Agrobiodiversity for Adaptive and Yield Traits in Romanian and Italian Barley Cultivars across Four Continental Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiments
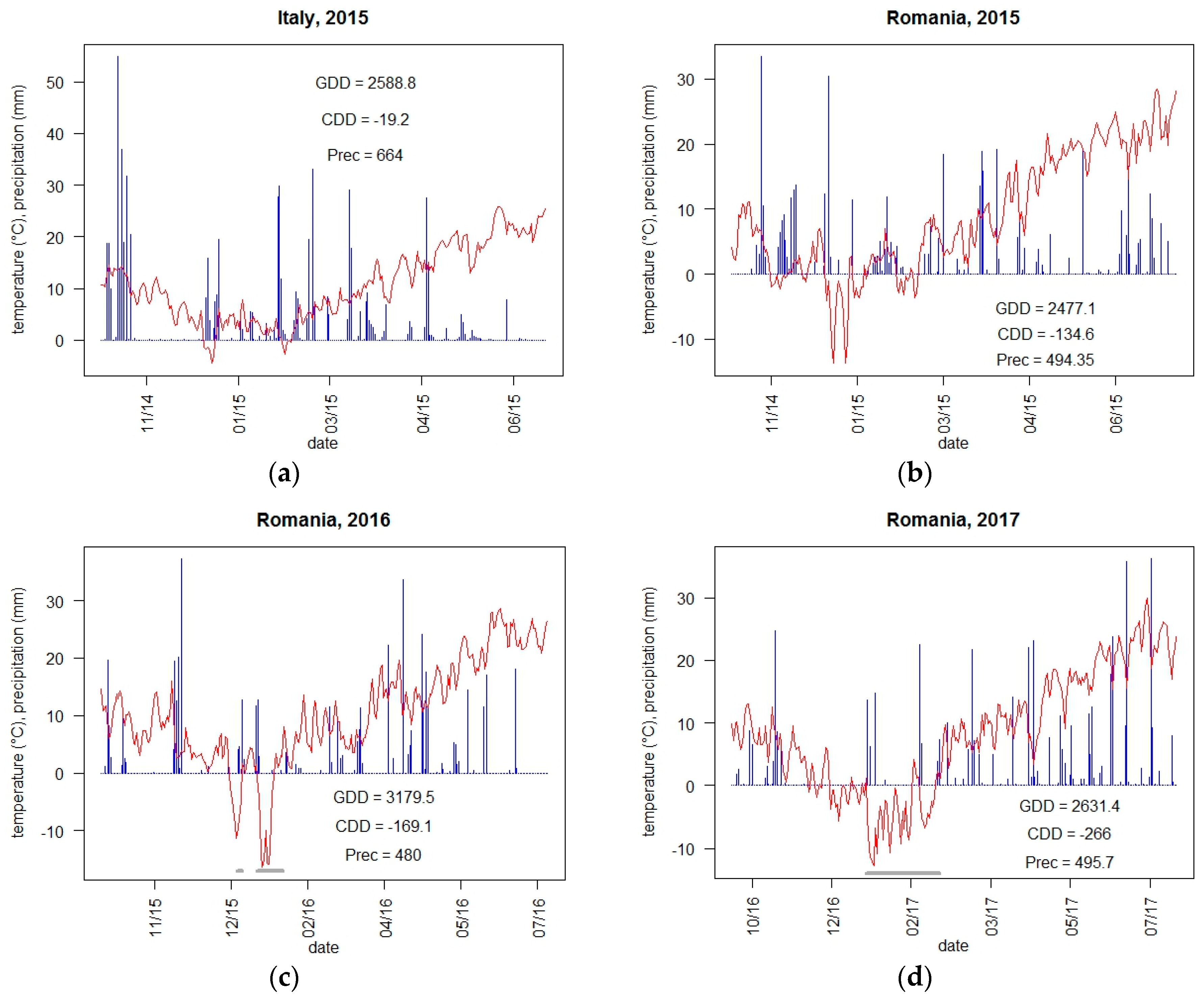
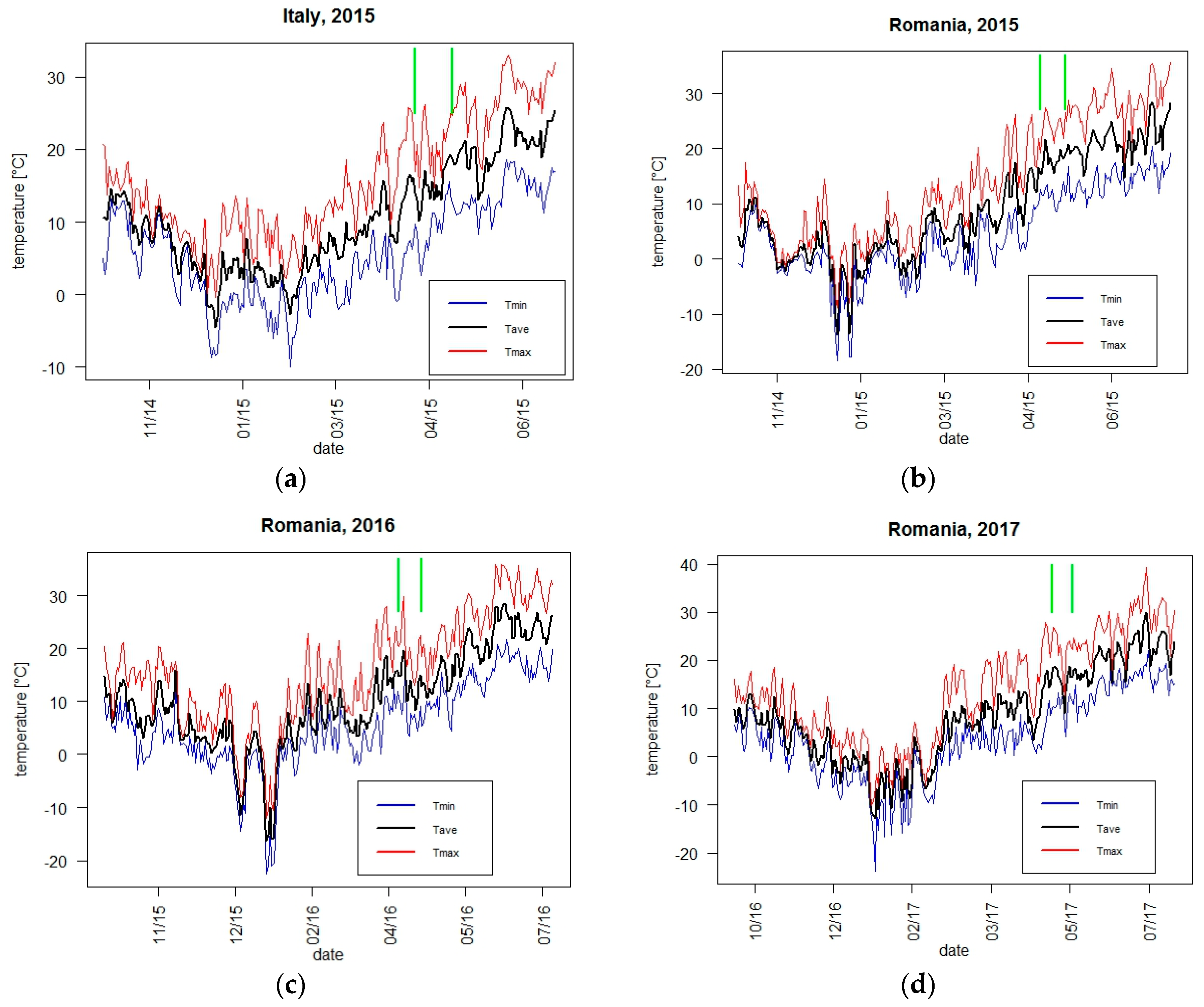
2.2. Meteorological Conditions and Climate Indicators
2.3. Laboratory Experiments
2.3.1. Low Temperature
2.3.2. High Temperature
2.4. Statistical Analyses
2.5. Molecular Marker Analysis
3. Results
3.1. Diversity in Field Trials
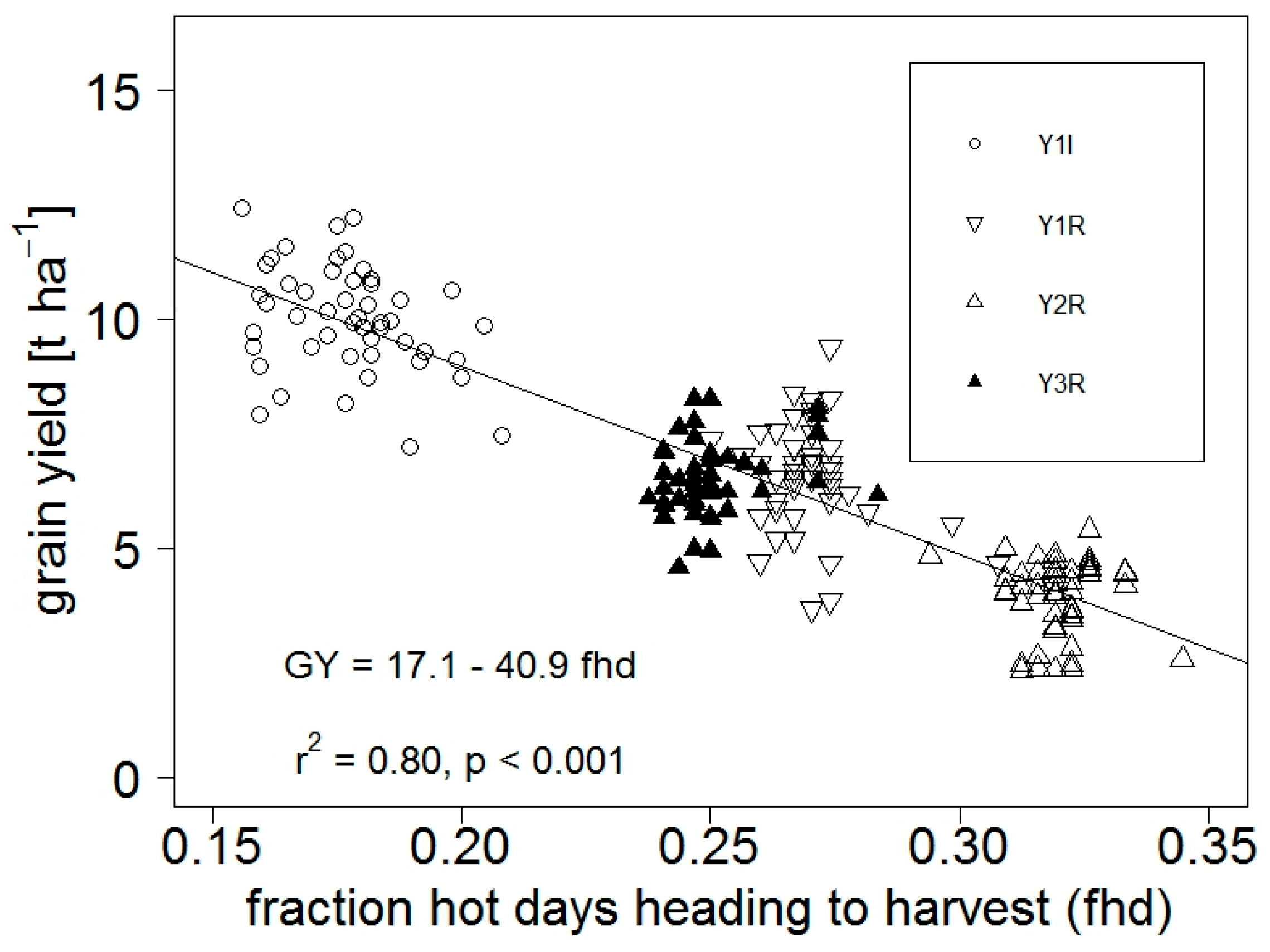
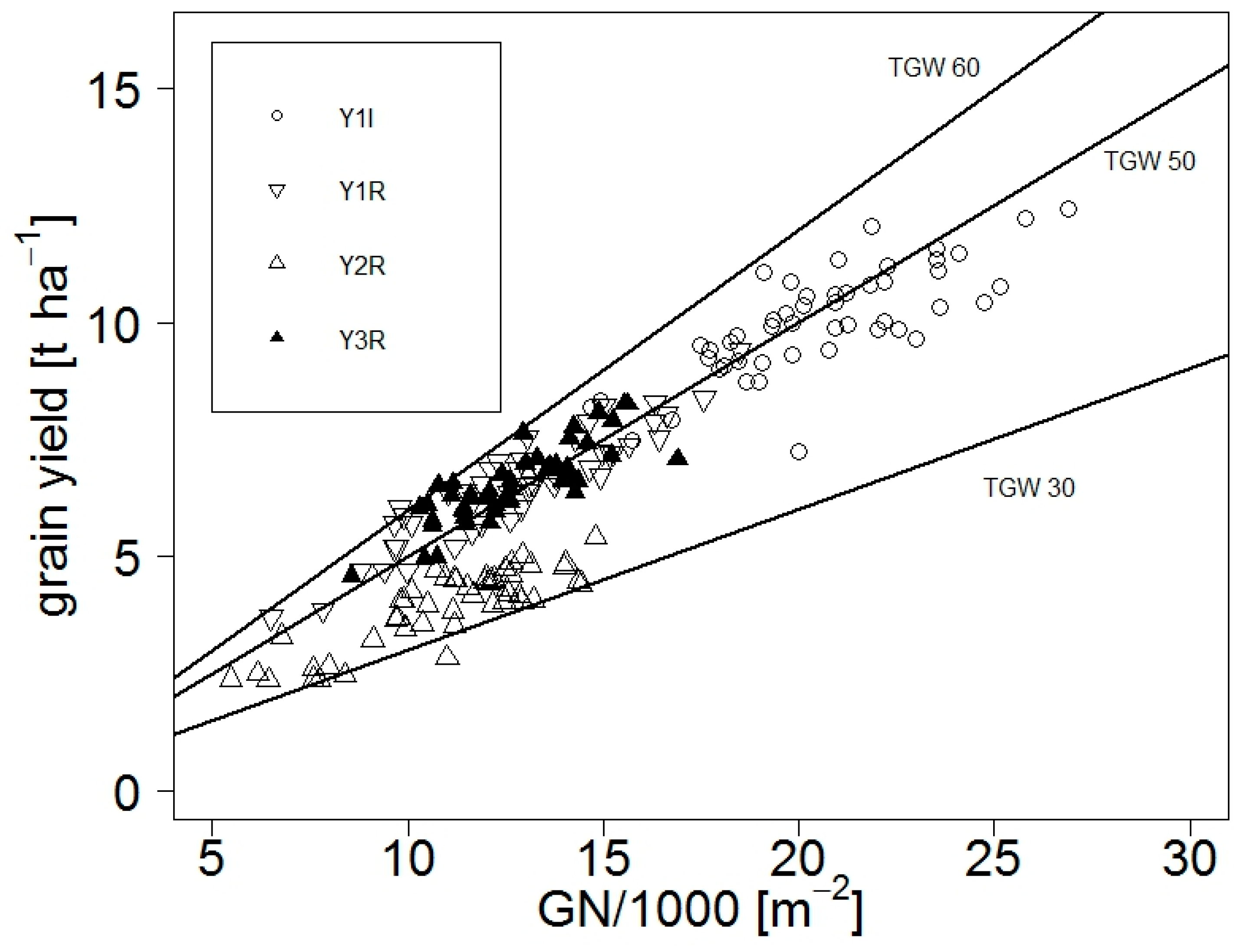
3.1.1. Yield and Adaptive Traits with Respect to Weather Conditions and Climate Indicators
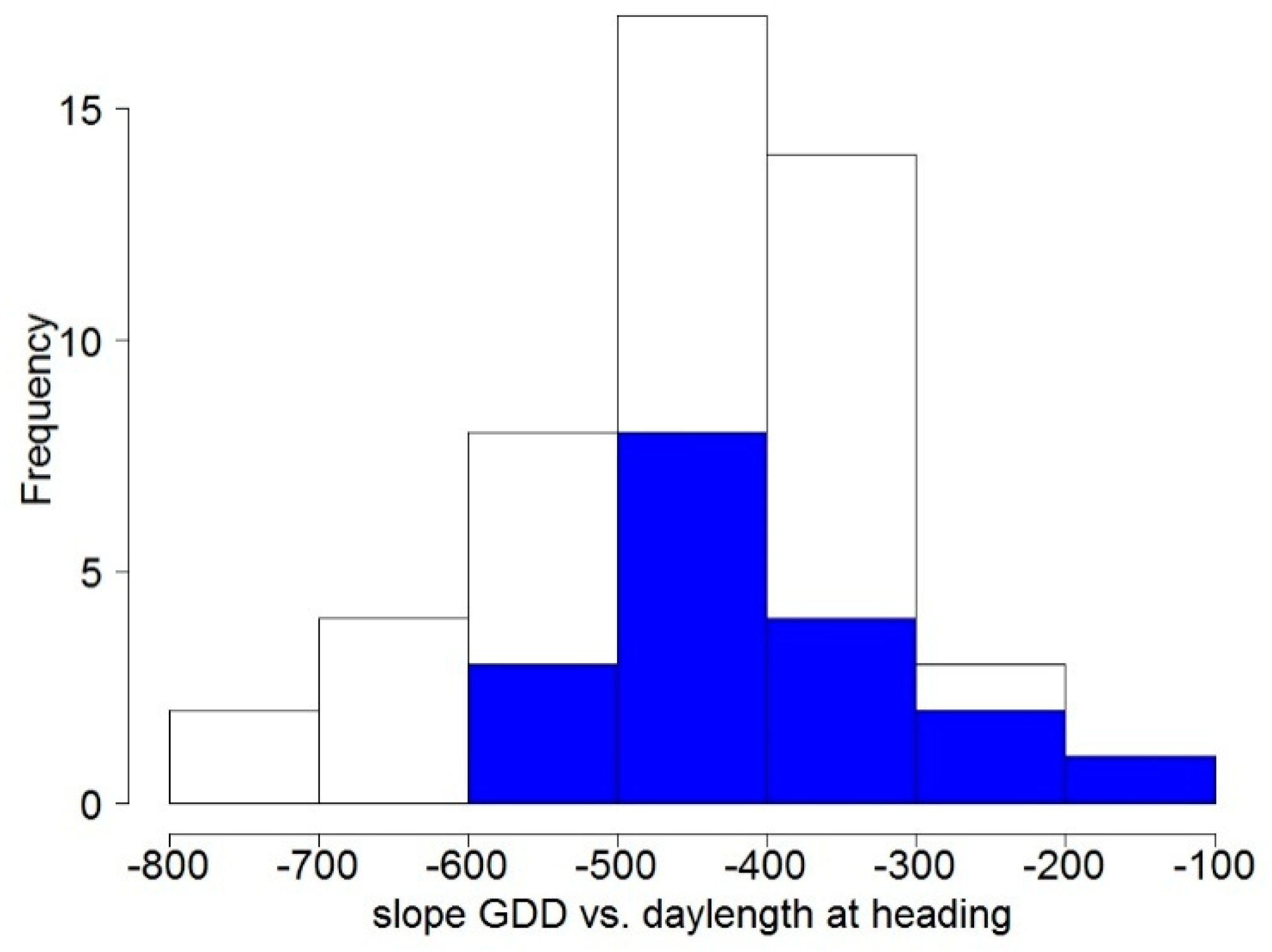
3.1.2. Flowering Phenology
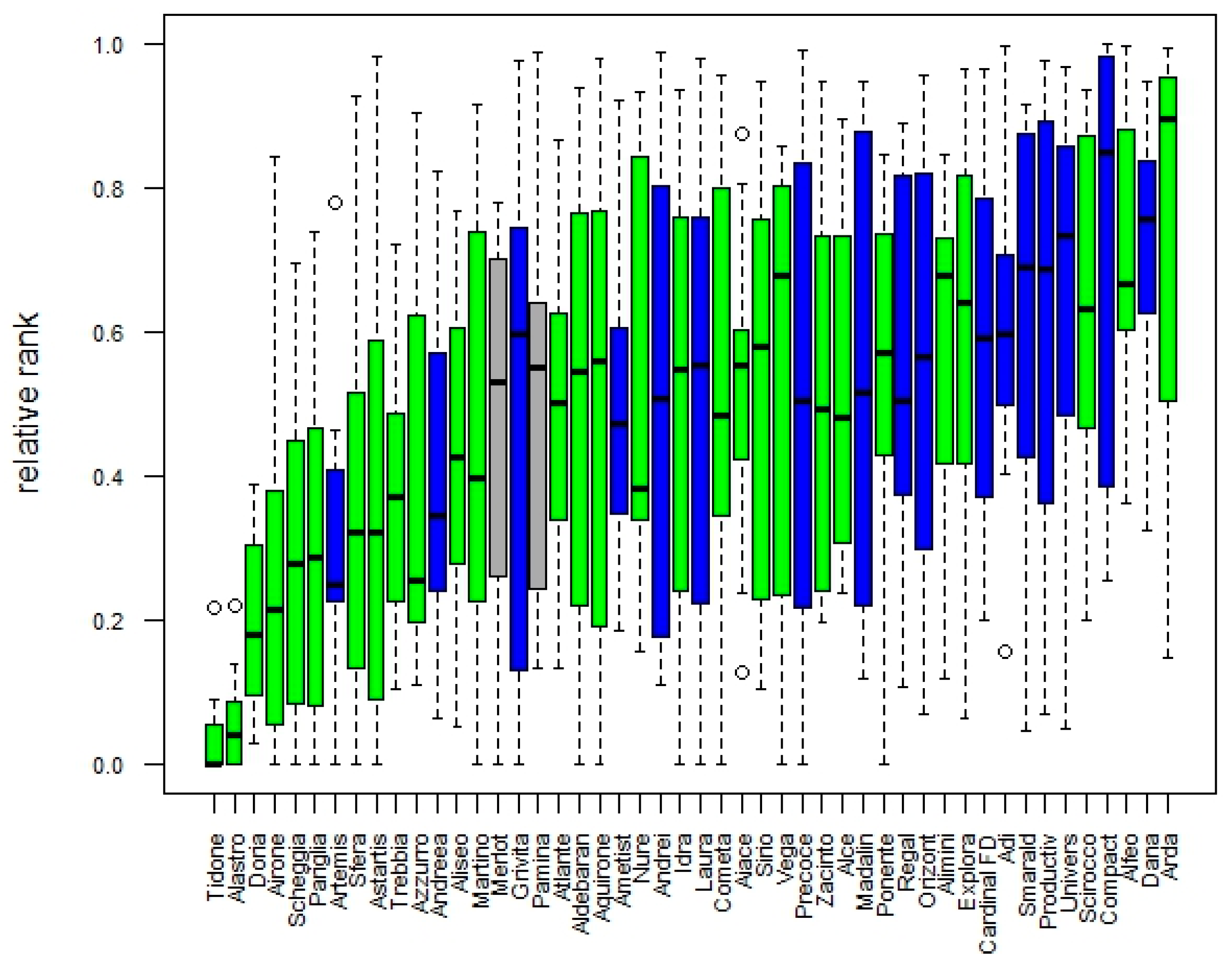
3.1.3. Genetic Gain and Selection Trends
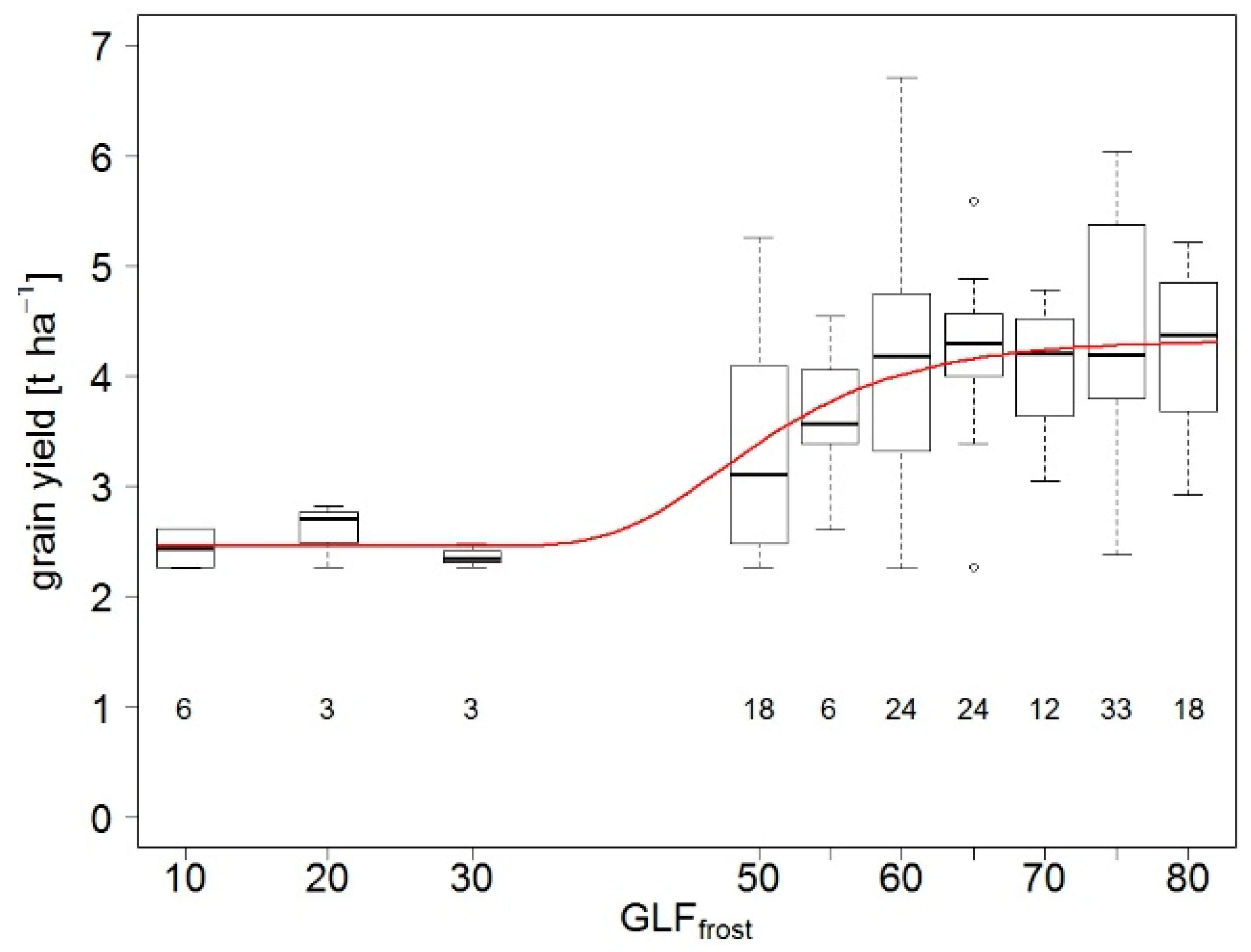
3.1.4. Winter Hardiness/Effects of Low Temperature Stress
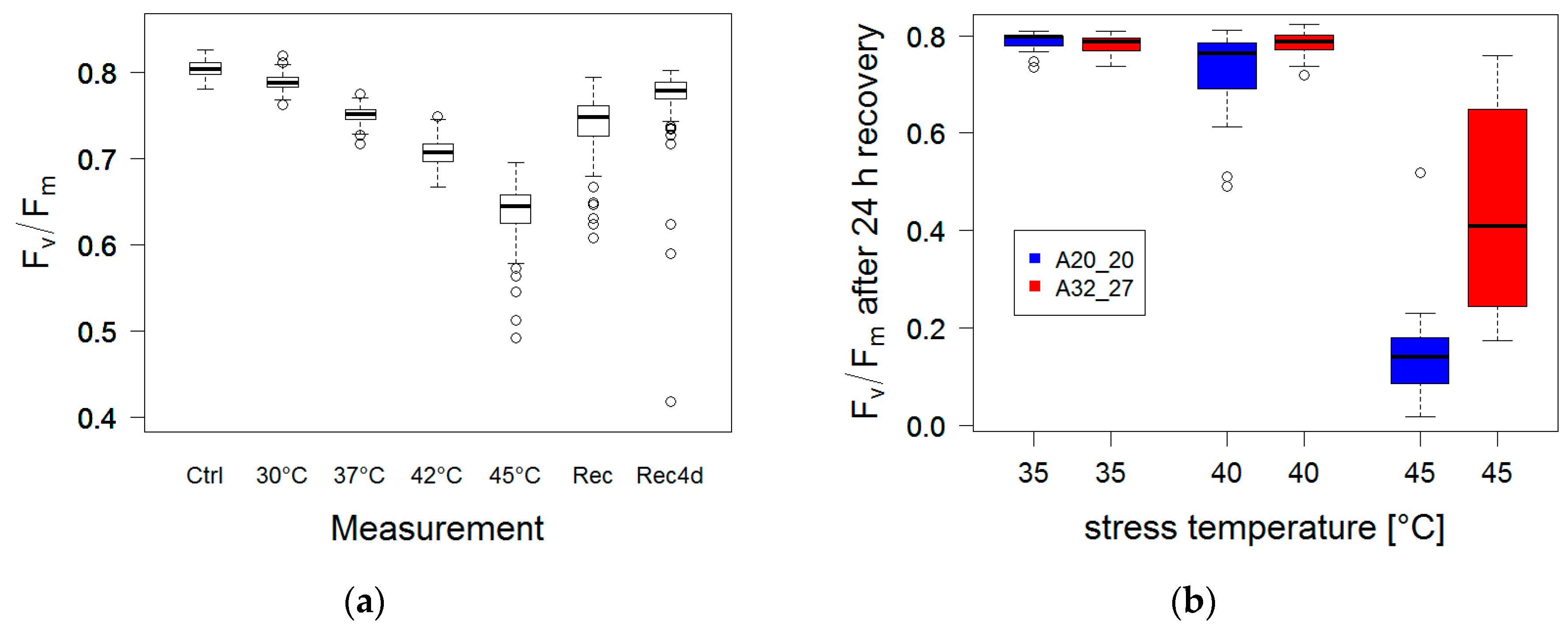
3.1.5. High Temperature Stress
3.2. Diversity in Laboratory Experiments
3.2.1. Low Temperature Stress
3.2.2. High Temperature Stress
3.3. Association between Traits and Alleles of Vernalisation and Photoperiod Genes
4. Discussion
4.1. Stress Frequencies Related to Continentality
4.1.1. Frost
4.1.2. Heat
4.1.3. Other Stresses
4.1.4. Limitations to Grain Yield
4.2. Differences in Agronomical Practice
4.3. Genetic Diversity and Continentality
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Stanca, A.M.; Francia, E.; Tondelli, A.; Badeck, F.W.; Terzi, V. Progress in Small Grain Cereals: A Case Study. In More Food: Road to Survival; Pilu, R., Gavazzi, G., Eds.; Bentham: Sharjah, UAE, 2017; pp. 578–604. [Google Scholar]
- Saulescu, N.N.; Braun, H.J. Cold tolerance. In Application of Physiology in Wheat Breeding; Reynolds, M.P., Ortiz-Monasterio, J.I., McNab, A., Eds.; CIMMYT: Mexico DF, NM, USA, 2001; pp. 111–123. [Google Scholar]
- Dawson, I.K.; Russell, J.; Powell, W.; Steffenson, B.; Thomas, W.T.B.; Waugh, R. Barley: A translational model for adaptation to climate change. New Phytol. 2015, 206, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.H.; Zhang, X.J.; Yang, X.H.; Francis, J.A. Cold winter extremes in northern continents linked to Arctic sea ice loss. Environ. Res. Lett. 2013, 8, 014036. [Google Scholar] [CrossRef]
- Olesen, J.E.; Carter, T.R.; Diaz-Ambrona, C.H.; Fronzek, S.; Heidmann, T.; Hickler, T.; Holt, T.; Minguez, M.I.; Morales, P.; Palutikof, J.P.; et al. Uncertainties in projected impacts of climate change on European agriculture and terrestrial ecosystems based on scenarios from regional climate models. Clim. Chang. 2007, 81, 123–143. [Google Scholar] [CrossRef]
- Rapacz, M.; Jurczyk, B.; Sasal, M. Deacclimation may be crucial for winter survival of cereals under warming climate. Plant Sci. 2017, 256, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Sasani, S.; Hemming, M.N.; Oliver, S.N.; Greenup, A.; Tavakkol-Afshari, R.; Mahfoozi, S.; Poustini, K.; Sharifi, H.-R.; Dennis, E.S.; Peacock, W.J. The influence of vernalization and daylength on expression of flowering-time genes in the shoot apex and leaves of barley (Hordeum vulgare). J. Exp. Bot. 2009, 60, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Karsai, I.; Meszaros, K.; Lang, L.; Hayes, P.M.; Bedo, Z. Multivariate analysis of traits determining adaptation in cultivated barley. Plant Breed. 2001, 120, 217–222. [Google Scholar] [CrossRef]
- Casao, M.C.; Karsai, I.; Igartua, E.; Gracia, M.P.; Veisz, O.; Casas, A.M. Adaptation of barley to mild winters: A role for PPDH2. BMC Plant Biol. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Yasuda, S. Genetics of earliness and growth habit in barley. In Barley Genetics II; Nilan, R.A., Ed.; Washington State University Press: Pullman, WA, USA, 1971; pp. 388–408. [Google Scholar]
- Laurie, D.A.; Pratchett, N.; Bezant, J.H.; Snape, J.W. Genetic analysis of a photoperiod response gene on the short arm of chromosome 2 (2H) of Hordeum vulgare (barley). Heredity 1994, 72, 619–627. [Google Scholar] [CrossRef]
- Laurie, D.A.; Pratchett, N.; Snape, J.W.; Bezant, J.H. RFLP mapping of five major genes and eight quantitative trait loci controlling flowering time in a winter × spring barley (Hordeum vulgare L.) cross. Genome 1995, 38, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.B.; Byrns, B.M.; Greer, K.J. Overwinter low-temperature responses of cereals: Analyses and simulation. Crop Sci. 2014, 54, 2395–2405. [Google Scholar] [CrossRef]
- Cuesta-Marcos, A.; Munoz-Amatriain, M.; Filichkin, T.; Karsai, I.; Trevaskis, B.; Yasuda, S.; Hayes, P.; Sato, K. The relationships between development and low temperature tolerance in barley near isogenic lines differing for flowering behavior. Plant Cell Physiol. 2015, 56, 2312–2324. [Google Scholar] [CrossRef] [PubMed]
- Distelfeld, A.; Li, C.; Dubcovsky, J. Regulation of flowering in temperate cereals. Curr. Opin. Plant Biol. 2009, 12, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Francia, E.; Tondelli, A.; Rizza, F.; Badeck, F.W.; Nicosia, O.L.D.; Akar, T.; Grando, S.; Al-Yassin, A.; Benbelkacem, A.; Thomas, W.T.B.; et al. Determinants of barley grain yield in a wide range of Mediterranean environments. Field Crops Res. 2011, 120, 169–178. [Google Scholar] [CrossRef]
- Rollins, J.A.; Drosse, B.; Mulki, M.A.; Grando, S.; Baum, M.; Singh, M.; Ceccarelli, S.; von Korff, M. Variation at the vernalisation genes Vrn-H1 and Vrn-H2 determines growth and yield stability in barley (Hordeum vulgare) grown under dryland conditions in Syria. Theor. Appl. Genet. 2013, 126, 2803–2824. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, M.; Ceglar, A.; Dentener, F.; Toreti, A. Wheat yield loss attributable to heat waves, drought and water excess at the global, national and subnational scales. Environ. Res. Lett. 2017, 12, 064008. [Google Scholar] [CrossRef]
- Semenov, M.A.; Shewry, P.R. Modelling predicts that heat stress, not drought, will increase vulnerability of wheat in Europe. Sci. Rep. 2011, 1. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.R.; Gawith, M. Temperatures and the growth and development of wheat: A review. Eur. J. Agron. 1999, 10, 23–36. [Google Scholar] [CrossRef]
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Pswarayi, A.; Van Eeuwijk, F.A.; Ceccarelli, S.; Grando, S.; Comadran, J.; Russell, J.R.; Pecchioni, N.; Tondelli, A.; Akar, T.; Al-Yassin, A. Changes in allele frequencies in landraces, old and modern barley cultivars of marker loci close to QTL for grain yield under high and low input conditions. Euphytica 2008, 163, 435–447. [Google Scholar] [CrossRef]
- Jones, H.; Civan, P.; Cockram, J.; Leigh, F.J.; Smith, L.M.J.; Jones, M.K.; Charles, M.P.; Molina-Cano, J.-L.; Powell, W.; Jones, G. Evolutionary history of barley cultivation in Europe revealed by genetic analysis of extant landraces. BMC Evolut. Boil. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- McNally, K.L.; Childs, K.L.; Bohnert, R.; Davidson, R.M.; Zhao, K.; Ulat, V.J.; Zeller, G.; Clark, R.M.; Hoen, D.R.; Bureau, T.E. Genomewide SNP variation reveals relationships among landraces and modern varieties of rice. Proc. Natl. Acad. Sci. USA 2009, 106, 12273–12278. [Google Scholar] [CrossRef] [PubMed]
- Cockram, J.; Horsnell, R.; Soh, E.-H.; Norris, C.; O’Sullivan, D.M. Molecular and phenotypic characterization of the alternative seasonal growth habit and flowering time in barley (Hordeum vulgare ssp. vulgare L.). Mol. Breed. 2015, 35, 165. [Google Scholar] [CrossRef]
- Meyer, R.S.; Purugganan, M.D. Evolution of crop species: Genetics of domestication and diversification. Nat. Rev. Genet. 2013, 14, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, C.R.; Chao, S.; Wang, S.; Huang, B.E.; Stephen, S.; Kiani, S.; Forrest, K.; Saintenac, C.; Brown-Guedira, G.L.; Akhunova, A. Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc. Natl. Acad. Sci. USA 2013, 110, 8057–8062. [Google Scholar] [CrossRef] [PubMed]
- Stroia, I.; Begea, M.; Baron, E.; Musu, S.; Baldea, G.; Vladescu, M.; Begea, P.; Ion, R.A.; Vasilescu, L. Malting behaviour of barley varieties cultivated in Romania for brewing from the harvest 2004—Characteristics of biological material. J. Agroaliment. Process. Technol. 2006, 12, 307–312. [Google Scholar]
- Cattivelli, L.; Faccini, N.; Gianinetti, A.; Alberici, R.; Amato, G.; Anastasi, U.; Attene, G.; Baravelli, M.; Barbiani, G.; Baronchelli, M. Orzo: Buoni risultati produttivi nonostante i mesi siccitosi. L’Inf. Agrar. 2017, 29, 36–41. [Google Scholar]
- Rizza, F.; Karsai, I.; Morcia, C.; Badeck, F.-W.; Terzi, V.; Pagani, D.; Kiss, T.; Stanca, A.M. Association between the allele compositions of major plant developmental genes and frost tolerance in barley (Hordeum vulgare L.) germplasm of different origin. Mol. Breed. 2016, 36. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; Svensgaard, J.; Christensen, S.; Roitsch, T. Plant phenomics and the need for physiological phenotyping across scales to narrow the genotype-to-phenotype knowledge gap. J. Exp. Bot. 2015, 66, 5429–5440. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, R.P.; Oxborough, K.; Pallett, K.E.; Baker, N.R. Rapid, noninvasive screening for perturbations of metabolism and plant growth using chlorophyll fluorescence imaging. Plant Physiol. 2003, 132, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Rizza, F.; Pagani, D.; Stanca, A.M.; Cattivelli, L. Use of chlorophyll fluorescence to evaluate the cold acclimation and freezing tolerance of winter and spring oats. Plant Breed. 2001, 120, 389–396. [Google Scholar] [CrossRef]
- Dai, F.; Zhou, M.; Zhang, G. The change of chlorophyll fluorescence parameters in winter barley during recovery after freezing shock and as affected by cold acclimation and irradiance. Plant Physiol. Biochem. 2007, 45, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Briantais, J.-M.; Dacosta, J.; Goulas, Y.; Ducruet, J.-M.; Moya, I. Heat stress induces in leaves an increase of the minimum level of chlorophyll fluorescence, Fo: A time-resolved analysis. Photosynth. Res. 1996, 48, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Fernandez, J.O.; Rosenqvist, E.; Ottosen, C.-O.; Andersen, S.B. Genotypic response of detached leaves versus intact plants for chlorophyll fluorescence parameters under high temperature stress in wheat. J. Plant Physiol. 2014, 171, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Cajanek, M.; Stroch, M.; Lachetova, I.; Kalina, J.; Spunda, V. Characterization of the photosystem II inactivation of heat-stressed barley leaves as monitored by the various parameters of chlorophyll a fluorescence and delayed fluorescence. J. Photochem. Photobiol. B Biol. 1998, 47, 39–45. [Google Scholar] [CrossRef]
- Woo, N.S.; Badger, M.R.; Pogson, B.J. A rapid, non-invasive procedure for quantitative assessment of drought survival using chlorophyll fluorescence. Plant Methods 2008, 4. [Google Scholar] [CrossRef] [PubMed]
- Rizza, F.; Badeck, F.W.; Cattivelli, L.; Lidestri, O.; Di Fonzo, N.; Stanca, A.M. Use of a water stress index to identify barley genotypes adapted to rainfed and irrigated conditions. Crop Sci. 2004, 44, 2127–2137. [Google Scholar] [CrossRef]
- Rizza, F.; Pagani, D.; Gut, M.; Prasil, I.T.; Lago, C.; Tondelli, A.; Orru, L.; Mazzucotelli, E.; Francia, E.; Badeck, F.W.; et al. Diversity in the Response to Low Temperature in Representative Barley Genotypes Cultivated in Europe. Crop Sci. 2011, 51, 2759–2779. [Google Scholar] [CrossRef]
- R. Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 21 November 2014).
- Nazari, L.; Pattori, E.; Terzi, V.; Morcia, C.; Rossi, V. Influence of temperature on infection, growth, and mycotoxin production by Fusarium langsethiae and F. sporotrichioides in durum wheat. Food Microbial. 2014, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Miflin, B. Crop improvement in the 21st century. J. Exp. Bot. 2000, 51, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Karsai, I.; Szücs, P.; Meszaros, K.; Filichkina, T.; Hayes, P.M.; Skinner, J.S.; Lang, L.; Bedo, Z. The Vrn-H2 locus is a major determinant of flowering time in a facultative winter growth habit barley (Hordeum vulgare L.) mapping population. Theor. Appl. Genet. 2005, 110, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Casao, M.C.; Igartua, E.; Karsai, I.; Lasa, J.M.; Gracia, M.P.; Casas, A.M. Expression analysis of vernalization and day-length response genes in barley (Hordeum vulgare L.) indicates that VRNH2 is a repressor of PPDH2 (HvFT3) under long days. J. Exp. Bot. 2011, 62, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Fisk, S.P.; Cuesta-Marcos, A.; Cistue, L.; Russell, J.; Smith, K.P.; Baenziger, S.; Bedo, Z.; Corey, A.; Filichkin, T.; Karsai, I.; et al. FR-H3: A new QTL to assist in the development of fall-sown barley with superior low temperature tolerance. Theor. Appl. Genet. 2013, 126, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Hemming, M.N.; Fieg, S.; Peacock, W.J.; Dennis, E.S.; Trevaskis, B. Regions associated with repression of the barley (Hordeum vulgare) VERNALIZATION1 gene are not required for cold induction. Mol. Genet. Genom. 2009, 282, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Trevaskis, B. The central role of the VERNALIZATION1 gene in the vernalization response of cereals. Funct. Plant Biol. 2010, 37, 479–487. [Google Scholar] [CrossRef]
- Prasil, I.T.; Prasilova, P.; Marik, P. Comparative study of direct and indirect evaluations of frost tolerance in barley. Field Crop. Res. 2007, 102, 1–8. [Google Scholar] [CrossRef]
- Garcia, G.A.; Dreccer, M.F.; Miralles, D.J.; Serrago, R.N.A. High night temperatures during grain number determination reduce wheat and barley grain yield: A field study. Glob. Chang. Biol. 2015, 21, 4153–4164. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.A.; Serrago, R.N.A.; Dreccer, M.F.; Miralles, D.J. Post-anthesis warm nights reduce grain weight in field-grown wheat and barley. Field Crop. Res. 2016, 195, 50–59. [Google Scholar] [CrossRef]
- Ugarte, C.; Calderini, D.F.; Slafer, G.A. Grain weight and grain number responsiveness to pre-anthesis temperature in wheat, barley and triticale. Field Crop. Res. 2007, 100, 240–248. [Google Scholar] [CrossRef]
- Cossani, C.M.; Slafer, G.A.; Savin, R. Yield and biomass in wheat and barley under a range of conditions in a Mediterranean site. Field Crop. Res. 2009, 112, 205–213. [Google Scholar] [CrossRef]
- Alzueta, I.; Abeledo, L.G.; Mignone, C.M.; Miralles, D.J. Differences between wheat and barley in leaf and tillering coordination under contrasting nitrogen and sulfur conditions. Eur. J. Agron. 2012, 41, 92–102. [Google Scholar] [CrossRef]
- Baethgen, W.E.; Christianson, C.B.; Lamothe, A.G. Nitrogen fertilizer effects on growth, grain yield, and yield components of malting barley. Field Crop. Res. 1995, 43, 87–99. [Google Scholar] [CrossRef]
- Sieling, K.; Schröder, H.; Finck, M.; Hanus, H. Yield, N uptake, and apparent N-use efficiency of winter wheat and winter barley grown in different cropping systems. J. Agric. Sci. 1998, 131, 375–387. [Google Scholar] [CrossRef]
- Delogu, G.; Cattivelli, L.; Pecchioni, N.; Defalcis, D.; Maggiore, T.; Stanca, A.M. Uptake and Agronomic Efficiency of Nitrogen in Winter Barley and Winter Wheat. Eur. J. Agron. 1998, 9, 11–20. [Google Scholar] [CrossRef]
- Laslo, L.; Ciobotaru, N.; Lupei, T.; Matei, M.; Velcea, A.M.; Boboc, M.; Badea, G.; Gyorgy, D. Drought and Irrigations of Romanian Agricultural Areas. RevCAD J. Geod. Cadastre 2017, 23, 117–124. [Google Scholar]


| Treatment | Y1I | Y1R | Y2R | Y3R |
|---|---|---|---|---|
| Site | Fiorenzuola | Fundulea | Fundulea | Fundulea |
| Agronomic year | 2014/2015 | 2014/2015 | 2015/2016 | 2016/2017 |
| Sowing date | 3 November 2014 | 5 November 2014 | 9 October 2015 | 15 October 2016 |
| Harvest date | 2 July 2015 | 20 July 2015 | 25 July 2016 | 20 July 2017 |
| Nitrogen, pre-sowing | 45 kg N ha−1 | none | none | none |
| Nitrogen, topdressing | 52 kg N ha−1 | 100 kg urea (47 kg N ha−1) applied in March | 100 kg urea (47 kg N ha−1) applied in March | 100 kg urea (47 kg N ha−1) applied in March |
| herbicide | Axial pronto + Trimmer SX | tribenuron metil + fluroxipir-meptil | tribenuron metil + fluroxipir-meptil | tribenuron metil + fluroxipir-meptil |
| fungicide | none | none | none | none |
| insecticide | none | none | none | none |
| Trait | GY (t ha−1) | GN (m−2) | TGW (g) | HD (DAS) | GPC (%) |
|---|---|---|---|---|---|
| Cultivar | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Environment | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| C × E | p < 0.001 | p < 0.01 | p < 0.001 | p < 0.001 | p < 0.001 |
| Mean Y1I | 10.02 a | 20525 a | 49.2 b | 174 d | 11.28 c |
| Mean Y1R | 6.53 b | 12803 b | 51.4 a | 184 c | 13.93 b |
| Mean Y2R | 3.94 c | 10990 c | 36.1 c | 197 b | 14.41 a |
| Mean Y3R | 6.53 b | 12732 b | 51.5 a | 202 a | 11.08 d |
| Trait | Fv/Fm Recovery | Frost Survival Y1I |
|---|---|---|
| Fv/Fm recovery | ||
| Frost survival Y1I | 0.324 (0.023) | |
| Frost survival Y2R | 0.496 (<0.001) | −0.004 (0.975) |
| Trait | F (2) | W(44) | S (3) | p | ppd-H2 (38) | Ppd-H2 (11) | p |
|---|---|---|---|---|---|---|---|
| Frost survival Y1I | 7.67 a | 6.71 b | 5.89 c | <0.02 | 6.68 a | 6.78 a | ns |
| GLF Y2R | 47.5 ab | 65.6 a | 28.3 b | <0.001 | 66.3 | 49.6 | <0.003 |
| Fv/Fm | 0.54 | 0.62 | 0.21 | <0.001 | 0.61 | 0.51 | <0.02 |
| Trial | ppd-H2 (38) | Ppd-H2 (11) | p | Trial | ppd-H2 (38) | Ppd-H2 (11) | p |
|---|---|---|---|---|---|---|---|
| Heading Y1I | 175.8 a | 167.4 b | p < 0.001 | GY Y1I | 10.28 a | 9.95 a | p = 0.413 |
| Heading Y1R | 183.8 a | 183.4 a | p = 0.630 | GY Y1R | 6.81 a | 5.57 b | p < 0.002 |
| Heading Y2R | 197.1 a | 196.8 a | p = 0.726 | GY Y2R | 4.17 a | 3.15 b | p < 0.001 |
| Heading Y3R | 203.4 a | 201.3 b | p = 0.021 | GY Y3R | 6.62 a | 6.24 a | p = 0.177 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizza, F.; Vasilescu, L.; Badeck, F.-W.; Morcia, C.; Alberici, R.; Bude, A.; Alionte, E.; Petcu, E.; Baronchelli, M.; Faccini, N.; et al. Agrobiodiversity for Adaptive and Yield Traits in Romanian and Italian Barley Cultivars across Four Continental Environments. Agronomy 2018, 8, 79. https://doi.org/10.3390/agronomy8060079
Rizza F, Vasilescu L, Badeck F-W, Morcia C, Alberici R, Bude A, Alionte E, Petcu E, Baronchelli M, Faccini N, et al. Agrobiodiversity for Adaptive and Yield Traits in Romanian and Italian Barley Cultivars across Four Continental Environments. Agronomy. 2018; 8(6):79. https://doi.org/10.3390/agronomy8060079
Chicago/Turabian StyleRizza, Fulvia, Liliana Vasilescu, Franz-W. Badeck, Caterina Morcia, Renzo Alberici, Alexandru Bude, Eliana Alionte, Eugen Petcu, Marina Baronchelli, Nadia Faccini, and et al. 2018. "Agrobiodiversity for Adaptive and Yield Traits in Romanian and Italian Barley Cultivars across Four Continental Environments" Agronomy 8, no. 6: 79. https://doi.org/10.3390/agronomy8060079
APA StyleRizza, F., Vasilescu, L., Badeck, F.-W., Morcia, C., Alberici, R., Bude, A., Alionte, E., Petcu, E., Baronchelli, M., Faccini, N., Pagani, D., & Terzi, V. (2018). Agrobiodiversity for Adaptive and Yield Traits in Romanian and Italian Barley Cultivars across Four Continental Environments. Agronomy, 8(6), 79. https://doi.org/10.3390/agronomy8060079






