B b | 4.24 ± 0.13 C b |
| 28 d | 4.13 ± 0.05 B a | 5.82 ± 0.06 A a | 5.60 ± 0.09 A a | 5.72 ± 0.07 A a | 5.55 ± 0.14 A a |
| 2018 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 1.01 ± 0.02 B e | 1.19 ± 0.04 AB e | 1.21 ± 0.07 A e | 1.26 ± 0.03 A e | 1.08 ± 0.02 AB e |
| 7 d | 1.66 ± 0.05 B d | 1.85 ± 0.04 AB d | 1.86 ± 0.05 AB d | 1.89 ± 0.04 A d | 1.83 ± 0.04 AB d |
| 14 d | 3.24 ± 0.08 B c | 3.82 ± 0.03 A c | 3.85 ± 0.06 A c | 3.63 ± 0.12 AB c | 3.35 ± 1.10 B c |
| 21 d | 5.43 ± 0.05 B b | 5.67 ± 0.09 AB b | 5.72 ± 0.03 AB b | 5.95 ± 0.04 A b | 5.47 ± 0.11 B b |
| 28 d | 6.25 ± 0.06 A a | 6.72 ± 0.09 A a | 6.63 ± 0.12 A a | 6.64 ± 0.11 A a | 6.43 ± 0.11 A a |
| Flavonoids content (mg/100g FW) | |||||
| 2017 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 101.51 ± 0.68 C a | 120.60 ± 0.48 A a | 116.57 ± 0.50 B a | 119.17 ± 0.57 AB a | 121.40 ± 0.81 A a |
| 7 d | 90.45 ± 0.55 B b | 100.62 ± 0.42 A b | 100.15 ± 0.32 A b | 98.11 ± 0.37 A b | 97.88 ± 1.12 A b |
| 14 d | 76.77 ± 0.55 C c | 84.61 ± 0.55 B c | 88.22 ± 0.33 A c | 85.59 ± 0.41 AB c | 86.98 ± 0.99 AB c |
| 21 d | 57.82 ± 1.05 C d | 69.48 ± 0.54 A d | 70.22 ± 0.98 A d | 71.54 ± 0.67 A d | 64.55 ± 0.47 B d |
| 28 d | 52.44 ± 0.64 C e | 60.33 ± 0.72 A e | 56.33 ± 0.53 B e | 59.61 ± 0.50 AB e | 57.35 ± 1.29 AB e |
| 2018 | Cont. | Mn | Zn | Fe | Ca |
| 0 d | 111.17 ± 1.00 B a | 115.53 ± 0.31 A a | 115.90 ± 1.01 A a | 115.31 ± 0.49 A a | 116.40 ± 0.67 A a |
| 7 d | 101.45 ± 0.57 B b | 105.53 ± 0.52 A b | 104.54 ± 0.55 A b | 107.07 ± 0.64 A b | 106.08 ± 0.63 A bb |
| 14 d | 91.35 ± 0.56 C c | 93.61 ± 0.85 BC c | 97.05 ± 0.34 A c | 95.10 ± 0.37 AB c | 94.65 ± 0.35 AB c |
| 21 d | 81.28 ± 0.60 B d | 83.82 ± 0.21 AB d | 85.73 ± 0.31 A d | 84.80 ± 0.86 A d | 82.42 ± 1.21 AB d |
| 28 d | 67.94 ± 0.93 A e | 69.46 ± 0.55 A e | 69.68 ± 0.56 A e | 69.56 ± 0.38 A e | 66.75 ± 0.79 A e |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Mogy, M.M.; Mahmoud, A.W.M.; El-Sawy, M.B.I.; Parmar, A. Pre-Harvest Foliar Application of Mineral Nutrients to Retard Chlorophyll Degradation and Preserve Bio-Active Compounds in Broccoli. Agronomy 2019, 9, 711. https://doi.org/10.3390/agronomy9110711
El-Mogy MM, Mahmoud AWM, El-Sawy MBI, Parmar A. Pre-Harvest Foliar Application of Mineral Nutrients to Retard Chlorophyll Degradation and Preserve Bio-Active Compounds in Broccoli. Agronomy. 2019; 9(11):711. https://doi.org/10.3390/agronomy9110711
Chicago/Turabian StyleEl-Mogy, Mohamed M., Abdel Wahab M. Mahmoud, Mohamed B. I. El-Sawy, and Aditya Parmar. 2019. "Pre-Harvest Foliar Application of Mineral Nutrients to Retard Chlorophyll Degradation and Preserve Bio-Active Compounds in Broccoli" Agronomy 9, no. 11: 711. https://doi.org/10.3390/agronomy9110711
APA StyleEl-Mogy, M. M., Mahmoud, A. W. M., El-Sawy, M. B. I., & Parmar, A. (2019). Pre-Harvest Foliar Application of Mineral Nutrients to Retard Chlorophyll Degradation and Preserve Bio-Active Compounds in Broccoli. Agronomy, 9(11), 711. https://doi.org/10.3390/agronomy9110711








