Terrestrial and Remote Indexes to Assess Moderate Deficit Irrigation in Early-Maturing Nectarine Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Automated Irrigation Feedback
2.3. Plant Water Status and Gas Exchange
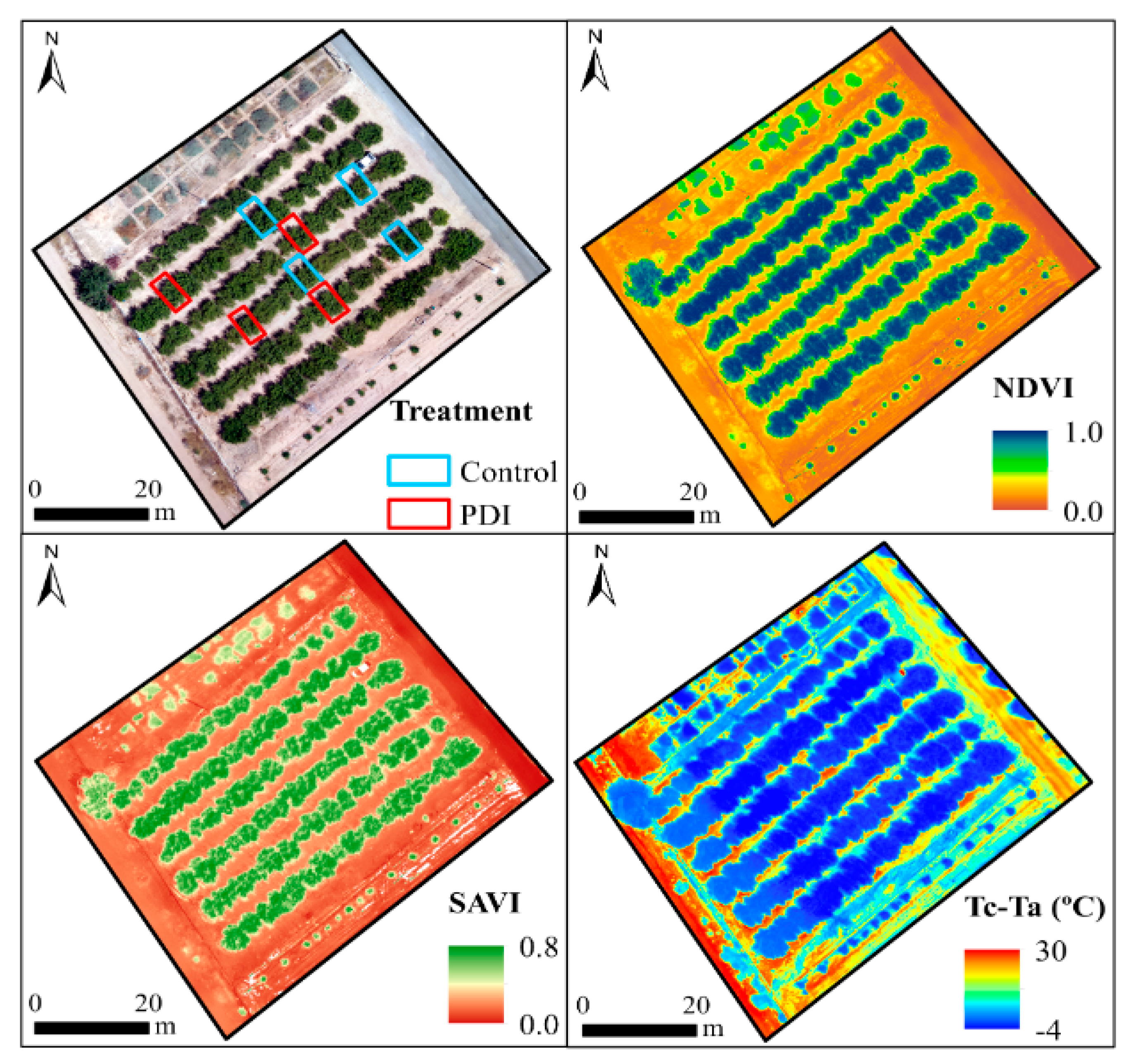
2.4. Airborne Campaign
2.5. Vegetative Growth, Yield, and Fruit Quality
2.6. Sensitivity Analysis
2.7. Statistical Analysis
3. Results and Discussion
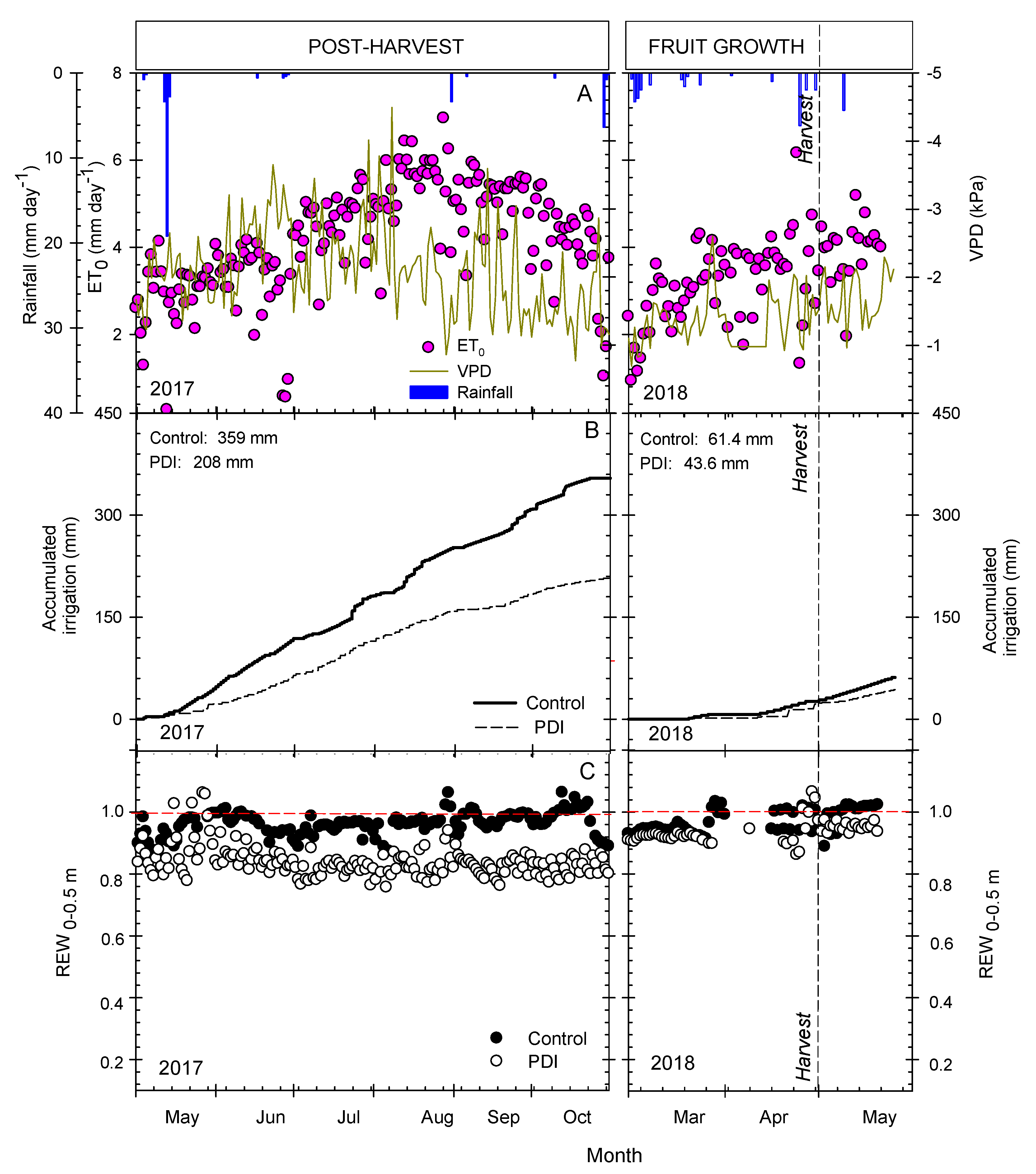
3.1. Environmental Conditions and Irrigation Applied
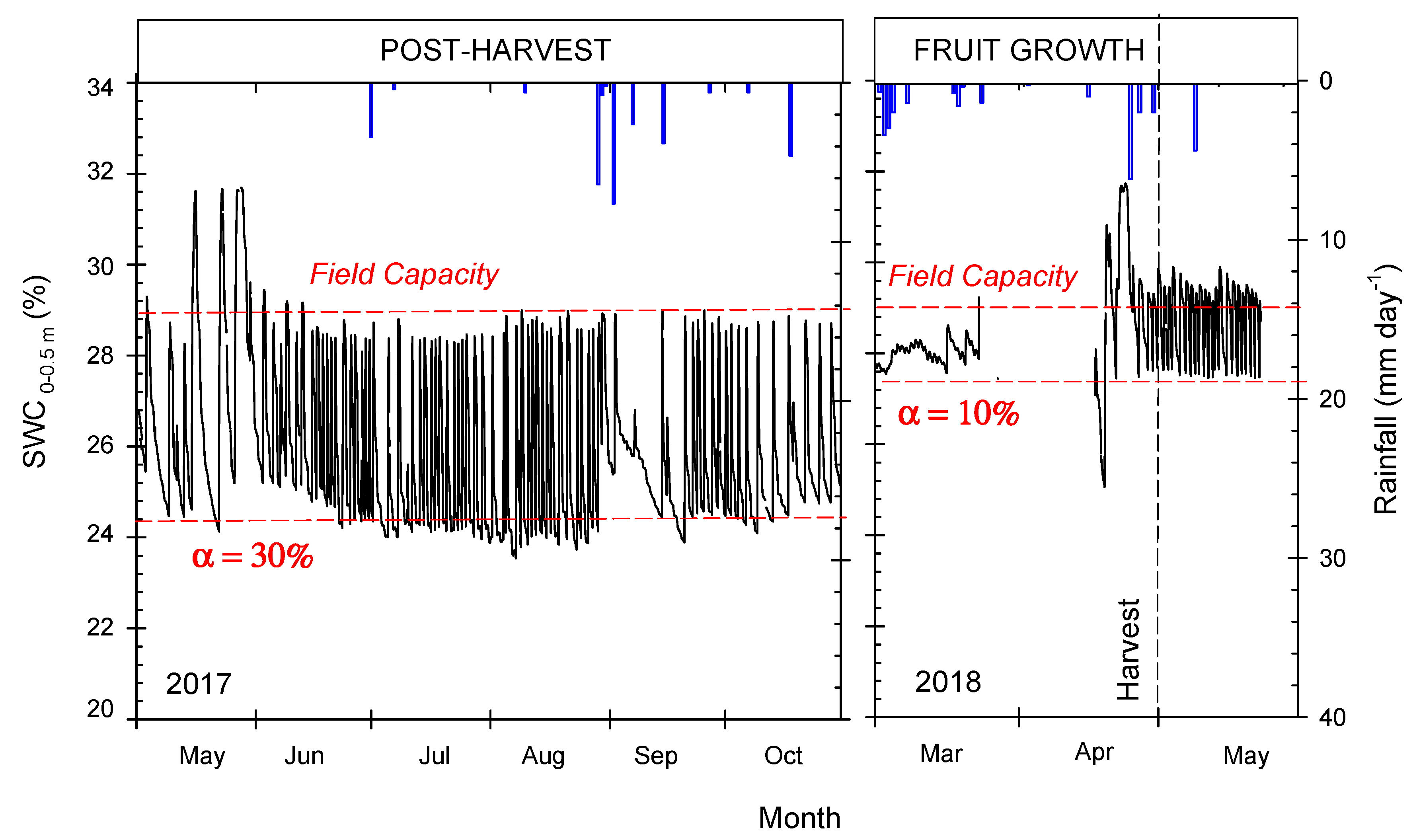
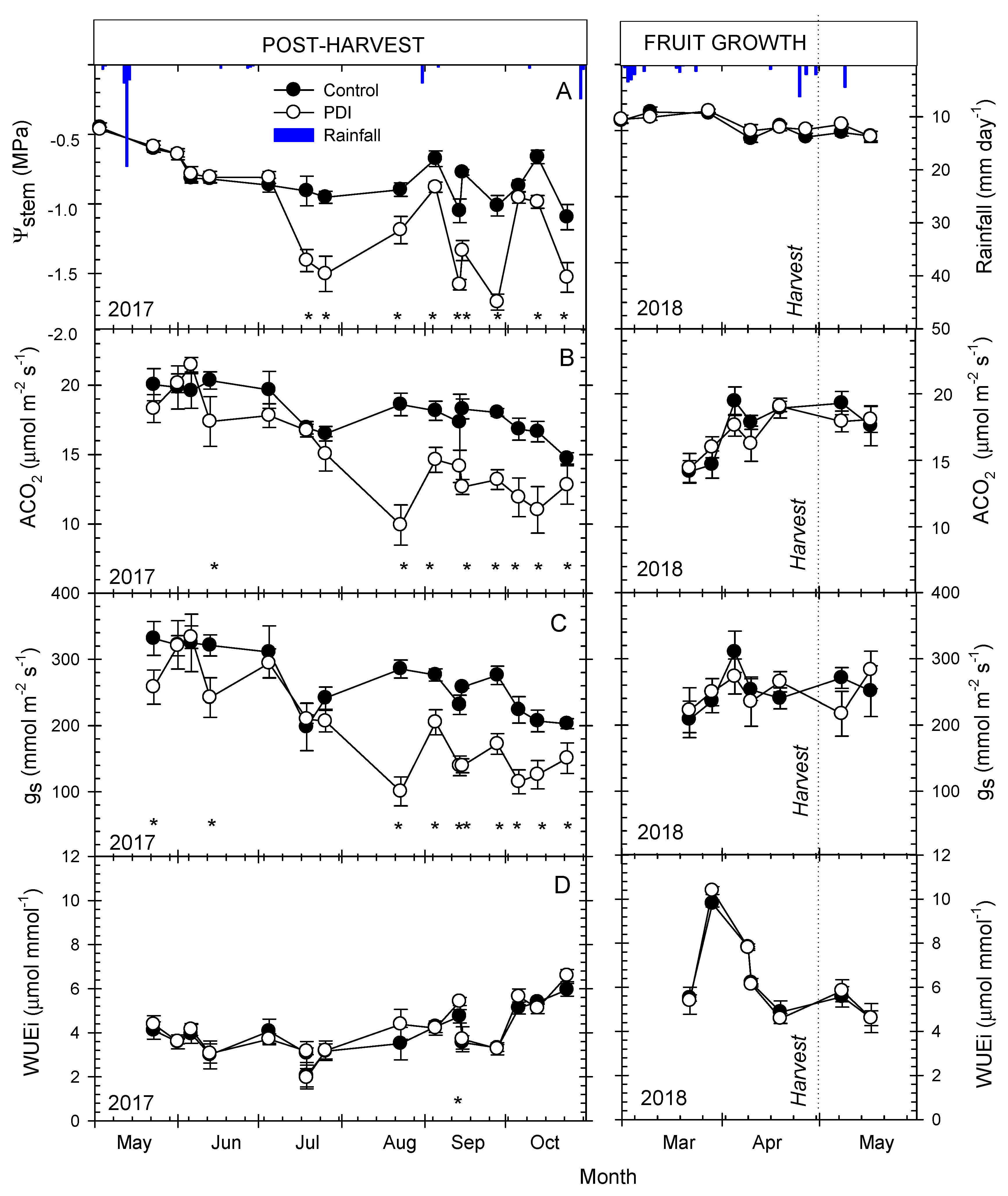
3.2. Seasonal Evolution of Terrestrial Soil and Plant Water-Status Indicators
3.3. Vegetative Growth, Yield, and Fruit Quality
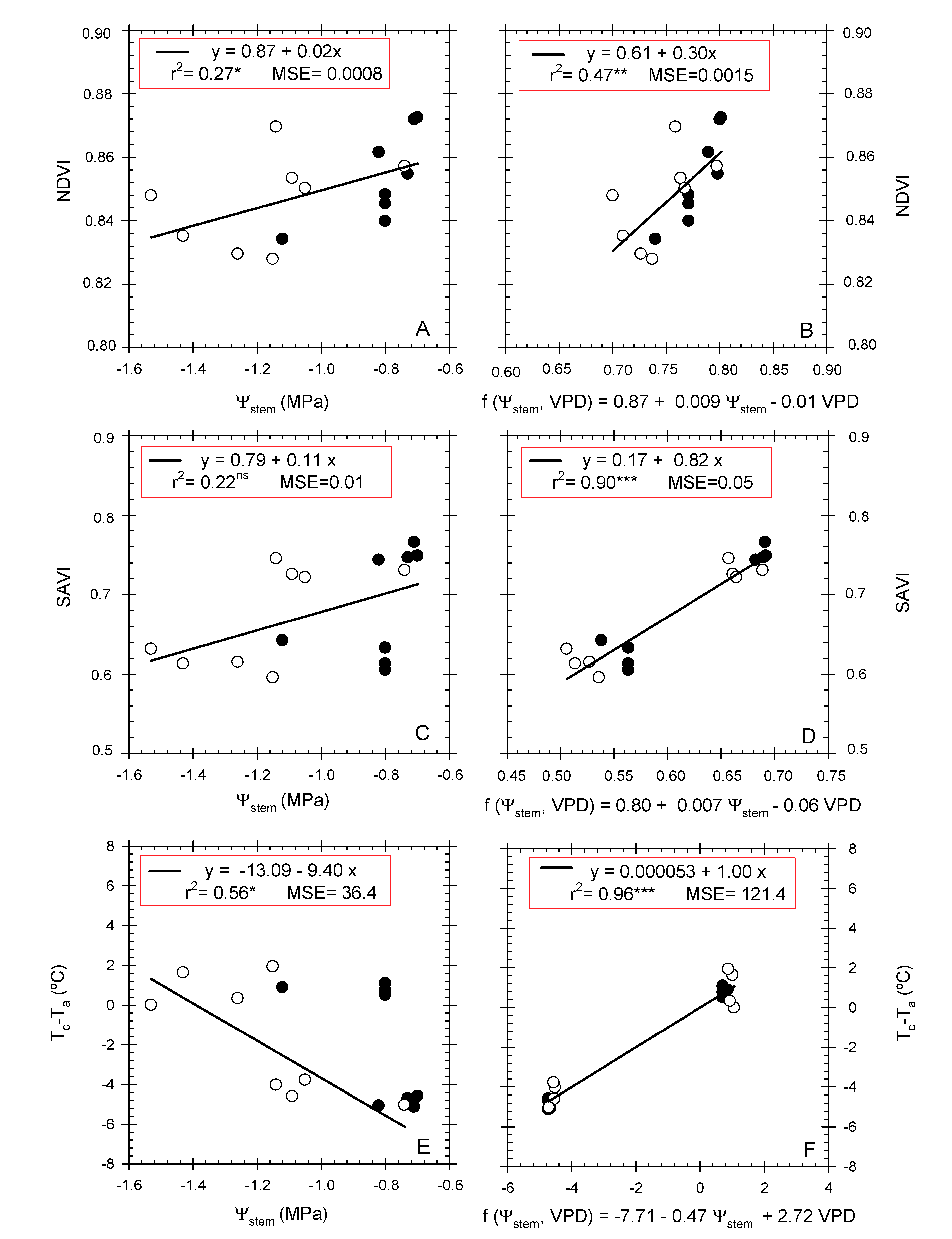
3.4. Remote and Terrestrial Plant-Water-Status Indicators
3.5. Role of NDVI, SAVI, and Tc-Ta indexes
3.6. Sensitivity of Remote and Terrestrial Plant Water Status Indicators
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations Education Scientific and Cultural Organization (UNESCO). Understanding the pressure of water (Part 1). In Water in a Changing World; The United Nations World Water Development, Ed.; UNESCO Publishing: Paris, France, 2019; pp. 29–75. ISBN 978-9-23104-095-5. [Google Scholar]
- Chai, Q.; Gan, Y.; Zhao, C.; Xu, H.L.; Waskom, R.M.; Niu, Y.; Siddique, K.H.M. Regulated deficit irrigation for crop production under drought stress: A review. Agron. Sustain. Dev. 2016, 36, 3. [Google Scholar] [CrossRef]
- Vera, J.; Conejero, W.; Conesa, M.R.; Ruiz-Sánchez, M.C. Irrigation Factor Approach Based on Soil Water Content: A Nectarine Orchard Case Study. Water 2019, 11, 589. [Google Scholar] [CrossRef]
- Vera, J.; Abrisqueta, I.; Abrisqueta, J.M.; Ruiz-Sánchez, M.C. Effect of deficit irrigation on early-maturing peach tree performance. Irrig. Sci. 2013, 31, 747–757. [Google Scholar] [CrossRef]
- De La Rosa, J.; Domingo, R.; Gómez-Montiel, J.; Pastor, A.P. Implementing deficit irrigation scheduling through plant water stress indicators in early nectarine trees. Agric. Water Manag. 2015, 152, 207–216. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, M.C.; Abrisqueta, I.; Conejero, W.; Vera, J. Deficit Irrigation Management in Early-Maturing Peach Crop. In Water Scarcity and Sustainable Agriculture in Semiarid Environment; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 111–129. [Google Scholar]
- Abrisqueta, I.; Abrisqueta, J.; Tapia, L.; Munguía, J.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M. Basal crop coefficients for early-season peach trees. Agric. Water Manag. 2013, 121, 158–163. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, M.C.; Domingo, R.; Castel, J.R. Review. Deficit irrigation in fruit trees and vines in Spain. Span. J. Agric. Res. 2010, 8, 5. [Google Scholar] [CrossRef]
- Lopez, G.; Mata, M.; Arbones, A.; Solans, J.R.; Girona, J.; Marsal, J. Mitigation of effects of extreme drought during stage III of peach fruit development by summer pruning and fruit thinning. Tree Physiol. 2006, 26, 469–477. [Google Scholar] [CrossRef]
- De La Rosa, J.; Domingo, R.; Aguayo, E.; Falagan, N.; Pérez-Pastor, A.; Conesa, M. Combined effects of deficit irrigation and crop level on early nectarine trees. Agric. Water Manag. 2016, 170, 120–132. [Google Scholar] [CrossRef]
- Conesa, M.; Martínez-López, L.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M. Summer pruning of early-maturing Prunus persica: Water implications. Sci. Hortic. 2019, 256, 108539. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.G.; Yoon, T.M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Girona, J.; Gelly, M.; Mata, M.; Arbones, A.; Rufat, J.; Marsal, J. Peach tree response to single and combined deficit irrigation regimes in deep soils. Agric. Water Manag. 2005, 72, 97–108. [Google Scholar] [CrossRef]
- Jones, H.G. Irrigation scheduling: Advantages and pitfalls of plant-based methods. J. Exp. Bot. 2004, 55, 2427–2436. [Google Scholar] [CrossRef]
- Naor, A. Irrigation Scheduling and Evaluation of Tree Water Status in Deciduous Orchards. Hortic. Rev. 2010, 32, 111–165. [Google Scholar]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap Pressure in Vascular Plants: Negative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Naor, A.; Cohen, S. Sensitivity and variability of maximum trunk shrinkage, midday stem water potential and transpiration rate in response to withholding irrigation from weld grown apple trees. HortScience 2003, 38, 547–551. [Google Scholar] [CrossRef]
- Bellvert, J.; Marsal, J.; Girona, J.; González-Dugo, V.; Fereres, E.; Ustin, S.L.; Zarco-Tejada, P.J. Airborne Thermal Imagery to Detect the Seasonal Evolution of Crop Water Status in Peach, Nectarine and Saturn Peach Orchards. Remote Sens. 2016, 8, 39. [Google Scholar] [CrossRef]
- Ramírez-Cuesta, J.M.; Mirás-Avalos, J.M.; Rubio-Asensio, J.S.; Intrigliolo, D.S. A novel ArcGIS toolbox for estimating crop water demands by integrating the dual crop coefficient approach with multi-satellite imagery. Water 2019, 11, 38. [Google Scholar] [CrossRef]
- Park, S.; Ryu, D.; Fuentes, S.; Chung, H.; Hernández-Montes, E.; O’Connell, M. Adaptative estimation of crop water stress in nectarine and peach orchards using high-resolution imagery from an Unmmanned Aerial Vehicle (UAV). Remote Sens. 2017, 9, 828. [Google Scholar] [CrossRef]
- Egea, G.; Padilla-Díaz, C.M.; Martinez-Guanter, J.; Fernández, J.E.; Pérez-Ruiz, M. Assessing a crop water stress index derived from aerial thermal imaging and infrared thermometry in super-high density olive orchards. Agric. Water Manag. 2017, 187, 210–221. [Google Scholar] [CrossRef]
- Idso, S.; Jackson, R.; Pinter, P.; Reginato, R.; Hatfield, J. Normalizing the stress-degree-day parameter for environmental variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Berni, J.; Zarco-Tejada, P.J.; Sepulcre-Cantó, G.; Fereres, E.; Villalobos, F.; Jimenez-Berni, J.A. Mapping canopy conductance and CWSI in olive orchards using high resolution thermal remote sensing imagery. Remote Sens. Environ. 2009, 113, 2380–2388. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; González-Dugo, V.; Williams, L.E.; Suárez, L.; Berni, J.A.J.; Goldhamer, D.; Fereres, E. A PRI-based water stress index combining structural and chlorophyll effects: Assessment using diurnal narrow-band air-borne imagery and the CWSI thermal index. Remote Sens. Environ. 2013, 138, 38–50. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Zarco-Tejada, P.J.; Fereres, E. Applicability and limitations of using the crop water stress index as an indicator of water deficits in citrus orchards. Agric. For. Meteorol. 2014, 198, 94–104. [Google Scholar] [CrossRef]
- Rouse, J.W.; Hass, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the 3rd ERTS Symposium, College Station, TX, USA, 1 January 1974; pp. 309–317. [Google Scholar]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indexes: A review of Developments and Applications. J. Sens. 2017, 2017, e1353691. [Google Scholar] [CrossRef]
- Huete, A.; Huete, A. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Viña, A.; Gitelson, A.A.; Nguy-Robertson, A.L.; Peng, Y. Comparison of different vegetation indices for the remote assessment of green leaf area index of crops. Remote Sens. Environ. 2011, 115, 3468–3478. [Google Scholar] [CrossRef]
- Stagakis, S.; Gonzalez-Dugo, V.; Cid, P.; Guillén-Climent, M.; Zarco-Tejada, P.J. Monitoring water stress and fruit quality in an orange orchard under regulated deficit irrigation using narrow-band structural and physiological remote sensing indices. ISPRS J. Photogramm. Remote Sens. 2012, 71, 47–61. [Google Scholar] [CrossRef]
- Romero-Trigueros, C.; Nortes, P.A.; Alarcón, J.J.; Hunink, J.E.; Parra, M.; Contreras, S.; Droogers, P.; Nicolás, E. Effects of saline reclaimed waters and deficit irrigation on Citrus physiology assessed by UAV remote sensing. Agric. Water Manag. 2017, 183, 60–69. [Google Scholar] [CrossRef]
- Vanella, D.; Ramírez-Cuesta, J.M.; Intrigliolo, D.S.; Consoli, S. Combining Electrical Resistivity Tomography and Satellite Images for Improving Evapotranspiration Estimates of Citrus Orchards. Remote Sens. 2019, 11, 373. [Google Scholar] [CrossRef]
- Gago, J.; Douthe, C.; Coopman, R.; Gallego, P.; Ribas-Carbo, M.; Flexas, J.; Escalona, J.; Medrano, H. UAVs challenge to assess water stress for sustainable agriculture. Agric. Water Manag. 2015, 153, 9–19. [Google Scholar] [CrossRef]
- Ramírez-Cuesta, J.M.; Allen, R.G.; Zarco-Tejada, P.J.; Kilic, A.; Santos, C.; Lorite, I.J. Impact of the spatial resolution on the energy balance components on an open-canopy olive orchard. Int. J. Appl. Earth Obs. Geoinf. 2019, 74, 88–102. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Zarco-Tejada, P.J.; Nicolas, E.; Nortes, P.A.; Alarcón, J.J.; Intrigliolo, D.S.; Fereres, E. Using high resolution UAV thermal imagery to assess the variability in the water status of five fruit tree species within a commercial orchard. Precis. Agric. 2013, 14, 660–678. [Google Scholar] [CrossRef]
- Usha, K.; Singh, B. Potential applications of remote sensing in horticulture—A review. Sci. Hortic. 2013, 153, 71–83. [Google Scholar] [CrossRef]
- Ballester, C.; Zarco-Tejada, P.J.; Nicolás, E.; Alarcón, J.J.; Fereres, E.; Intrigliolo, D.S.; González-Dugo, V. Evaluating the performance of xanthophyll chlorophyll and structure-sensitive spectral indices to detect water stress in five fruit tree species. Precis. Agric. 2018, 19, 178–193. [Google Scholar] [CrossRef]
- Goldhamer, D.; Fereres, E. Irrigation scheduling protocols using continuously recorded trunk diameter measurements. Irrig. Sci. 2001, 20, 115–125. [Google Scholar] [CrossRef]
- De La Rosa, J.M.; Conesa, M.R.; Domingo, R.; Pérez-Pastor, A. A new approach to ascertain the sensitivity to water stress of different plant water indicators in extra-early nectarine trees. Sci. Hortic. 2014, 169, 147–153. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Méndez, A.; Blaya, J.M.; López-Torres, F.J.; Rodriguez, E.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Distribución de raíces de nectarino en distintas condiciones de riego. In Proceedings of the II Congreso IDIES, Murcia, Spain, 22 June 2015. [Google Scholar]
- Evett, S.R.; Tolk, J.A.; Howell, T.A. Soil Profile Water Content Determination. Vadose Zone J. 2006, 5, 894. [Google Scholar] [CrossRef]
- Abrisqueta, I.; Vera, J.; Tapia, L.; Abrisqueta, J.; Ruiz-Sánchez, M. Soil water content criteria for peach trees water stress detection during the postharvest period. Agric. Water Manag. 2012, 104, 62–67. [Google Scholar] [CrossRef]
- Abrisqueta, I.; Conejero, W.; López-Martínez, L.; Vera, J.; Sánchez, M.C.R. Root and aerial growth in early-maturing peach trees under two crop load treatments. Span. J. Agric. Res. 2017, 15, e0803. [Google Scholar] [CrossRef]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef]
- Hsiao, T.C. Measurement of plant water status. In Irrigation of Agricultural Crops. Agronomy Monograph No.30; Steward, B.A., Nielsen, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1990; pp. 243–279. [Google Scholar]
- McCutchan, H.; Shackel, K. Stem-water Potential as a Sensitive Indicator of Water Stress in Prune Trees (Prunus domestica L. cv. French). J. Am. Soc. Hortic. Sci. 1992, 117, 607–611. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Greenwave Effect) of Natural Vegetation; NASA/GSFC Type II Final Report 371; Remote Sensing Center, Texas A&M University: College Station, TX, USA, 1973. [Google Scholar]
- European Union. Commission Regulation (EC) No 1221/2008 of 5 December, 2008. Amending Regulation (EC) No 1580/2007 laying down implementing rules of Council Regulations (EC) No 2200/96, (EC) No 2201/96 and (EC) No 1182/2007 in the fruit and vegetable sector as regards marketing standards. Off. J. Eur. Union. 2008, 51, 80. [Google Scholar]
- Agencia Española de Meteorología (AEMET). Available online: http://www.aemet.es/es (accessed on 18 June 2019).
- Naor, A.; Stern, R.; Peres, M.; Greenblat, Y.; Gal, Y.; Flaishman, M.A. Timing and Severity of Postharvest Water Stress Affect Following-year Productivity and Fruit Quality of Field-grown ‘Snow Queen’ Nectarine. J. Am. Soc. Hortic. Sci. 2005, 130, 806–812. [Google Scholar] [CrossRef]
- Lopez, G.; Echeverria, G.; Bellvert, J.; Mata, M.; Behboudian, M.H.; Girona, J.; Marsal, J. Water stress for a short period before harvest in nectarine: Yield, fruit composition, sensory quality, and consumer acceptance of fruit. Sci. Hortic. 2016, 211, 1–7. [Google Scholar] [CrossRef]
- Bhusal, N.; Bhusal, S.J.; Yoon, T.M. Comparisons of physiological and anatomical characteristics between two cultivars in bi-leader apple trees (Malus×domestica Borkh.). Sci. Hortic. 2018, 231, 73–81. [Google Scholar] [CrossRef]
- Pérez-Pastor, A.; Domingo, R.; Torrecillas, A.; Ruiz-Sánchez, M.C. Response of apricot trees to deficit irrigation strategies. Irrig. Sci. 2009, 27, 231–242. [Google Scholar] [CrossRef]
- Osório, M.L.; Breia, E.; Rodrigues, A.; Osório, J.; Roux, X.; Daudet, F.; Ferreira, I.; Chaves, M.M. Limitations to carbon assimilation by mild drought in nectarine trees growing under field conditions. Environ. Exp. Bot. 2006, 55, 235–247. [Google Scholar] [CrossRef]
- Dichio, B.; Xiloyannis, C.; Sofo, A.; Montanaro, G. Effects of postharvest regulated deficit irrigation on carbohydrate and nitrogen partitioning, yield quality and vegetative growth of peach trees. Plant Soil 2007, 290, 127–137. [Google Scholar] [CrossRef]
- Conesa, M.; Dodd, I.; Temnani, A.; De La Rosa, J.; Pérez-Pastor, A. Physiological response of post-veraison deficit irrigation strategies and growth patterns of table grapes (cv. Crimson Seedless). Agric. Water Manag. 2018, 208, 363–372. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Escalona, J.M.; Pou, A.; Fuentes, S.; Flexas, J.; Bota, J. Improving water use efficiency of vineyards in semi-arid regions: A review. Agron. Sustain. Dev. 2015, 35, 499–517. [Google Scholar] [CrossRef]
- Marsal, J.; Girona, J. Relationship between Leaf Water Potential and Gas Exchange Activity at Different Phenological Stages and Fruit Loads in Peach Trees. J. Am. Soc. Hortic. Sci. 1997, 122, 415–421. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Escalona, J.M.; Sampol, B.; Medrano, H. Effects of drought on photosynthesis and electron transport rate regulation in grapevine. Plant Cell Environ. 2002, 22, 39–48. [Google Scholar] [CrossRef]
- Shackel, K.; Lampinen, B.; Sibbett, S.; Olson, W. The Relation of Midday Stem Water Potential to the Growth and Physiology of Fruit Trees Under Water Limited Conditions. Acta Hortic. 2000, 537, 425–430. [Google Scholar] [CrossRef]
- Galindo, A.; Rodríguez, P.; Collado-González, J.; Cruz, Z.; Torrecillas, E.; Ondoño, S.; Corell, M.; Moriana, A.; Torrecillas, A.; González, M.C. Rainfall intensifies fruit peel cracking in water stressed pomegranate trees. Agric. For. Meteorol. 2014, 194, 29–35. [Google Scholar] [CrossRef]
- Alcobendas, R.; Mirás-Avalos, J.M.; Alarcón, J.J.; Pedrero, F.; Nicolás, E. Combined effects of irrigation, crop load and fruit position on size, color and firmness of fruits in an extra-early cultivar of peach. Sci. Hortic. 2012, 142, 128–135. [Google Scholar] [CrossRef]
- Leinonen, I.; Jones, H.G. Combining thermal and visible imagery for estimating canopy temperature and identifying plant stress. J. Exp. Bot. 2004, 55, 1423–1431. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Zarco-Tejada, P.J.; Berni, J.; Suarez, L.; Goldhamer, D.; Fereres, E.; Jimenez-Berni, J.A. Almond tree canopy temperature reveals intra-crown variability that is water stress-dependent. Agric. For. Meteorol. 2012, 154, 156–165. [Google Scholar] [CrossRef]
- Rahmati, M.; Davarynejad, G.H.; Génard, M.; Bannayan, M.; Azizi, M.; Vercambre, G. Peach Water Relations, Gas Exchange, Growth and Shoot Mortality under Water Deficit in Semi-Arid Weather Conditions. PLoS ONE 2015, 10, e0120246. [Google Scholar] [CrossRef]
- Valdés-Vela, M.; Abrisqueta, I.; Conejero, W.; Vera, J.; Sánchez, M.C.R. Soft computing applied to stem water potential estimation: A fuzzy rule based approach. Comput. Electron. Agric. 2015, 115, 150–160. [Google Scholar] [CrossRef]
- Drew, M.C. Stress physiology. In Plant Physiology, 4th ed.; Taiz, L., Zeiger, E., Eds.; Sinauer Associates: Sunderland, MA, USA, 2006; p. 764. [Google Scholar]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Durán-Zuazo, V.H.; Muriel-Fernández, J.L.; Jiménez-Bocanegra, J.A. Linking canopy temperature and trunk diameter fluctuations with other physiological water status tools for water stress management in citrus orchards. Funct. Plant Boil. 2011, 38, 106. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Gonzalez-Dugo, V.; Berni, J.; Jimenez-Berni, J.A. Fluorescence, temperature and narrow-band indices acquired from a UAV platform for water stress detection using a micro-hyperspectral imager and a thermal camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
- Ferrer, M.; Echeverria, G.; Pereyra, G.; González-Neves, G.; Pans, D.; Mirás-Avalos, J.M. Mapping vineyard vigour using airborne remote sensing: Relations with yield, berry composition and sanitary status under humid climate conditions. Precis. Agric. 2019, 1–20. [Google Scholar] [CrossRef]
- Rojas, O.; Vrieling, A.; Rembold, F. Assessing drought probability for agricultural areas in Africa with coarse resolution remote sensing imagery. Remote Sens. Environ. 2011, 115, 343–352. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil adjusted vegetation indexes. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Dobrowski, S.; Ustin, S.; Wolpert, J. Grapevine dormant pruning weight prediction using remotely sensed data. Aust. J. Grape Wine Res. 2003, 9, 177–182. [Google Scholar] [CrossRef]
- Hogrefe, K.R.; Patil, V.P.; Ruthrauff, D.R.; Meixell, B.W.; Budde, M.E.; Hupp, J.W.; Ward, D.H. Normalized Difference Vegetation Index as an Estimator for Abundance and Quality of Avian Herbivore Forage in Arctic Alaska. Remote Sens. 2017, 9, 1234. [Google Scholar] [CrossRef]
- Jackson, R.D. Canopy Temperature and Crop Water Stress. In Advances in Irrigation; Elsevier BV: Amsterdam, The Netherlands, 1982; Volume 1, pp. 43–85. [Google Scholar]
- González-Dugo, M.P.; Moran, M.S.; Mateos, L.; Bryant, R. Canopy temperature variability as an indicator of crop water stress severity. Irrig. Sci. 2006, 24, 233. [Google Scholar] [CrossRef]
- González-Dugo, V.; Goldhamer, D.; Zarco-Tejada, P.J.; Fereres, E. Improving the precision of irrigation in a pistachio farm using an unmanned airborne thermal system. Irrig. Sci. 2015, 33, 43–52. [Google Scholar] [CrossRef]
- Camino, C.; Zarco-Tejada, P.J.; González-Dugo, V. Effects of Heterogeneity within Tree Crowns on Airborne-Quantified SIF and the CWSI as Indicators of Water Stress in the Context of Precision Agriculture. Remote Sens. 2018, 10, 604. [Google Scholar] [CrossRef]
- Abrisqueta, I.; Conejero, W.; Valdés-Vela, M.; Vera, J.; Ortuño, M.F.; Ruiz-Sánchez, M.C.; Ortuño, M.F. Stem water potential estimation of drip-irrigated early-maturing peach trees under Mediterranean conditions. Comput. Electron. Agric. 2015, 114, 7–13. [Google Scholar] [CrossRef]
- Blanco, V.; Domingo, R.; Pérez-Pastor, A.; Blaya-Ros, P.J.; Torres-Sánchez, R. Soil and plant water indicators for deficit irrigation management of field-grown sweet cherry trees. Agric. Water Manag. 2018, 208, 83–94. [Google Scholar] [CrossRef]
 ) and PDI (
) and PDI ( ) treatments at t1 (10: 00 GTM) and t2 (12:00 GTM) flights. Values are means ± SE of 4 replicates. Different letters on the bars indicate significant differences between treatments at p ≤ 0.05 within t1 or t2.
) treatments at t1 (10: 00 GTM) and t2 (12:00 GTM) flights. Values are means ± SE of 4 replicates. Different letters on the bars indicate significant differences between treatments at p ≤ 0.05 within t1 or t2.
 ) and PDI (
) and PDI ( ) treatments at t1 (10: 00 GTM) and t2 (12:00 GTM) flights. Values are means ± SE of 4 replicates. Different letters on the bars indicate significant differences between treatments at p ≤ 0.05 within t1 or t2.
) treatments at t1 (10: 00 GTM) and t2 (12:00 GTM) flights. Values are means ± SE of 4 replicates. Different letters on the bars indicate significant differences between treatments at p ≤ 0.05 within t1 or t2.
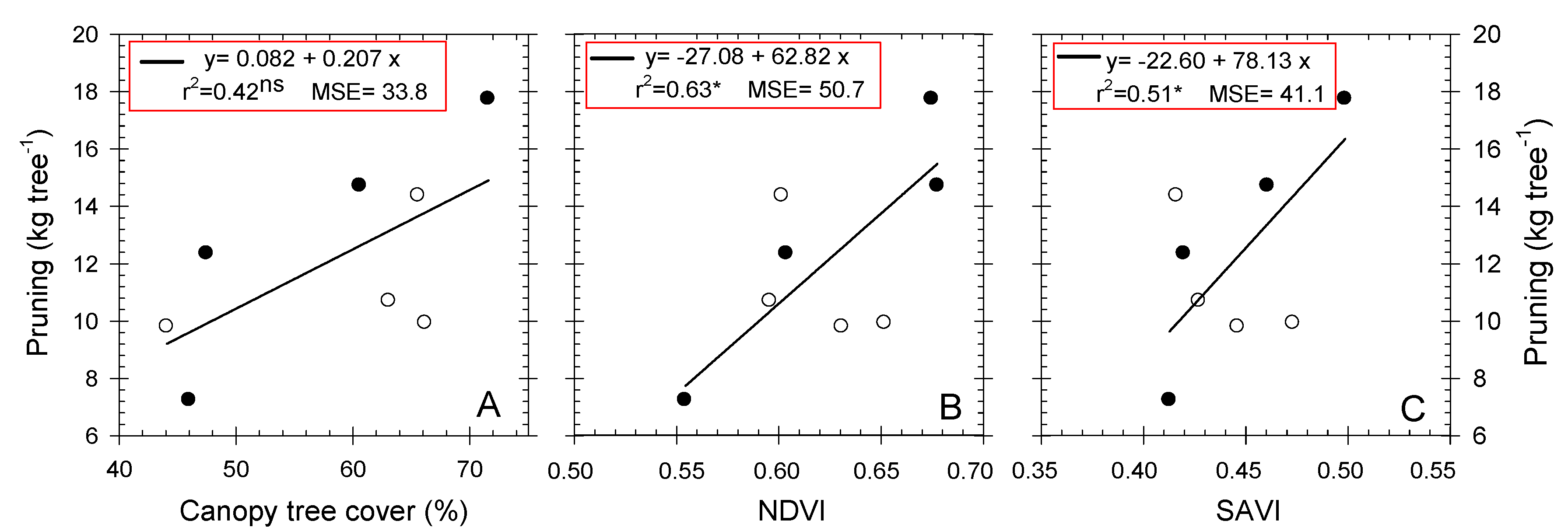
| Vegetative Components | Control | PDI | ANOVA |
|---|---|---|---|
| Pruning (kg tree−1) | 13.01 ± 2.22 | 11.20 ± 1.08 | ns |
| Canopy tree cover (%) | 56.43 ± 6.03 | 59.75 ± 5.26 | ns |
| Trunk cross-section area (TCSA, cm2) | 242.28 ± 31.78 | 214.72 ± 20.02 | ns |
| Yield components | |||
| Yield (t ha−1) | 8.37 ± 1.15 | 6.24 ± 0.64 | ns |
| N° fruits (fruits tree−1) | 145.67 ± 21.95 | 118 ± 13.65 | ns |
| Cracking (%) | 22.67 ± 3.84 | 23.67 ± 10.17 | ns |
| Fruit mass (g) | 131.20 ± 2.44 | 120.70 ± 5.81 | ns |
| Crop load (fruits cm−2 TCSA) | 0.55 ± 0.07 | 0.51 ± 0.07 | ns |
| Crop water use efficiency (WUE, kg m−3) | 4.74 ± 0.65 | 5.01 ± 0.52 | ns |
| Fruit quality components | |||
| Fruit diameter z (mm) | 64.09 ± 0.67 | 63.42 ± 0.19 | ns |
| Soluble solid content (SSC, °Brix) | 11.65 ± 0.31 | 12.16 ± 0.18 | ns |
| Lightness (L) | 39.33 ± 1.15 | 44.09 ± 2.11 | ns |
| Hue angle (°hue) | 40.49 ± 0.18 | 40.15 ± 1.19 | ns |
| Skin Chroma (*C) | 31.90 ± 1.78 | 41.98 ± 3.39 | * |
| t1 | t2 | |||||||
|---|---|---|---|---|---|---|---|---|
| SI | CV | S | S* | SI | CV | S | S* | |
| Ѱstem | 1.36 | 0.18 | 7.56 | 1.99 | 1.53 | 0.13 | 12.05 | 4.15 |
| ACO2 | 0.99 | 0.05 | 21.13 | ‒0.25 | 0.90 | 0.15 | 6.18 | ‒0.71 |
| gs | 1.06 | 0.05 | 20.00 | 1.14 | 0.90 | 0.19 | 4.84 | ‒0.55 |
| WUEi | 1.03 | 0.01 | 70.61 | 1.,97 | 0.94 | 0.16 | 5.99 | ‒0.37 |
| NDVI | 0.99 | 0.01 | 100.58 | ‒0.89 | 0.99 | 0.01 | 91.28 | ‒0.74 |
| SAVI | 0.97 | 0.01 | 68.49 | ‒1.91 | 0.98 | 0.02 | 41.01 | ‒0.64 |
| Tc | 1.02 | 0.02 | 43.22 | 0.93 | 1.01 | 0.03 | 34.33 | 0.17 |
| Tc-Ta | 0.90 | 0.13 | 6.96 | ‒0.81 | 1.21 | 1.00 | 1.21 | 0.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conesa, M.R.; Conejero, W.; Vera, J.; Ramírez-Cuesta, J.M.; Ruiz-Sánchez, M.C. Terrestrial and Remote Indexes to Assess Moderate Deficit Irrigation in Early-Maturing Nectarine Trees. Agronomy 2019, 9, 630. https://doi.org/10.3390/agronomy9100630
Conesa MR, Conejero W, Vera J, Ramírez-Cuesta JM, Ruiz-Sánchez MC. Terrestrial and Remote Indexes to Assess Moderate Deficit Irrigation in Early-Maturing Nectarine Trees. Agronomy. 2019; 9(10):630. https://doi.org/10.3390/agronomy9100630
Chicago/Turabian StyleConesa, María R., Wenceslao Conejero, Juan Vera, Juan M. Ramírez-Cuesta, and M. Carmen Ruiz-Sánchez. 2019. "Terrestrial and Remote Indexes to Assess Moderate Deficit Irrigation in Early-Maturing Nectarine Trees" Agronomy 9, no. 10: 630. https://doi.org/10.3390/agronomy9100630
APA StyleConesa, M. R., Conejero, W., Vera, J., Ramírez-Cuesta, J. M., & Ruiz-Sánchez, M. C. (2019). Terrestrial and Remote Indexes to Assess Moderate Deficit Irrigation in Early-Maturing Nectarine Trees. Agronomy, 9(10), 630. https://doi.org/10.3390/agronomy9100630







