Foliar Zn Spraying Simultaneously Improved Concentrations and Bioavailability of Zn and Fe in Maize Grains Irrespective of Foliar Sucrose Supply
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Crop Management
2.3. Plant Sampling and Nutrient Analysis
2.4. Statistical Analysis
3. Results
3.1. Maize Agronomic Traits and Grain Zn and Fe Accumulation in Quzhou
3.2. Maize Agronomic Traits in Licheng
3.3. Maize Grain Zn and Fe Concentrations, Contents, and Bioavailability in Licheng
3.4. Concentrations of Carbon, Nitrogen, Total and Phytate Phosphorus and Calcium, and Ratios of C/N and Phytate P/Total P in Maize Grains in Licheng
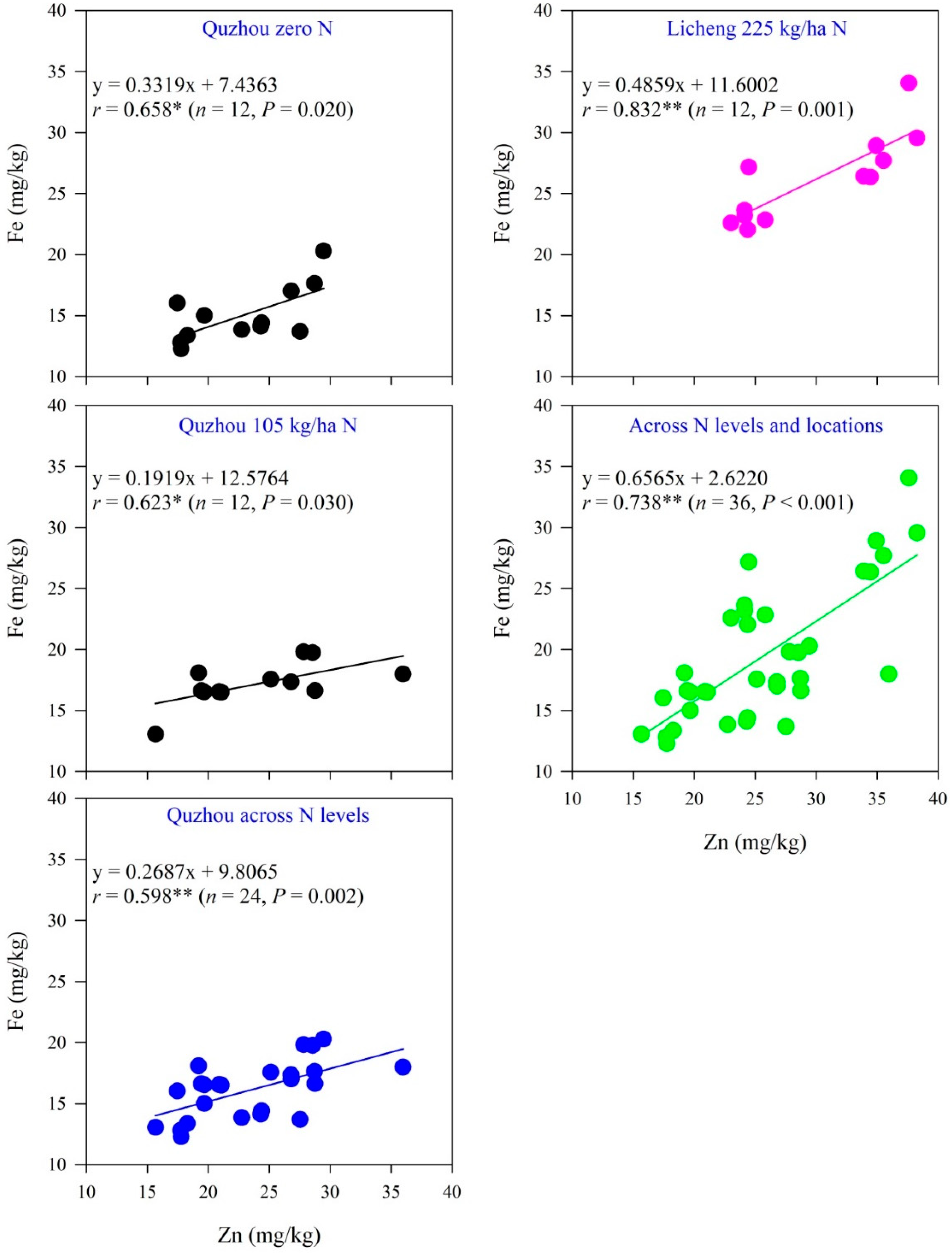
3.5. Relationships between Zn and Fe Concentrations in Maize Grains
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nuss, E.T.; Tanumihardjo, S.A. Maize: A paramount staple crop in the context of global nutrition. Compr. Rev. Food Sci. Food Saf. 2010, 9, 417–436. [Google Scholar] [CrossRef]
- Xue, Y.; Yue, S.; Zhang, W.; Liu, D.; Cui, Z.; Chen, X.; Ye, Y.; Zou, C. Zinc, iron, manganese and copper uptake requirement in response to nitrogen supply and the increased grain yield of summer maize. PLoS ONE 2014, 9, e93895. [Google Scholar] [CrossRef]
- Xia, H.; Xue, Y.; Liu, D.; Kong, W.; Xue, Y.; Tang, Y.; Li, J.; Li, D.; Mei, P. Rational application of fertilizer nitrogen to soil in combination with foliar Zn spraying improved Zn nutritional quality of wheat grains. Front. Plant Sci. 2018, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Takkar, P.N.; Walker, C.D. The distribution and correction of zinc deficiency. In Zinc in Soils and Plants; Robson, A.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; Volume 55, pp. 151–165. [Google Scholar]
- Yang, X.; Chen, W.; Feng, Y. Improving human micronutrient nutrition through biofortification in the soil-plant system: China as a case study. Environ. Geochem. Health 2007, 29, 413–428. [Google Scholar] [CrossRef]
- Wang, S.; Gao, L.; Liu, Z.; Huang, J.; Chen, L.; Liu, R.; Wang, H. Effect of zinc on maize leaf cell ultra-structure under different soil moistures. Chin. J. Eco-Agric. 2013, 21, 959–965. (In Chinese) [Google Scholar] [CrossRef]
- Cakmak, I. Plant nutrition research: Priorities to meet human needs for food in sustainable ways. Plant Soil 2002, 247, 3–24. [Google Scholar] [CrossRef]
- Garvin, D.F.; Welch, R.M.; Finley, J.W. Historical shifts in the seed mineral micronutrient concentration of US hard red winter wheat germplasm. J. Sci. Food Agric. 2006, 86, 2213–2220. [Google Scholar] [CrossRef]
- Scagel, C.; Bi, G.; Fuchigami, L.; Regan, R. Irrigation frequency alters nutrient uptake in container-grown rhododendron plants grown with different rates of nitrogen. HortScience 2012, 47, 189–197. [Google Scholar] [CrossRef]
- Saha, B.; Saha, S.; Poddar, P.; Murmu, S.; Singh, A.K. Uptake of nutrients by wheat as influenced by long-term phosphorus fertilization. Bioscan 2013, 8, 1331–1335. [Google Scholar]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Pellny, T.K.; Lovegrove, A. Is modern wheat bad for health? Nat. Plants 2016, 2, 16097. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Li, X.; Ulfat, A.; Lv, Z.; Fang, L.; Jiang, D.; Liu, F. Effect of multigenerational exposure to elevated atmospheric CO2 concentration on grain quality in wheat. Environ. Exp. Bot. 2019, 157, 310–319. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Plaza, C.; Saiz, H.; Manzano, R.; Flagmeier, M.; Maestre, F.T. Aridity and reduced soil micronutrient availability in global drylands. Nat. Sustain. 2019, 2, 371–377. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.; Ezzati, M.; Hoorn, S.V.; Lopez, A.D.; Lin, R.-B.; Murray, C.J.L.; Collaborating, G.C.R.A. Distribution of major health risks: Findings from the global burden of disease study. PLoS Med. 2004, 1, e27. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Jin, Y.; Piao, J.; Kok, F.; Guusje, B.; Jacobsen, E. Phytate, calcium, iron, and zinc contents and their molar ratios in foods commonly consumed in China. J. Agric. Food Chem. 2005, 53, 10285–10290. [Google Scholar] [CrossRef]
- Clemens, S. Zn and Fe biofortification: The right chemical environment for human bioavailability. Plant Sci. 2014, 225, 52–57. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Tong, Y.; Xue, Y.; Liu, D.; Zhang, W.; Deng, Y.; Meng, Q.; Yue, S.; Yan, P.; et al. Harvesting more grain zinc of wheat for human health. Sci. Rep. 2017, 7, 7016. [Google Scholar] [CrossRef]
- Orabi, A.A.; Mashadi, H.; Abdallah, A.; Morsy, M. Effect of zinc and phosphorus on the grain yield of corn (Zea mays L.) grown on a calcareous soil. Plant Soil 1981, 63, 291–294. [Google Scholar] [CrossRef]
- Hossain, M.A.; Jahiruddin, M.; Islam, M.R.; Mian, M.H. The requirement of zinc for improvement of crop yield and mineral nutrition in the maize–mungbean–rice system. Plant Soil 2008, 306, 13–22. [Google Scholar] [CrossRef]
- Kanwal, S.; Rahmatullah, A.R.; Ahmad, R. Zinc partitioning in maize grain after soil fertilization with zinc sulfate. Int. J. Agric. Biol. 2010, 12, 299–302. [Google Scholar]
- Peck, A.W.; McDonald, G.K.; Graham, R.D. Zinc nutrition influences the protein composition of flour in bread wheat (Triticum aestivum L.). J. Cereal Sci. 2008, 47, 266–274. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Sun, Y.-X.; Ye, Y.-L.; Karim, M.R.; Xue, Y.-F.; Yan, P.; Meng, Q.-F.; Cui, Z.-L.; Cakmak, I.; Zhang, F.-S.; et al. Zinc biofortification of wheat through fertilizer applications in different locations of China. Field Crops Res. 2012, 125, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Mao, H.; Zhao, H.; Huang, D.; Wang, Z. Different increases in maize and wheat grain zinc concentrations caused by soil and foliar applications of zinc in Loess Plateau, China. Field Crops Res. 2012, 135, 89–96. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Agronomic biofortification of cereal grains with iron and zinc. Adv. Agron. 2014, 125, 55–91. [Google Scholar]
- Niyigaba, E.; Twizerimana, A.; Mugenzi, I.; Ngnadong, W.A.; Ye, Y.P.; Wu, B.M.; Hai, J.B. Winter wheat grain quality, zinc and iron concentration affected by a combined foliar spray of zinc and iron fertilizers. Agronomy 2019, 9, 250. [Google Scholar] [CrossRef]
- Saha, S.; Mandal, B.; Hazra, G.C.; Dey, A.; Chakraborty, M.; Adhikari, B.; Mukhopadhyay, S.K.; Sadhukhan, R. Can agronomic biofortification of zinc be benign for iron in cereals? J. Cereal Sci. 2015, 65, 186–191. [Google Scholar] [CrossRef]
- Pearson, J.N.; Rengel, Z.; Jenner, C.F.; Graham, R.D. Manipulation of xylem transport affects Zn and Mn transport into developing wheat grains of cultured ears. Physiol. Plant. 1996, 98, 229–234. [Google Scholar] [CrossRef]
- Ma, Y.Z.; MacKown, C.T.; Van Sanford, D.A. Differential effects of partial spikelet removal and defoliation on kernel growth and assimilate partitioning among wheat cultivars. Field Crops Res. 1996, 47, 201–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, N.; Su, D.; Xue, Q.; Stewart, B.; Wang, Z. Effect of source-sink manipulation on accumulation of micronutrients and protein in wheat grains. J. Plant Nutr. Soil Sci. 2012, 175, 622–629. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, Y.; Wang, B.; Xue, Y.; Yu, P.; Zhang, Q.; Wang, Z. Is grain zinc concentration in wheat limited by source? Aust. J. Crop Sci. 2014, 8, 1534–1541. [Google Scholar]
- Xia, H.; Xue, Y.; Kong, W.; Tang, Y.; Li, J.; Li, D. Effects of source/sink manipulation on grain zinc accumulation by winter wheat genotypes. Chil. J. Agric. Res. 2018, 78, 117–125. [Google Scholar] [CrossRef]
- Welch, R.M.; Graham, R.D. Breeding for micronutrients in staple food crops from a human nutrition perspective. J. Exp. Bot. 2004, 55, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Brinch-Pedersen, H.; Borg, S.; Tauris, B.; Holm, P.B. Molecular genetic approaches to increasing mineral availability and vitamin content of cereals. J. Cereal Sci. 2007, 46, 308–326. [Google Scholar] [CrossRef]
- Cakmak, I.; Pfeiffer, W.H.; McClafferty, B. Biofortification of durum wheat with zinc and iron. Cereal Chem. 2010, 87, 10–20. [Google Scholar] [CrossRef]
- Morris, E.R.; Ellis, R. Phytate, wheat bran, and bioavailability of dietary iron. ACS Symp. Ser. 1982, 203, 121–141. [Google Scholar]
- Ryan, M.H.; McInerney, J.K.; Record, I.R.; Angus, J.F. Zinc bioavailability in wheat grain in relation to phosphorus fertiliser, crop sequence and mycorrhizal fungi. J. Sci. Food Agric. 2008, 88, 1208–1216. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.; Zhang, W.; Chen, X.; Zou, C. Agronomic approach of zinc biofortification can increase zinc bioavailability in wheat flour and thereby reduce zinc deficiency in humans. Nutrients 2017, 9, 465. [Google Scholar]
- World Health Organization (WHO). Trace Elements in Human Nutrition and Health; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Glahn, R.P.; Wortley, G.M.; South, P.K.; Miller, D.D. Inhibition of iron uptake by phytic acid, tannic acid, and ZnCl2: Studies using an in vitro digestion/Caco-2 cell model. J. Agric. Food Chem. 2002, 50, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.; Kelsay, J.L.; Reynolds, R.D.; Morris, E.R.; Moser, P.B.; Frazier, C.W. Phytate: zinc and phytate × calcium:zinc millimolar ratios in self-selected diets of Americans, Asian Indians, and Nepalese. J. Am. Diet. Assoc. 1987, 87, 1043–1047. [Google Scholar] [PubMed]
- Zhang, S.; Yue, S.; Yan, P.; Qiu, M.; Chen, X.; Cui, Z. Testing the suitability of the end-of-season stalk nitrate test for summer corn (Zea mays L.) production in China. Field Crops Res. 2013, 154, 153–157. [Google Scholar] [CrossRef]
- Xia, H.; Wang, L.; Xue, Y.; Kong, W.; Xue, Y.; Yu, R.; Wang, X.; Wang, J.; Liu, Z.; Guo, X. Impact of increasing maize densities on agronomic performances and the community stability of productivity of maize/peanut intercropping systems. Agronomy 2019, 9, 150. [Google Scholar] [CrossRef]
- Haug, W.; Lantzsch, H. Sensitive method for the rapid determination of phytate in cereals and cereal products. J. Sci. Food Agric. 1983, 34, 1423–1426. [Google Scholar] [CrossRef]
- Mohsin, A.U.; Ahmad, A.U.H.; Farooq, M.; Ullah, S. Influence of zinc application through seed treatment and foliar spray on growth, productivity and grain quality of hybrid maize. J. Anim. Plant Sci. 2014, 24, 1494–1503. [Google Scholar]
- Bouis, H.E. Micronutrient fortification of plants through plant breeding: Can it improve nutrition in man at low cost? Proc. Nutr. Soc. 2003, 62, 403–411. [Google Scholar] [CrossRef]
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisoy, Z.; Erdem, H.; Yazici, A.; Gokmen, O.; et al. Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef]
- Zhao, A.Q.; Tian, X.H.; Cao, Y.X.; Lu, X.C.; Liu, T. Comparison of soil and foliar zinc application for enhancing grain zinc content of wheat when grown on potentially zinc-deficient calcareous soils. J. Sci. Food Agric. 2014, 94, 2016–2022. [Google Scholar] [CrossRef]
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Karim, M.; Zhang, Y.; Zhao, R.; Chen, X.; Zhang, F.; Zou, C. Alleviation of drought stress in winter wheat by late foliar application of zinc, boron, and manganese. J. Plant Nutr. Soil Sci. 2012, 175, 142–151. [Google Scholar] [CrossRef]
- Sasaki, H.; Edo, E.; Uehara, N.; Ishimaru, T.; Kawamitsu, Y.; Suganuma, S.; Ueda, D.; Ohsugi, R. Effect of sucrose on activity of starch synthesis enzymes in rice ears in culture. Physiol. Plant. 2005, 124, 301–310. [Google Scholar] [CrossRef]
- Haslett, B.S.; Reid, R.J.; Rengel, Z. Zinc mobility in wheat: Uptake and distribution of zinc applied to leaves or roots. Ann. Bot. 2001, 87, 379–386. [Google Scholar] [CrossRef]
- Kutman, U.B.; Yildiz, B.; Ozturk, L.; Cakmak, I. Biofortification of durum wheat with zinc through soil and foliar applications of nitrogen. Cereal Chem. 2010, 87, 1–9. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, Y.; Rashid, A.; Ram, H.; Savasli, E.; Arisoy, R.; Ortiz-Monasterio, I.; Simunji, S.; Wang, Z.; Sohu, V.; et al. Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil 2012, 361, 119–130. [Google Scholar] [CrossRef]
- Chomba, E.; Westcott, C.M.; Westcott, J.E.; Mpabalwani, E.M.; Krebs, N.F.; Patinkin, Z.W.; Palacios, N.; Hambidge, K.M. Zinc absorption from biofortified maize meets the requirements of young rural Zambian children. J. Nutr. 2015, 145, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.D.; Welch, R.M.; Saunders, D.A.; Ortiz-Monasterio, I.; Bouis, H.E.; Bonierbale, M.; De Haan, S.; Burgos, G.; Thiele, G.; Liria, R.; et al. Nutritious subsistence food systems. Adv. Agron. 2007, 92, 1–74. [Google Scholar]
- Maqbool, M.A.; Beshir, A. Zinc biofortification of maize (Zea mays L.): Status and challenges. Plant Breed. 2019, 138, 1–28. [Google Scholar] [CrossRef]
- Gomez-Coronado, F.; Poblaciones, M.J.; Almeida, A.S.; Cakmak, I. Zinc (Zn) concentration of bread wheat grown under Mediterranean conditions as affected by genotype and soil/foliar Zn application. Plant Soil 2016, 401, 331–346. [Google Scholar] [CrossRef]
- Foy, C.D.; Montenegro, G.; Barber, S.A. Foliar feeding of corn with urea nitrogen. Soil Sci. Soc. Am. 1953, 17, 387–390. [Google Scholar] [CrossRef]
- Pearson, J.N.; Jenner, C.F.; Rengel, Z.; Graham, R.D. Differential transport of Zn, Mn and sucrose along the longitudinal axis of developing wheat grains. Physiol. Plant. 1996, 97, 332–338. [Google Scholar] [CrossRef]
- Fernández, V.; Bahamonde, H.A.; Peguero-Pina, J.J.; Gil-Pelegrín, E.; Sancho-Knapik, D.; Gil, L.; Goldbach, H.E.; Eichert, T. Physico-chemical properties of plant cuticles and their functional and ecological significance. J. Exp. Bot. 2017, 68, 5293–5306. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, L. Polar paths of diffusion across plant cuticles: New evidence for an old hypothesis. Ann. Bot. 2005, 95, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Leakey, A.; Ainsworth, E.; Bernacchi, C.; Rogers, A.; Long, S. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef] [PubMed]
- Giordano, P.M.; Mortvedt, J.J. Rice response to zinc in flooded and nonflooded soil. Agron. J. 1972, 64, 521–524. [Google Scholar] [CrossRef]
- Chakraborti, M.; Prasanna, B.; Hossain, F.; Singh, A.; Guleria, S. Genetic evaluation of kernel Fe and Zn concentrations and yield performance of selected Maize (Zea mays L.) genotypes. Range Manag. Agrofor. 2009, 30, 109–114. [Google Scholar]
- Aref, F. Zinc and boron fertilization on concentration and uptake of iron and manganese in the corn grain. J. Anim. Sci. 2010, 6, 236–242. [Google Scholar]
- Zeidan, M.S.; Mohamed, M.F.; Hamouda, H.A. Effect of foliar fertilization of Fe, Mn and Zn on wheat yield and quality in low sandy soils fertility. World J. Agric. Sci. 2010, 6, 696–699. [Google Scholar]
- Zhao, A.; Bao, Q.; Tian, X.; Lu, X.; Gale, W. Combined effect of iron and zinc on micronutrient levels in wheat (Triticum aestivum L.). J. Environ. Biol. 2011, 32, 235–239. [Google Scholar]
- Qin, H.; Cai, Y.; Liu, Z.; Wang, G.; Wang, J.; Guo, Y.; Wang, H. Identification of QTL for zinc and iron concentration in maize kernel and cob. Euphytica 2012, 187, 345–358. [Google Scholar] [CrossRef]
| Year | Experimental Site | Geographic Coordinates | Soil Type | pH (2.5:1 Water:Soil Ratio) | Organic Matter | Total Nitrogen | Alkaline Hydrolysis | Olsen P | Exchangeable K | DTPA-Extractable Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| (g/kg) | (g/kg) | Nitrogen (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | |||||
| 2010 | Quzhou | 115°0′ E, 36°54′ N | Calcareous alluvial soil | 8.3 | 14.5 | 0.92 | - | 14.9 | 130.5 | 2.8 |
| 2016 | Licheng | 117°04′ E, 36°42′ N | Calcareous alluvial soil | 8.0 | 15.7 | - | 90.5 | 20.2 | 142.6 | 1.5 |
| Treatments | Solution Composition | |
|---|---|---|
| T1 | Control | Deionized water |
| T2 | Sucrose | 10.0% (w/v) |
| T3 | ZnSO4·7H2O (Zn) | 0.2% in Quzhou and 0.3% in Licheng (w/v) |
| T4 | Zn + Sucrose | A combination of T2 and T3 |
| Parameters | Treatments | N application Rates (kg/ha) | ANOVA | ||||
|---|---|---|---|---|---|---|---|
| 0 | 105 | Mean | |||||
| GDW | T1 | Deionized water | 67.6 ± 1.7 a | 104.9 ± 16.4 a | 86.2 ± 7.3 A | N | 0.0052 |
| (g per ear) | T2 | Sucrose | 66.8 ± 19.3 a | 104.6 ± 13.6 a | 85.7 ± 16.3 A | T | 0.4983 |
| T3 | ZnSO4·7H2O (Zn) | 75.6 ± 19.0 a | 114.6 ± 25.2 a | 95.1 ± 22.1 A | N × T | 0.9898 | |
| T4 | Sucrose + Zn | 65.9 ± 10.0 a | 99.7 ± 10.9 a | 82.8 ± 10.5 A | |||
| Mean | 69.0 ± 11.3 B | 106.0 ± 8.9 A | 87.5 ± 9.9 | ||||
| TDW | T1 | Deionized water | 136.5 ± 12.0 a | 224.3 ± 26.6 a | 180.4 ± 9.0 A | N | <0.0001 |
| (g per plant) | T2 | Sucrose | 134.3 ± 30.2 a | 206.0 ± 12.2 a | 170.2 ± 20.9 A | T | 0.4732 |
| T3 | ZnSO4·7H2O (Zn) | 143.4 ± 33.9 a | 221.7 ± 29.5 a | 182.5 ± 30.7 A | N × T | 0.8051 | |
| T4 | Sucrose + Zn | 134.1 ± 13.1 a | 201.5 ± 10.0 a | 167.8 ± 11.3 A | |||
| Mean | 137.1 ± 20.7 B | 213.4 ± 8.8 A | 175.2 ± 14.4 | ||||
| HGDW | T1 | Deionized water | 28.3 ± 2.9 a | 32.5 ± 2.5 a | 30.4 ± 0.5 A | N | 0.0001 |
| (g) | T2 | Sucrose | 28.1 ± 1.3 a | 30.7 ± 1.1 a | 29.4 ± 0.6 A | T | 0.2814 |
| T3 | ZnSO4·7H2O (Zn) | 27.3 ± 1.5 a | 31.4 ± 1.4 a | 29.4 ± 0.9 A | N × T | 0.6057 | |
| T4 | Sucrose + Zn | 27.5 ± 2.2 a | 31.0 ± 1.4 a | 29.2 ± 0.7 A | |||
| Mean | 27.8 ± 1.9 B | 31.4 ± 1.3 A | 29.6 ± 0.5 | ||||
| Grain Zn | T1 | Deionized water | 18.4 ± 1.1 b | 19.9 ± 1.0 b | 19.1 ± 0.5 B | N | 0.4941 |
| concentration | T2 | Sucrose | 19.5 ± 2.8 b | 18.7 ± 2.7 b | 19.1 ± 2.4 B | T | <0.0001 |
| (mg/kg) | T3 | ZnSO4·7H2O (Zn) | 28.6 ± 1.0 a | 28.0 ± 1.1 a | 28.3 ± 0.3 A | N × T | 0.2453 |
| T4 | Sucrose + Zn | 25.2 ± 1.4 ab | 29.6 ± 5.6 a | 27.4 ± 2.4 A | |||
| Mean | 22.9 ± 0.6 A | 24.1 ± 2.2 A | 23.5 ± 1.0 | ||||
| Grain Zn | T1 | Deionized water | 1.2 ± 0.1 b | 2.1 ± 0.3 b | 1.7 ± 0.1 B | N | 0.0357 |
| content | T2 | Sucrose | 1.3 ± 0.2 b | 1.9 ± 0.2 b | 1.6 ± 0.1 B | T | 0.0008 |
| (mg per plant) | T3 | ZnSO4·7H2O (Zn) | 2.2 ± 0.6 a | 3.2 ± 0.6 a | 2.7 ± 0.6 A | N × T | 0.5642 |
| T4 | Sucrose + Zn | 1.7 ± 0.3 ab | 2.9 ± 0.6 a | 2.3 ± 0.3 A | |||
| Mean | 1.6 ± 0.3 B | 2.5 ± 0.2 A | 2.1 ± 0.2 | ||||
| Grain Fe | T1 | Deionized water | 13.4 ± 1.4 a | 17.1 ± 0.9 ab | 15.2 ± 1.2 B | N | 0.0091 |
| concentration | T2 | Sucrose | 14.4 ± 1.4 a | 15.4 ± 2.0 b | 14.9 ± 0.3 B | T | 0.0587 |
| (mg/kg) | T3 | ZnSO4·7H2O (Zn) | 17.2 ± 3.3 a | 17.9 ± 1.6 ab | 17.6 ± 2.1 A | N × T | 0.1656 |
| T4 | Sucrose + Zn | 15.2 ± 1.6 a | 18.5 ± 1.2 a | 16.8 ± 0.6 AB | |||
| Mean | 15.0 ± 1.8 B | 17.2 ± 0.9 A | 16.1 ± 0.8 | ||||
| Grain Fe | T1 | Deionized water | 0.9 ± 0.1 a | 1.8 ± 0.2 ab | 1.3 ± 0.1 B | N | 0.0325 |
| content | T2 | Sucrose | 1.0 ± 0.4 a | 1.6 ± 0.1 b | 1.3 ± 0.2 B | T | 0.0470 |
| (mg per plant) | T3 | ZnSO4·7H2O (Zn) | 1.3 ± 0.6 a | 2.0 ± 0.4 a | 1.7 ± 0.5 A | N × T | 0.7565 |
| T4 | Sucrose + Zn | 1.0 ± 0.3 a | 1.8 ± 0.1 ab | 1.4 ± 0.2 AB | |||
| Mean | 1.1 ± 0.3 B | 1.8 ± 0.1 A | 1.4 ± 0.2 | ||||
| Treatments | Grain Dry Weight (g/ear) | Hundred Grain Weight (g) | Number of Kernels per Ear | Ear Diameter (mm) | Ear Length (cm) | Bald Tip Length (cm) | Bald Tip Length/Ear Length (%) | |
|---|---|---|---|---|---|---|---|---|
| T1 | Deionized water | 142.8 ± 15.7 a | 37.8 ± 2.2 a | 485.6 ± 119.5 a | 50.9 ± 3.1 a | 20.8 ± 3.5 a | 1.5 ± 0.4 a | 7.2 ± 2.2 a |
| T2 | Sucrose | 139.6 ± 10.4 a | 38.6 ± 1.7 a | 518.4 ± 103.4 a | 48.5 ± 5.2 a | 21.9 ± 1.5 a | 2.2 ± 1.1 a | 10.0 ± 2.0 a |
| T3 | ZnSO4·7H2O (Zn) | 143.6 ± 13.2 a | 36.1 ± 1.3 a | 523.4 ± 96.6 a | 46.0 ± 4.8 a | 21.3 ± 1.3 a | 2.2 ± 0.4 a | 10.3 ± 4.9 a |
| T4 | Sucrose + Zn | 150.9 ± 20.1 a | 37.9 ± 1.3 a | 500.0 ± 120.7 a | 50.0 ± 2.7 a | 20.0 ± 1.2 a | 1.6 ± 1.1 a | 8.0 ± 5.5 a |
| Treatments | Zn Concentrations (mg/kg) | Zn Contents (mg/plant) | PA/Zn | PA × Ca/Zn | Fe Concentrations (mg/kg) | Fe Contents (mg/plant) | PA/Fe | PA × Ca/Fe | |
|---|---|---|---|---|---|---|---|---|---|
| T1 | Deionized water | 24.9 ± 0.8 b | 3.6 ± 0.1 b | 34.9 ± 3.6 a | 70.1 ± 3.5 a | 24.0 ± 2.8 b | 3.4 ± 0.3 b | 31.4 ± 4.3 a | 63.0 ± 5.2 a |
| T2 | Sucrose | 23.8 ± 0.6 b | 3.3 ± 0.1 b | 32.6 ± 2.8 a | 37.9 ± 1.9 b | 23.1 ± 0.5 b | 3.2 ± 0.1 b | 28.9 ± 2.8 ab | 33.6 ± 2.1 b |
| T3 | ZnSO4·7H2O (Zn) | 36.8 ± 2.0 a | 5.3 ± 0.3 a | 22.1 ± 2.0 b | 35.8 ± 7.6 b | 30.0 ± 3.9 a | 4.3 ± 0.5 a | 23.7 ± 4.1 bc | 37.9 ± 7.1 b |
| T4 | Sucrose + Zn | 34.8 ± 0.8 a | 5.3 ± 0.2 a | 18.9 ± 2.4 b | 36.7 ± 11.5 b | 27.7 ± 1.2 ab | 4.2 ± 0.2 a | 20.4 ± 2.3c | 39.5 ± 10.8 b |
| Treatments | Carbon Concentration (%) | Nitrogen Concentration (%) | C/N | Phytate-P Concentration (g/kg) | Total P Concentration (g/kg) | Phytate P/Total P (%) | Ca Concentration (mg/kg) | |
|---|---|---|---|---|---|---|---|---|
| T1 | Deionized water | 42.8 ± 0.5 a | 1.78 ± 0.02 a | 24.07 ± 0.06 b | 2.49 ± 0.18 a | 3.24 ± 0.08 a | 76.8 ± 7.5 a | 80.8 ± 6.1 a |
| T2 | Sucrose | 42.7 ± 0.6 a | 1.69 ± 0.03 b | 25.27 ± 0.27 a | 2.22 ± 0.25 ab | 3.01 ± 0.10 ab | 73.8 ± 6.8 a | 46.6 ± 1.7 b |
| T3 | ZnSO4·7H2O (Zn) | 42.8 ± 0.1 a | 1.76 ± 0.03 a | 24.36 ± 0.40 b | 2.33 ± 0.17 ab | 3.07 ± 0.25 ab | 76.2 ± 7.2 a | 65.4 ± 17.0 ab |
| T4 | Sucrose + Zn | 42.7 ± 0.3 a | 1.70 ± 0.01 b | 25.11 ± 0.12 a | 1.88 ± 0.27 b | 2.79 ± 0.07 b | 67.4 ± 8.2 a | 77.5 ± 19.0 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, H.; Kong, W.; Wang, L.; Xue, Y.; Liu, W.; Zhang, C.; Yang, S.; Li, C. Foliar Zn Spraying Simultaneously Improved Concentrations and Bioavailability of Zn and Fe in Maize Grains Irrespective of Foliar Sucrose Supply. Agronomy 2019, 9, 386. https://doi.org/10.3390/agronomy9070386
Xia H, Kong W, Wang L, Xue Y, Liu W, Zhang C, Yang S, Li C. Foliar Zn Spraying Simultaneously Improved Concentrations and Bioavailability of Zn and Fe in Maize Grains Irrespective of Foliar Sucrose Supply. Agronomy. 2019; 9(7):386. https://doi.org/10.3390/agronomy9070386
Chicago/Turabian StyleXia, Haiyong, Weilin Kong, Lan Wang, Yanhui Xue, Wenlong Liu, Chunyan Zhang, Shenggang Yang, and Chong Li. 2019. "Foliar Zn Spraying Simultaneously Improved Concentrations and Bioavailability of Zn and Fe in Maize Grains Irrespective of Foliar Sucrose Supply" Agronomy 9, no. 7: 386. https://doi.org/10.3390/agronomy9070386
APA StyleXia, H., Kong, W., Wang, L., Xue, Y., Liu, W., Zhang, C., Yang, S., & Li, C. (2019). Foliar Zn Spraying Simultaneously Improved Concentrations and Bioavailability of Zn and Fe in Maize Grains Irrespective of Foliar Sucrose Supply. Agronomy, 9(7), 386. https://doi.org/10.3390/agronomy9070386






