Physiological Response of Wheat to Chemical Desiccants Used to Simulate Post-Anthesis Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments
2.2. Physiological Parameters
2.3. Agronomic Traits
2.4. Statistical Analysis
3. Results
3.1. Response of ‘Norin 61’ to Control, Desiccant, and Drought Conditions
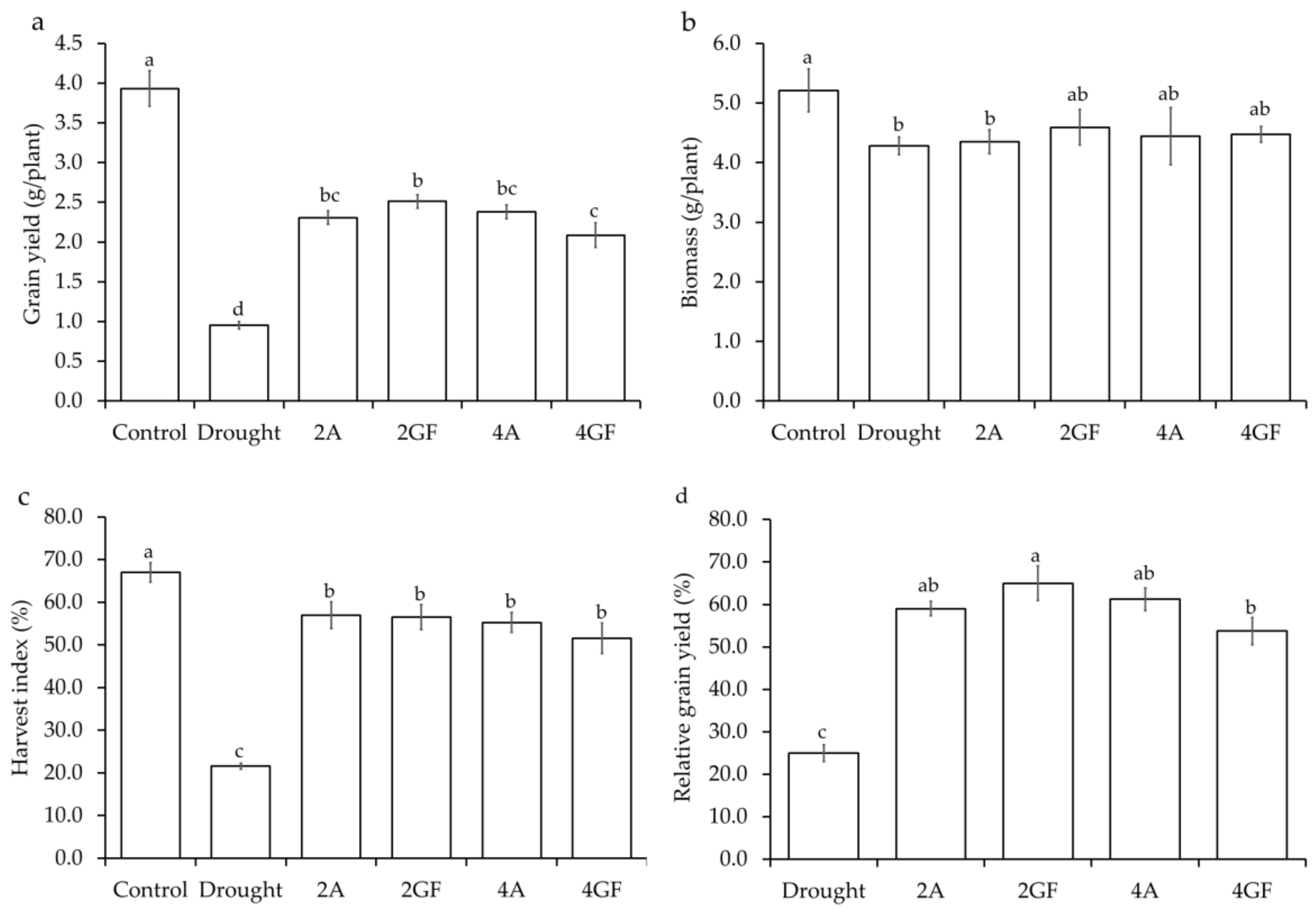
3.2. Effects of Drought and Desiccant on Grain Yield, Biomass, and Harvest Index
3.3. Chlorophyll Content and Stomatal Conductance
3.4. Carbon Isotope Composition (δ13C)
3.5. Correlation between Traits
4. Discussion
4.1. Use of Chemical Desiccants to Simulate Drought
4.2. Effects of Desiccation and Drought on Stomatal Conductance, Chlorophyll Content, and δ13C
4.2.1. Effect on Stomatal Conductance and δ13C
4.2.2. Effect on Chlorophyll Content
4.3. Effect of Desiccation on Aboveground Fresh Biomass, Harvest Index, and Grain Yield
4.3.1. Analysis of Yield Reduction under Desiccation Treatments
4.3.2. Biomass and Harvest Index
4.4. Association between Chemical Desiccant and Drought Stress in Effects on Yield Traits
4.5. Relationship between Response to Chemical Desiccation and Drought Resistance
4.6. Relationship between δ13C and Yield Characters
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Learnmore, M.; Hussein, S.; Ernest, D.; Mark, D.; Toi, J. Breeding wheat for drought tolerance: Progress and technologies. J. Integr. Agric. 2016, 15, 935–943. [Google Scholar] [CrossRef]
- Cuculeanu, V.M.A.; Simota, C. Climate change impact on agricultural crops and adaptation options in Romania. Clim. Res. 1999, 12, 153–160. [Google Scholar] [CrossRef]
- Zhu, J.K.; Hasegawa, P.M.; Bressan, R.A. Molecular aspects of osmotic stress in plants. Crit. Rev. Plant Sci. 1997, 16, 253–277. [Google Scholar] [CrossRef]
- Hossain, A.B.S.; Sears, P.G.; Cox, J.S.; Paulesen, G.M. Desiccation tolerance and its relationship to assimilate partitioning in winter wheat. Crop Sci. 1990, 30, 622–627. [Google Scholar] [CrossRef]
- Nicolas, M.E.; Turner, N.C. Use of chemical desiccants and senescing agent to select wheat lines maintaining lines stable grain size during post-anthesis drought. Field Crop Res. 1993, 31, 155–171. [Google Scholar] [CrossRef]
- Budakli, E.; Celik, N.; Turk, M.; Bayram, G.; Tas, B. Effect of Post-Anthesis Drought Stress on the Stem-Reserve Mobilization Supporting Grain Filling of Two-Rowed Barley Cultivars at Different Levels of Nitrogen. J. Biol. Sci. 2007, 7, 949–953. [Google Scholar]
- Richards, R.A.; Rebetzke, G.J.; Condon, A.G.; van Herwaarden, A.F. Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Sci. 2002, 42, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Poiarkova, H.; Golan, G.; Mayer, J. Chemical desiccation of wheat plants as a simulator of post-anthesis stress. I. Effect on translocation on kernel growth. Field Crop. Res. 1983, 6, 51–58. [Google Scholar] [CrossRef]
- Blum, A.; Mayer, J.; Golan, G. Chemical desiccation of wheat plants as a simulator of post-anthesis stress. II. Relations to stage of kernel growth. While chemical desiccation destroys the drought stress. Field Crop. Res. 1983, 6, 149–155. [Google Scholar] [CrossRef]
- Flexas, J.; Miquel, R.C.; Antonio, D.E.; Jeroni, G.; Hipolito, M. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef] [PubMed]
- Inaki Iturbe-Ormaetxe, I.; Escuredo, P.R.; Arrese-Igor, C.; Becana, M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998, 116, 173–181. [Google Scholar] [CrossRef]
- Smirnoff, N. Antioxidant systems and plant response to the environment. In Environment and Plant Metabolism: Flexibility and Acclimation; Smirnoff, N., Ed.; Bios Scientific Publishers: Oxford, UK, 1995; pp. 217–243. [Google Scholar]
- Zobayed, S.; Afreen, F.; Kozai, T. Temperature stress can alter the photosynthetic efficiency and secondary metabolite concentrations in St. John’s Wort. Plant Physiol. Biochem. 2005, 43, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, K.; Seyfit, T. Biomass as a Selection Criterion for Drought Tolerance in Wheat. Rom. Biotechnol. Lett. 2016, 21, 11505–11512. [Google Scholar]
- Edmeades, G.O.; Lafitte, H.R.; Bolanos, J.; Chapman, S.C.; Banziger, M.; Deutsch, J.A. Developing maize that tolerates drought or low nitrogen conditions. In Stress Tolerance Breeding: Maize that Resists Insects, Drought, Low Nitrogen, and Acid Soils; Edmeades, G.O., Deutsch, J.A., Eds.; CIMMYT: Mexico City, Mexico, 1994; pp. 21–84. [Google Scholar]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Boyer, J.S. Advances in drought tolerance in plants. Adv. Agron. 1996, 56, 187–218. [Google Scholar]
- Plaut, Z.; Butow, B.J.; Blumenthal, C.S.; Wrigley, C.W. Transport of dry matter into developing wheat kernels and its contribution to grain yield under post-anthesis water deficit and elevated temperature. Field Crop. Res. 2004, 86, 185–198. [Google Scholar] [CrossRef]
- Turner, N.; Begg, J. Plant water relations and adaptation to stress. Plant Soil 1981, 58, 97–131. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Richards, R.A. Isotope Composition of Plant Carbon Correlates with Water-Use Efficiency of Wheat Genotypes. Aust. J. Plant Physiol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Shailesh, K. Carbon-13 Discrimination (Delta) and Its Relationship with Water Use Efficiency (WUE) in Wheat. Master’s Thesis, Indian Agricultural Research Institute, New Delhi, India, 2004. [Google Scholar]
- Patrick, O.O.; Jeffrey, J.V.; Ejeta, G. Selection for drought tolerance in sorghum using desiccants to simulate post-anthesis drought stress. Field Crop. Res. 2016, 198, 1–322. [Google Scholar] [CrossRef]
- Dogan, R.; Kacar, O.; Budakli, C.; Goksu, E. Effects of drought stress post-anthesis stage on mobilization of stem-reserves supporting grain filling of some triticale cultivar and lines. Bulg. J. Agric. Sci. 2012, 18, 325–329. [Google Scholar]
- Herrett, R.A.; Hatfield, H.H.; Crosby, D.G.; Vlitos, A.J. Leaf abscission induced by the iodide ion. Plant Physiol. 1962, 37, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Veena, S.; Singh, D.P. Effect of chemical desiccation at the post-anthesis stage on some physiological and biochemical changes in the flag leaf of contrasting wheat genotypes. Field Crop. Res. 2002, 77, 1–6. [Google Scholar] [CrossRef]
- Jiemei, L.; Ruitao, Y.; Huicong, W.; Xuming, H. Stress Effects of Chlorate on Longan (Dimocarpus longan Lour.) Trees: Changes in Nitrogen and Carbon Nutrition. Hortic. Plant J. 2017, 3, 237–246. [Google Scholar] [CrossRef]
- Haley, S.D.; Quick, J.S. Early generation selection for chemical desiccation tolerance in winter wheat. Crop Sci. 1993, 33, 1217–1223. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubic, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Phys. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Strtontip, C.; Tiyayon, P. Influence of water regimes and potassium chlorate on floral induction, leaf photosynthesis, and leaf water potential in Longan. J. Agric. Sci. 2013, 5, 211–220. [Google Scholar] [CrossRef]
- Ludlow, M.; Muchow, R.C. A critical evaluation of traits for improving crop yields in water limited environments. Adv. Agron. 1990, 43, 107–153. [Google Scholar]
- Nicolas, T. Plant water relation and irrigation management. Agric. Water Manag. 1990, 17, 59–93. [Google Scholar]
- Hoda, H.S.; Singh, D.O.; Pannu, R.K. Effect of environments and Potassium Iodine desiccant spray on remobilization of assimilates and yield attributes of wheat genotypes Haryana. J. Agron. 1996, 12, 125–131. [Google Scholar]
- Sritontip, C.; Khaosumain, Y.; Changjaraja, S.; Poruksa, R. Effects of potassium chlorate, potassium nitrate, sodium hypochlorite and thiourea on off-season flowering and photosynthesis of ‘Do’ longan. Acta Hortic. 2005, 665, 291–296. [Google Scholar] [CrossRef]
- Morgan, J.M. Osmoregulation as a criterion for drought tolerance in wheat Aust. J. Agric. Res. 1984, 34, 607–614. [Google Scholar] [CrossRef]
- Lin, T.; Wolf, S.; Schwartz, A.; Saranga, Y. Silver leaf whitefly stress impairs sugar export from cotton source leaves. Phsyiol. Plant 2000, 109, 291–297. [Google Scholar] [CrossRef]
- Sanchez-Bragado, R.; Molero, G.; Reynolds, M.P.; Araus, J.L. Relative contribution of shoot and ear photosynthesis to grain filling in wheat under good agronomical conditions assessed by differential organ δ13C. J. Exp. Bot. 2014, 65, 5401–5413. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the relationship between carbon isotope discrimination and intercellular carbon dioxide concentration in leaves. Aust. J. Plant Physiol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Foulkes, M.J.; Desilva, J.; Gaju, P.; Carvalho, P. Relationship between δ13C, δ18O and grain yield variation in bread wheat genotypes under favourable irrigated and rain fed conditions. Field Crops Res. 2016, 196, 237–250. [Google Scholar] [CrossRef]
- Kano, M.; Inukai, Y.; Kitano, H.; Yamauchi, A. Root Plasticity as the Key Root Trait for Adaptation to Various Intensities of Drought Stress in Rice. Plant Soil 2011, 342, 117–128. [Google Scholar] [CrossRef]
- Nakata, M.K.; Tatsum, J.; Inukai, Y.; Asanuma, S.; Yamauchi, A. Effect of Various Intensities of Drought Stress on δ13C Variation among Plant Organs in Rice: Comparison of Two Cultivars. Am. J. Plant Sci. 2014, 5, 1686–1693. [Google Scholar] [CrossRef][Green Version]
- Xu, X.; Yuan, H.; Li, S.; Trethowan, R.; Monneveux, P. Relationship between Carbon Isotope Discrimination and Grain Yield in Spring Wheat Cultivated under Different Water Regimes. J. Integr. Plant Biol. 2007, 49, 1497–1507. [Google Scholar] [CrossRef]
- Zhu, L.; Li, S.H.; Liang, Z.S.; Xu, X.; Li, Y. Relationship between Carbon Isotope Discrimination, Mineral Content and Gas Exchange Parameters in Vegetative Organs of Wheat Grown under Three Different Water Regimes. J. Agron. Crop Sci. 2009, 196, 175–184. [Google Scholar] [CrossRef]
- Kondo, M.; Pablico, P.P.; Aragones, D.V.; Agbisit, R. Genotypic variations in carbon isotope discrimination, transpiration efficiency, and biomass production in rice as affected by soil water conditions and N. Plant Soil 2004, 267, 165–177. [Google Scholar] [CrossRef]
- Fahimeh, S.; Julien, L.R.; Benjamin, L.; Beata, S.; Priyanka, K.; Saba, M.; Joanne, T.; Delphine, F. Genetic association of stomatal traits and yield in wheat grown in low rainfall environments. BMC Plant Biol. 2016, 16, 150. [Google Scholar] [CrossRef]
- Nyachiro, J.M.; Briggs, K.G.; Hoddinott, J.; Johnson-Flanagan, A.M. Chlorophyll content, chlorophyll fluorescence and water deficit in spring wheat. Cereal Res. Commun. 2001, 29, 135–142. [Google Scholar]
- Kpyoarissis, A.; Petropoulou, Y.; Manetas, Y. Summer survival of leaves in a soft-leaved shrub (Phlomis fruticosa L., Labiatae) under Mediterranean field conditions: Avoidance of photoinhibitory damage through decreased chlorophyll contents. J. Exp. Bot. 1995, 46, 1825–1831. [Google Scholar] [CrossRef]
- Ommen, O.E.; Donnelly, A.; Vanhoutvin, S.; van Oijen, M.; Manderscheid, R. Chlorophyll content of spring wheat flag leaves grown under elevated CO2 Concentrations and other environmental stresses within the ESPACE-wheat Project. Eur. J. Agron. 1999, 10, 197–203. [Google Scholar] [CrossRef]
- Palta, J.A.; Kobata, T.; Turner, N.C.; Fillery, I.R. Remobilization of carbon and nitrogen in wheat as induced by pos-tanthesis water deficits. Crop Sci. 1994, 34, 118–124. [Google Scholar] [CrossRef]
- Nicolas, M.E.; Gleadow, R.M.; Dalling, M.J. Effects of drought and high temperature on grain growth in wheat. J. Plant Physiol. 1984, 11, 553–566. [Google Scholar] [CrossRef]
- Nicolas, M.E.; Gleadow, R.M.; Dalling, M.J. Effects of post-anthesis drought on cell division and starch accumulation in developing wheat grains. Ann. Bot. (Lond.) 1985, 55, 433–444. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high wateruse efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Dodig, D.; Zoriæ, M.; Kandiæ, V.; Peroviæ, D.; Šurlan-Momiroviæ, G. Comparison of responses to drought stress of 100 wheat accessions and landraces to identify opportunities for improving wheat drought resistance. Plant Breed. 2012, 131, 369–379. [Google Scholar] [CrossRef]
- Sareen, S.; Bhudev, S.T.; Ashok, K.S.; Vinod, T.; Indu, S.S. Trait analysis, diversity, and genotype × environment interaction in some wheat landraces evaluated under drought and heat stress conditions. Chilean J. Agric. Res. 2014, 74. [Google Scholar] [CrossRef]
- Monneveux, P.; Rekika, D.; Acevedo, E.; Merah, O. Effect of drought on leaf gas exchange, carbon isotope discrimination, transpiration effcincy and productivity in field grown durum wheat genotypes. Plant Sci. 2005. [Google Scholar] [CrossRef]
- Merah, O.; Deleens, E.; Teulat, P.; Monneveux, P. Grain yield, carbon Isotope discrimination, dry matter production and harvest index in durum wheat. J. Plant Physiol. 2001, 158, 723–729. [Google Scholar] [CrossRef]
- Merah, O.; Deleens, E.; Teulat, B.; Monneveux, P. Association between yield and carbon Isotope discrimination value in different organs of durum wheat under drought. J. Agron. Crop Sci. 2002, 1888, 426–434. [Google Scholar] [CrossRef]
- Cabuslay, G.S.; Ito, A.A. Physiological evaluation of responses of rice (Oryza sativa L.). To water deficit. Plant Sci. 2002, 163, 815–827. [Google Scholar] [CrossRef]
- Saranga, Y.; Flash, A.; Paterson, H.; Yakir, D. Carbon isotope ratio in cotton varieties with growth stage and plant organ. Plant Sci. 2002, 142, 47–56. [Google Scholar] [CrossRef]
- Condon, A.G.; Richard, R.A. Exploring genetic variation in transpiration efficiency in wheat: An agronomic view. In Stable Isotope and Plant Carbon-Water Relations; Ehleringer, J.R., Hall, A.E., Farquhar, G.D., Eds.; Academic Press: New York, NY, USA, 1993; pp. 435–450. [Google Scholar]
- Leidi, O.; Lopez, E.; Gorham, J.; Gutierrez, C. Variation in carbon isotope discrimination and other traits related to drought tolerance in upland cotton cultivars under dry land conditions. Field Crop. Res. 1999, 61, 109–123. [Google Scholar] [CrossRef]
- Acevedo, E. Potential of Carbon isotope discrimination as a selection criterion in barley breeding. In Stable Isotopes and Plant Carbon Water Relations; Ehleringer, J.R., Hall, A.E., Farquhar, G.D., Eds.; Academic Press: New York, NY, USA, 1993; pp. 399–417. [Google Scholar]
- Ehdaie, B.; Waines, G. Variation in water-use efficiency and its components in wheat. I. Well-watered pot experiment. Crop Sci. 1993, 33, 294–299. [Google Scholar] [CrossRef]
- Merah, O.; Deléens, E.; Monneveux, P. Grain yield, carbon isotope discrimination, mineral and silicon content in durum wheat under different precipitation regimes. Physiol. Plant. 1999, 107, 387–394. [Google Scholar] [CrossRef]


| Traits | SE± | P | l.s.d. |
|---|---|---|---|
| Grain yield | 0.14 | <0.0001 | 0.30 |
| Relative grain yield | 2.59 | <0.0001 | 7.58 |
| Aboveground fresh biomass | 0.12 | <0.0001 | 0.78 |
| Harvest index | 2.80 | <0.0001 | 10.64 |
| Chlorophyll content (SPAD unit) | 1.72 | <0.0001 | 3.33 |
| Stomatal conductance (mmol m−2 s−1) | 6.14 | <0.0001 | 14.52 |
| Carbon isotope composition δ13C | 0.07 | 0.004 | 0.47 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, N.M.; Gorafi, Y.S.A.; Mega, R.; Tsujimoto, H. Physiological Response of Wheat to Chemical Desiccants Used to Simulate Post-Anthesis Drought Stress. Agronomy 2018, 8, 44. https://doi.org/10.3390/agronomy8040044
Kamal NM, Gorafi YSA, Mega R, Tsujimoto H. Physiological Response of Wheat to Chemical Desiccants Used to Simulate Post-Anthesis Drought Stress. Agronomy. 2018; 8(4):44. https://doi.org/10.3390/agronomy8040044
Chicago/Turabian StyleKamal, Nasrein Mohamed, Yasir Serag Alnor Gorafi, Ryosuke Mega, and Hisashi Tsujimoto. 2018. "Physiological Response of Wheat to Chemical Desiccants Used to Simulate Post-Anthesis Drought Stress" Agronomy 8, no. 4: 44. https://doi.org/10.3390/agronomy8040044
APA StyleKamal, N. M., Gorafi, Y. S. A., Mega, R., & Tsujimoto, H. (2018). Physiological Response of Wheat to Chemical Desiccants Used to Simulate Post-Anthesis Drought Stress. Agronomy, 8(4), 44. https://doi.org/10.3390/agronomy8040044





