Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses
Abstract
1. Introduction
2. Biological Functions of Potassium in Plants
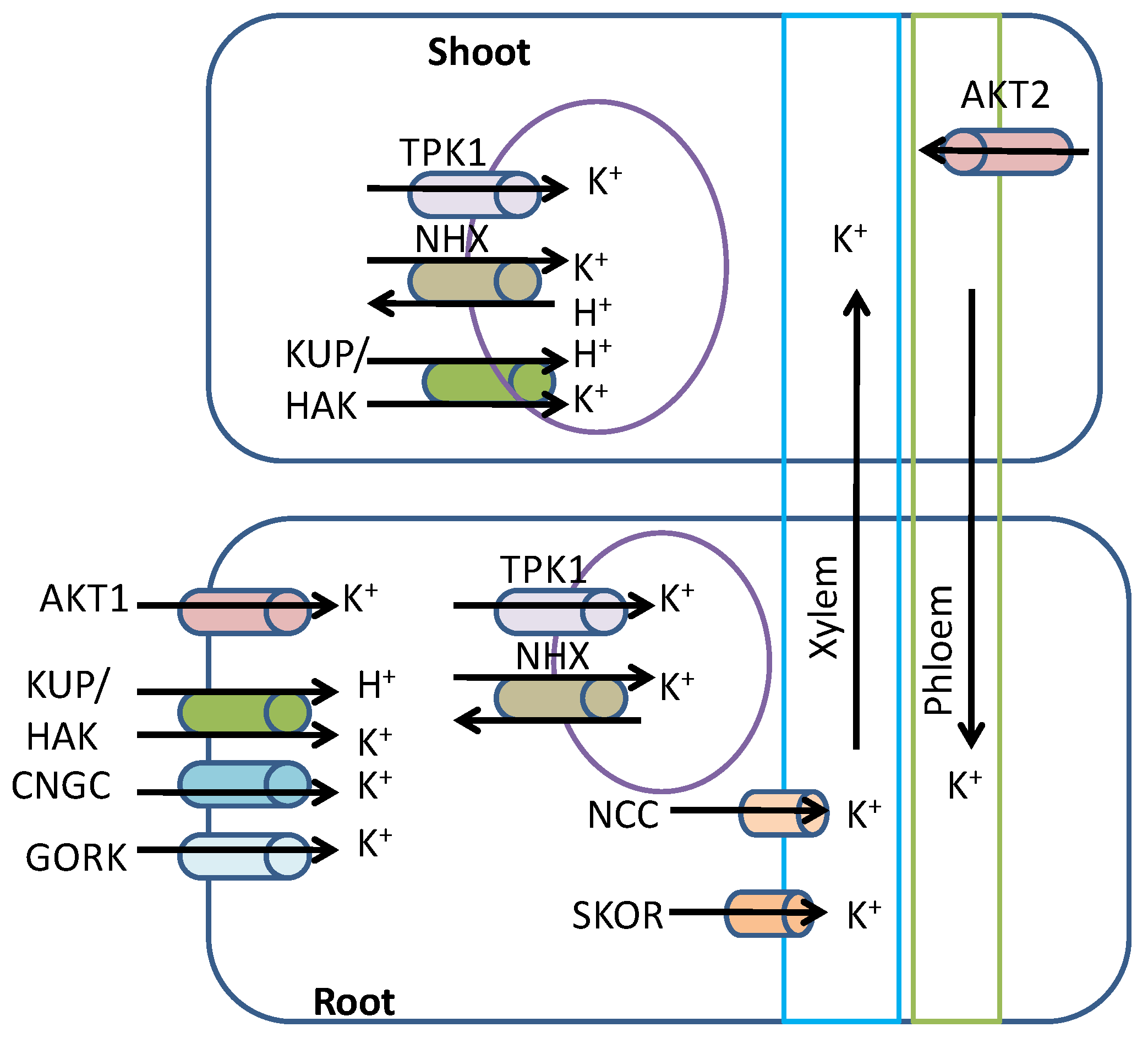
3. Potassium Uptake, Transport, and Assimilation in Plants
4. Potassium and Plant Responses
4.1. Seed Germination and Emergence
4.2. Growth
4.3. Stomatal Regulation
4.4. Water Uptake
4.5. Photosynthesis
4.6. Nutrient Balance
4.7. Reproductive Development
4.8. Yield
4.9. Crop Quality
5. Potassium-Induced Abiotic Stress Tolerance
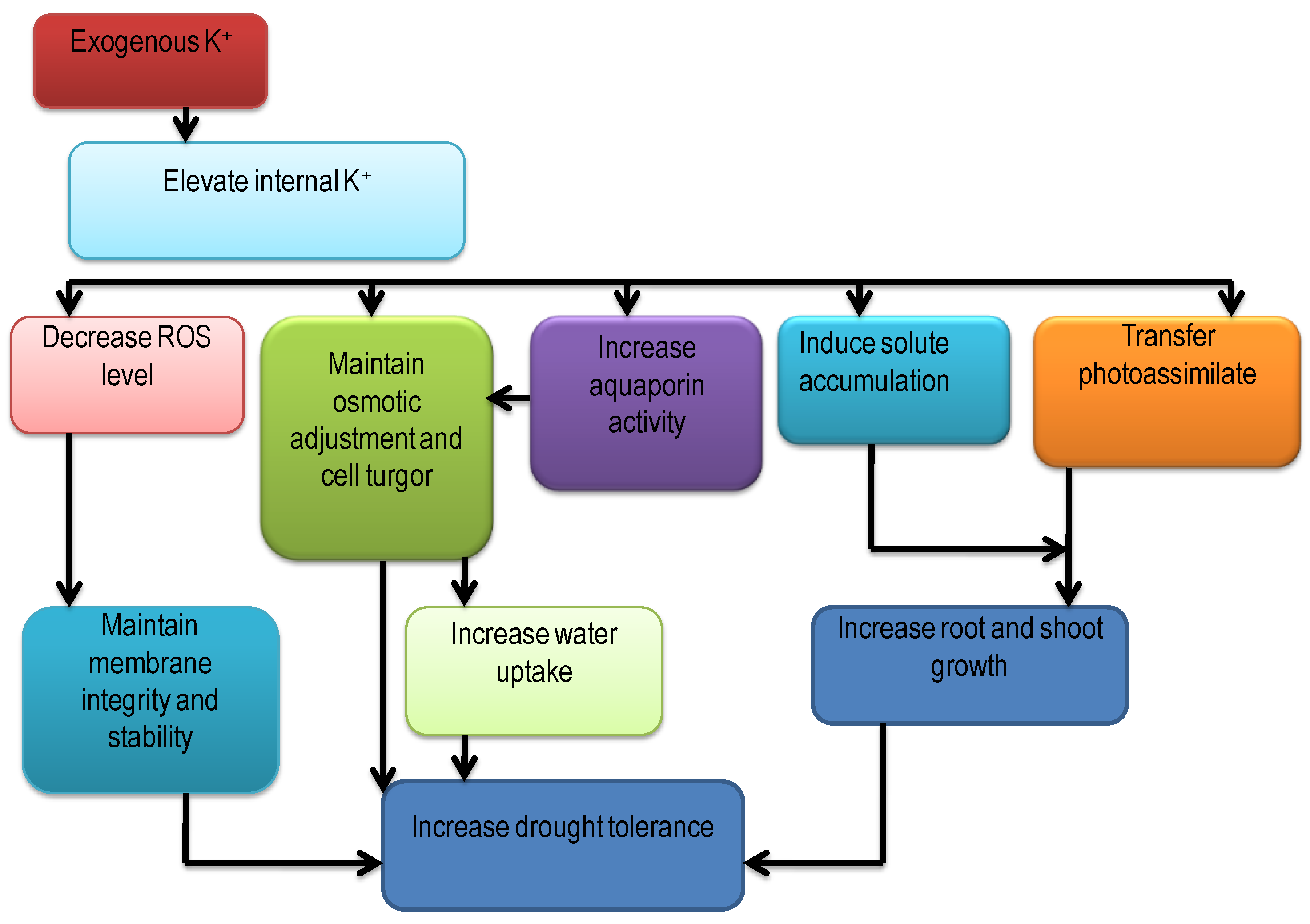
5.1. Drought
5.2. Salinity
5.3. Extreme Temperature
5.4. Toxic Metals/Metalloids
5.5. High Light
5.6. Waterlogging
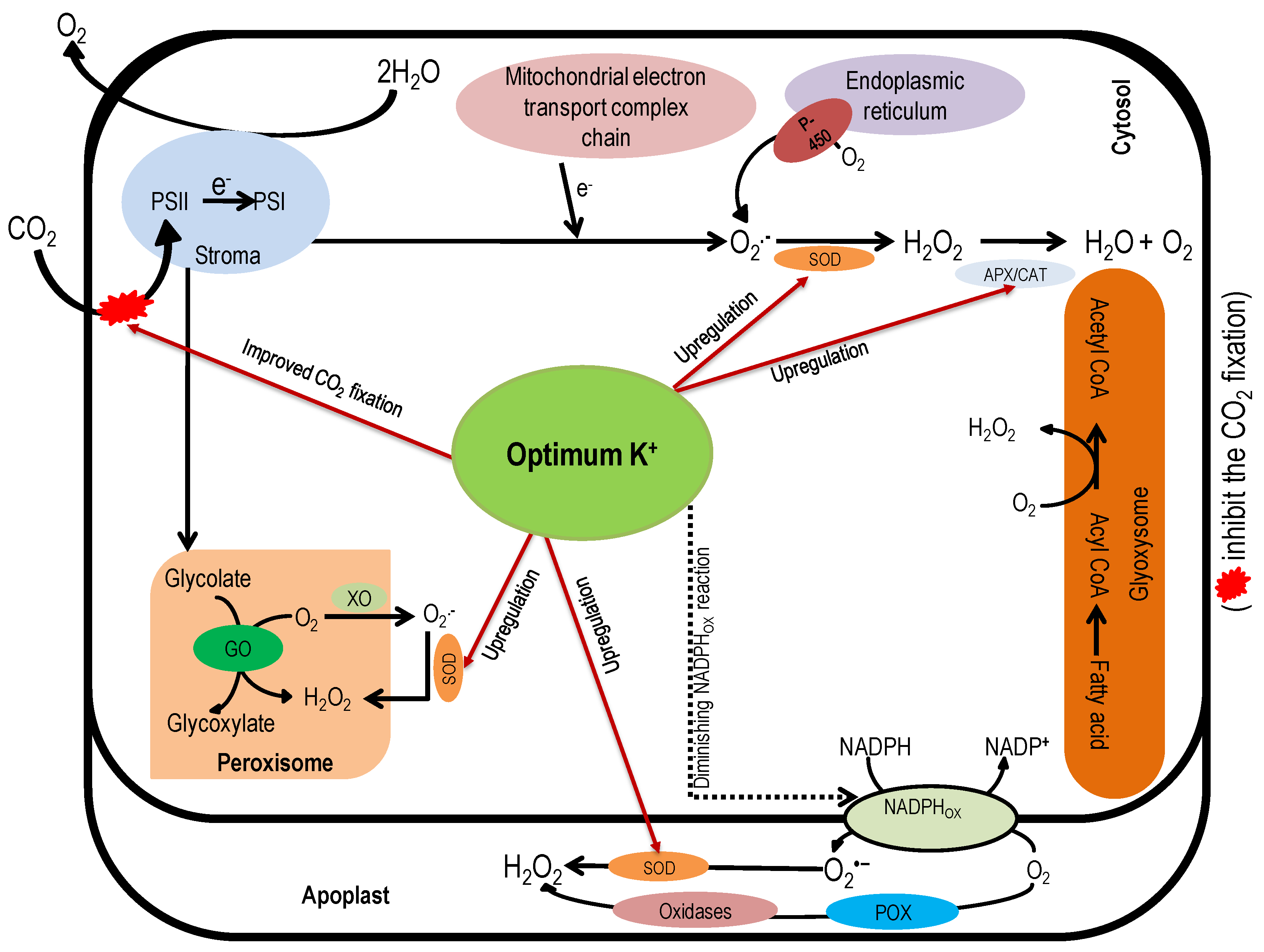
6. Role of Potassium in the Detoxification of Reactive Oxygen Species
7. Interaction of Potassium with Other Biomolecules
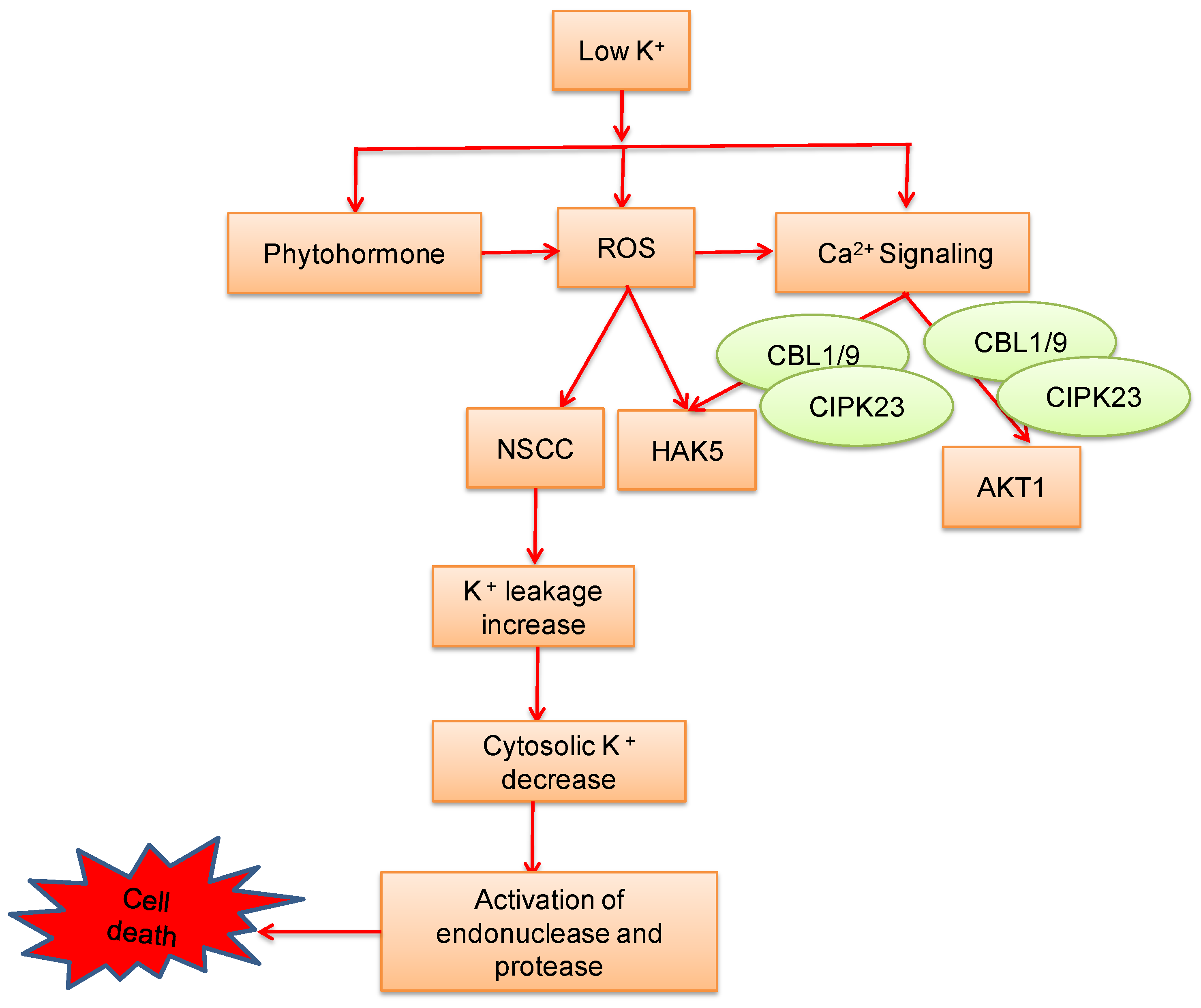
8. Potassium-Induced Abiotic Stress Signaling
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wani, S.H.; Sah, S.K. Biotechnology and abiotic stress tolerance in rice. J. Rice Res. 2014, 2, 1000–1105. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2013; pp. 25–87. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Jedmowski, C.; Ashoub, A.; Momtaz, O.; Brüggemann, W. Impact of drought, heat, and their combination on chlorophyll fluorescence and yield of wild barley (Hordeum spontaneum). J. Bot. 2015. [Google Scholar] [CrossRef]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Halim, A.; Aziz, T. Alleviation of temperature stress by nutrient management in crop plants: A review. J. Soil Sci. Plant Nutr. 2012, 12, 221–244. [Google Scholar] [CrossRef]
- O’Neill, S.D.; Spanswick, R.M. Characterization of native and reconstituted plasma membrane H+-ATPase from the plasma membrane of Beta vulgaris. J. Membr. Biol. 1984, 79, 245–256. [Google Scholar] [CrossRef]
- Halford, N.G. New insights on the effects of heat stress on crops. J. Exp. Bot. 2009, 60, 4215–4216. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Shani, E.; Salehin, M.; Zhang, Y.; Sanchez, S.E.; Doherty, C.; Wang, R.; Mangado, C.C.; Song, L.; Tal, I.; Pisanty, O.; et al. Plant stress tolerance requires auxin-sensitive Aux/IAA transcriptional repressors. Curr. Biol. 2017, 27, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Huang, S.C.; Wise, A.; Castanon, R.; Nery, J.R.; Chen, H.; Watanabe, M.; Thomas, J.; Bar-Joseph, Z.; Ecker, J.R. A transcription factor hierarchy defines an environmental stress response network. Science 2016, 354. [Google Scholar] [CrossRef] [PubMed]
- Zwack, P.J.; Rashotte, A.M. Interactions between cytokinin signalling and abiotic stress responses. J. Exp. Bot. 2015, 66, 4863–4871. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Fageria, N.K. The Use of Nutrients in Crop Plants; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Schroeder, D. Structure and weathering of potassium containing minerals. In Proceedings of the 11th Congress of the International Potash Institute, Tokyo, Japan, 27–28 August 1978; International Potash Institute: Bern, Switzerland, 1978. [Google Scholar]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Kafkafi, U.; Xu, G.; Imas, P.; Magen, H.; Tarchitzky, J. Potassium and Chloride in Crops and Soils: The Role of Potassium Chloride Fertilizer in Crop Nutrition; IPI Research Topics No. 22; International Potash Institute: Horgen, Switzerlands, 2001; p. 220. [Google Scholar]
- Ishizaki, H.; Akiya, T. Effects of chlorine on growth and quality of tobacco. Jpn. Agric. Res. Q. 1978, 12, 1–6. [Google Scholar]
- Masood, S.; Bano, A. Mechanism of potassium solubilization in the agricultural soils by the help of soil microorganisms. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V., Maurya, B., Verma, J., Meena, R., Eds.; Springer: New Delhi, India, 2016; pp. 137–147. [Google Scholar]
- Sarkar, R.K.; Malik, G.C. Effect of foliar spray of potassium nitrate and calcium nitrate on grasspea (Lathyrus sativus L.) grown in rice fallows. Lathyrus Lathyrism Newsl. 2001, 2, 47–48. [Google Scholar]
- Ashraf, M.A.; Ahmad, M.S.A.; Ashraf, M.; Al-Qurainy, F.; Ashraf, M.Y. Alleviation of waterlogging stress in upland cotton (Gossypium hirsutum L.) by exogenous application of potassium in soil and as a foliar spray. Crop Past. Sci. 2011, 6, 25–38. [Google Scholar] [CrossRef]
- Ling, F.; Silberbush, M. Response of maize to foliar vs. soil application of nitrogen–phosphorus–potassium fertilizers. J. Plant Nutr. 2002, 25, 2333–2342. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Alemán, F.; Martínez, V.; Rubio, F. K+ uptake in plant roots. The systems involved, their regulation and parallels in other organisms. J. Plant Physiol. 2014, 171, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Santa-María, G.E.; Rubio, F.; Dubcovsky, J.; Rodríguez-Navarro, A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell 1997, 9, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.E.; Lewis, B.D.; Spalding, E.P.; Sussman, M.R. A role for the AKT1 potassium channel in plant nutrition. Science 1998, 280, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Anschütz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Pyo, Y.J.; Gierth, M.; Schroeder, J.I.; Cho, M.H. High-affinity K+ transport in Arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and postgermination growth under low-potassium conditions. Plant Physiol. 2010, 153, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Long, Y.; Qi, G.N.; Xu, Z.J.; Wu, W.H.; Wang, Y. The Os-AKT1 channel is critical for K+ uptake in rice roots and is modulated by the rice CBL1-CIPK23 complex. Plant Cell 2014, 26, 3387–3402. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, S.; Hu, Y.; Wu, F.; Hu, Q.; Chen, G.; Cai, J.; Wu, T.; Moran, N.; Yu, L.; et al. The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol. 2014, 166, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Hosy, E.; Vavasseur, A.; Mouline, K.; Dreyer, I.; Gaymard, F. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA 2003, 100, 5549–5554. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Isayenkov, S.; Voelker, C.; Czempinski, K.; Maathuis, F.J.M. The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc. Natl. Acad. Sci. USA 2007, 104, 10726–10731. [Google Scholar] [CrossRef] [PubMed]
- Bassil, E.; Tajima, H.; Liang, Y.C.; Ohto, M.A.; Ushijima, K. The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 2011, 23, 3482–3497. [Google Scholar] [CrossRef] [PubMed]
- Barragan, V.; Leidi, E.O.; Andrés, Z.; Rubio, L.; De Luca, A.; Fernandez, A.; Cubero, B.; Pardo, J.M. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 2012, 24, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Maathuis, F.J. Cellular and tissue distribution of potassium: Physiological relevance, mechanisms and regulation. J. Plant Physiol. 2014, 171, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; pp. 178–189. [Google Scholar]
- Farooq, M.; Basra, S.M.A.; Rehman, H.; Saleem, B.A. Seed priming enhances the performance of late sown wheat (Triticum aestivum L.) by improving chilling tolerance. J. Agron. Crop Sci. 2008, 194, 55–60. [Google Scholar] [CrossRef]
- Copeland, L.O.; McDonald, M.B. Seed Science and Technology; Chapman & Hill: New York, NY, USA, 2001. [Google Scholar]
- Mohammadi, G.R. The effect of seed priming on plant traits of late-spring seeded soybean (Glycine max L.). Am. Eur. J. Agric. Environ. Sci. 2009, 5, 322–326. [Google Scholar]
- Çokkizgin, H.; Bölek, Y. Priming treatments for improvement of germination and emergence of cotton seeds at low temperature. Plant Breed. Seed Sci. 2015, 71, 121–134. [Google Scholar] [CrossRef]
- Esmeili, M.A.; Heidarzade, A. Investigation of different osmopriming techniques on seed and seedling properties of rice (Oryza sativa) genotypes. Int. Res. J. Basic Appl. Sci. 2012, 3, 242–246. [Google Scholar]
- Tang, Z.H.; Zhang, A.J.; Wei, M.; Chen, X.G.; Liu, Z.H.; Li, H.M.; Ding, Y.F. Physiological response to potassium deficiency in three sweet potato (Ipomoea batatas [L.] Lam.) genotypes differing in potassium utilization efficiency. Acta Physiol. Plant. 2015, 37, 184. [Google Scholar] [CrossRef]
- Iqbal, A.; Hidayat, Z. Potassium management for improving growth and grain yield of maize (Zea mays L.) under moisture stress condition. Sci. Rep. 2016, 6, 34627. [Google Scholar] [CrossRef]
- Hussain, F.; Malik, A.U.; Haji, M.A.; Malghani, A.L. Growth and yield response of two cultivars of mungbean (Vigna radiata L.) to different potassium levels. J. Anim. Plant Sci. 2011, 21, 622–625. [Google Scholar]
- Zelelew, D.Z.; Lal, S.; Kidane, T.T.; Ghebreslassie, B.M. Effect of potassium levels on growth and productivity of potato varieties. Am. J. Plant Sci. 2016, 7, 1629–1638. [Google Scholar] [CrossRef]
- Gerardeaux, E.; Jordan-Meille, L.; Constantin, J.; Pellerin, S.; Dingkuhn, M. Changes in plant morphology and dry matter partitioning caused by potassium deficiency in Gossypium hirsutum L. Environ. Exp. Bot. 2010, 67, 451–459. [Google Scholar] [CrossRef]
- Divito, G.A.; Sadras, V.O. How do phosphorus, potassium and sulphur affect plant growth and biological nitrogen fixation in crop and pasture legumes? A meta-analysis. Field Crops Res. 2014, 156, 161–171. [Google Scholar] [CrossRef]
- Thomas, T.C.; Thomas, A.C. Vital role of potassium in the osmotic mechanism of stomata aperture modulation and its link with potassium deficiency. Plant Signal. Behav. 2009, 4, 240–243. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer: Sunderland, MA, USA, 2010. [Google Scholar]
- Wang, Y.; Wu, W.H. Regulation of potassium transport and signaling in plants. Curr. Opin. Plant Biol. 2017, 39, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Schwartzkopf, C.A.R.L. Potassium, Calcium, Magnesium—How They Relate to Plant Growth. Available online: http://gsrpdf.lib.msu.edu/ticpdf.py?file=/1970s/1972/721101.pdf (accessed on 11 March 2018).
- Martineau, E.; Domec, J.C.; Bosc, A.; Denoroy, P.; Fandino, V.A.; Lavres, J., Jr.; Jordan-Meille, L. The effects of potassium nutrition on water use in field-grown maize (Zea mays L.). Environ. Exp. Bot. 2017, 134, 62–71. [Google Scholar] [CrossRef]
- Oddo, E.; Inzerillo, S.; Grisafi, F.; Sajeva, M.; Salleo, S.; Nardini, A. Does short-term potassium fertilization improve recovery from drought stress in laurel? Tree Physiol. 2014, 34, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Zorb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Lu, J.; Pan, Y.; Lu, P.; Li, X.; Cong, R.; Ren, T. Anatomical variation of mesophyll conductance under potassium deficiency has a vital role in determining leaf photosynthesis. Plant Cell Environ. 2016, 39, 2428–2439. [Google Scholar] [CrossRef] [PubMed]
- Bednarz, C.W.; Oosterhuis, D.M.; Evans, R.D. Leaf photosynthesis and carbon isotope discrimination of cotton in response to potassium deficiency. Environ. Exp. Bot. 1998, 39, 131–139. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M.; Bednarz, C.W. Influence of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosynthetica 2001, 39, 103–109. [Google Scholar] [CrossRef]
- Shingles, R.; McCarty, R.E. Direct measurement of ATP-dependent proton concentration changes and characterization of a K-stimulated ATPase in pea chloroplast inner envelope vesicles. Plant Physiol. 1994, 106, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.H.; Gierth, M.; Herdean, A.; Satoh-Cruz, M.; Kramer, D.M.; Spetea, C.; Schroeder, J.I. Plastidial transporters KEA1, -2, and -3 are essential for chloroplast osmoregulation, integrity, and pH regulation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 7480–7485. [Google Scholar] [CrossRef] [PubMed]
- Dana, S.; Herdean, A.; Lundin, B.; Spetea, C. Each of the chloroplast potassium efflux antiporters affects photosynthesis and growth of fully developed Arabidopsis rosettes under short day photoperiod. Physiol. Plant. 2016, 158, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Sicilia, M.N.; Aboukila, A.; Armbruster, U.; Cagnac, O.; Schumann, T.; Kunz, H.H.; Jahns, P.; Rodrı´guez-Rosales, M.P.; Sze, H.; Venema, K. Envelope K+/H+ antiporters AtKEA1 and AtKEA2 function in plastid development. Plant Physiol. 2016, 172, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, U.; Carrillo, L.R.; Venema, K.; Pavlovic, L.; Schmidtmann, E.; Kornfeld, A.; Jahns, P.; Berry, J.A.; Kramer, D.M.; Jonikas, M.C. Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat. Commun. 2014, 5, 5439. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yamamoto, H.; Narumiya, F.; Munekage, Y.N.; Finazzi, G.; Szabo, I.; Shikanai, T. Fine-tuned regulation of the K+/H+ antiporter KEA3 is required to optimize photosynthesis during induction. Plant J. 2017, 89, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Britto, D.T.; Kronzucker, H.J. The nitrogen–potassium intersection: Membranes, metabolism, and mechanism. Plant Cell Environ. 2017, 40, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Ganie, A.H.; Ahmad, A.; Yousuf, P.Y.; Pandey, R.; Ahmad, S.; Aref, I.M.; Iqbal, M. Nitrogen-regulated changes in total amino acid profile of maize genotypes having contrasting response to nitrogen deficit. Protoplasma 2017, 254, 2143–2153. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.K.; Hadacek, F.; Sassmann, S.; Lang, I. Iron deprivation-induced reactive oxygen species generation leads to non-autolytic PCD in Brassica napus leaves. Environ. Exp. Bot. 2013, 91, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Omer, F.A.; Abbas, D.N.; Khalaf, A.S. Effect of molybdenum and potassium application on nodulation, growth and yield of lentil (Lens culinaris Medic). Pak. J. Bot. 2016, 48, 2255–2259. [Google Scholar]
- Fan, L.; Wang, Y.; Wang, H.; Wu, W. In vitro Arabidopsis pollen germination an characterization of inward potassium currents in Arabidopsis pollen grain protoplasts. J. Exp. Bot. 2001, 52, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Mondal, S.; Mandal, S. Studies on in vitro pollen germination of Carissa carandus L. Sci. Cult. 2013, 79, 128–130. [Google Scholar]
- Makhdum, M.I.; Pervez, H.; Ashraf, M. Dry matter accumulation and partitioning in cotton (Gossypium hirsutum L.) as influenced by potassium fertilization. Biol. Fertil. Soils 2007, 43, 295–301. [Google Scholar] [CrossRef]
- Sadiq, S.A.; Jan, A. Effect of Graded Application of Potash on Kharif Maize Sown at Different Fertility Levels. M.Sc (Hons) Thesis, Department of Agron, KPK Agricultural University, Peshawar, Pakistan, 2001. [Google Scholar]
- Asif, M.; Anwar, M. Phenology, leaf area and yield of spring maize (Cv. Azam) as affected by levels and timings of potassium application. World Appl. Sci. J. 2007, 2, 299–303. [Google Scholar]
- Zou, T.X.; Dai, T.B.; Jiang, D.; Jing, Q.; Cao, W.X. Effects of nitrogen and potassium application levels on flag leaf photosynthetic characteristics after anthesis in winter wheat. Acta Agron. Sin. 2007, 33, 1667–1673. [Google Scholar]
- Islam, A.; Muttaleb, A. Effect of potassium fertilization on yield and potassium nutrition of Boro rice in a wetland ecosystem of Bangladesh. Arch. Agron. Soil Sci. 2016, 62, 1530–1540. [Google Scholar] [CrossRef]
- Cheema, M.A.; Wahid, M.A.; Sattar, A.; Rasul, F.; Saleem, M.F. Influence of different levels of potassium on growth, yield and quality of canola (Brassica napus L.) cultivars. Pak. J. Agric. Sci. 2012, 49, 163–168. [Google Scholar]
- Uddin, S.; Sarkar, M.A.R.; Rahman, M.M. Effect of nitrogen and potassium on yield of dry direct seeded rice cv. Nerica 1 in Aus season. Int. J. Agron. Plant Prod. 2013, 4, 69–75. [Google Scholar]
- Duan, Y.; Shi, X.; Li, S.; Sun, X.; He, X. Nitrogen use efficiency as affected by phosphorus and potassium in long-term rice and wheat. J. Integr. Agric. 2014, 13, 588–596. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Saleem, M.F.; Shah, G.M.; Khan, I.H.; Raza, A. Exogenous application of glycinebetaine and potassium for improving water relations and grain yield of wheat under drought. J. Soil Sci. Plant Nutr. 2014, 14, 348–364. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Hur, R.G.M.; Ahmad, A.; Mahmood, N. Response of foliar application of KNO3 on yield, yield components and lint quality of cotton (Gossypium hirsutum L.). Afr. J. Agric. Res. 2011, 6, 5457–5463. [Google Scholar]
- Colpan, E.; Zengin, M.; Özbahçe, A. The effects of potassium on the yield and fruit quality components of stick tomato. Hortic. Environ. Biotechnol. 2013, 54, 20–28. [Google Scholar] [CrossRef]
- Khan, R.; Gurmani, A.R.; Gurmani, A.H.; Zia, M.S. Effect of potassium application on crop yields under wheat-rice system. Sarhad J. Agric. 2007, 23, 277–279. [Google Scholar]
- Hussain, F.; Yasin, M. Soil Fertility Monitoring and Management in Rice-Wheat System; Annual Report LRRP; National Agricultural Research Centre (NARC): Islamabad, Pakistan, 2003; pp. 1–16.
- Islam, A.; Chandrabiswas, J.; Karim, A.J.M.S.; Salmapervin, M.; Saleque, M.A. Effects of potassium fertilization on growth and yield of wetland rice in grey terrace soils of Bangladesh. Res. Crop Ecophysiol. 2015, 10, 64–82. [Google Scholar]
- Tahir, M.; Tanveer, A.; Ali, A.; Ashraf, M.; Wasaya, A. Growth and yield response of two wheat (Triticum aestivum L.) varieties to different potassium levels. Pak. J. Life Soc. Sci. 2008, 6, 92–95. [Google Scholar]
- Maurya, P.; Kumar, V.; Maurya, K.K.; Kumawat, N.; Kumar, R. Effect of potassium application on growth and yield of wheat varieties. Bioscan 2014, 9, 1371–1373. [Google Scholar]
- Jahan, S.A.; Alim, M.A.; Hasan, M.M.; Kabiraj, U.K.; Hossain, M.B. Effect of potassium levels on the growth, yield and yield attributes of lentil. Int. J. Sustain. Crop Prod. 2009, 4, 1–6. [Google Scholar]
- El-Bassiony, A.M.; Fawzy, Z.F.; El-Samad, E.A.; Riad, G.S. Growth, yield and fruit quality of sweet pepper plants (Capsicum annuum L.) as affected by potassium fertilization. Am. J. Sci. 2010, 6, 722–729. [Google Scholar]
- Gormus, O.; Yucel, C. Different planting date and potassium fertility effects on cotton yield and fiber properties in the Cukurova region, Turkey. Field Crop Res. 2002, 78, 141–149. [Google Scholar] [CrossRef]
- Dong, H.; Kong, X.; Li, W.; Tang, W.; Zhang, D. Effects of plant density and nitrogen and potassium fertilization on cotton yield and uptake of major nutrients in two fields with varying fertility. Field Crops Res. 2010, 119, 106–113. [Google Scholar] [CrossRef]
- Bansal, S.K.; Trehan, S.P. Effect of potassium on yield and processing quality attributes of potato. Karnataka J. Agric. Sci. 2011, 24, 48–54. [Google Scholar]
- Al-Moshileh, A.M.; Errebi, M.A. Effect of various potassium sulfate rates on growth, yield and quality of potato grown under sandy soil and arid conditions. In Proceedings of the InIPI Regional Workshop on Potassium and Fertigation Development in West Asia and North Africa, Rabat, Morocco, 24–28 November 2004; pp. 24–28. [Google Scholar]
- Pervez, M.A.; Ayyub, C.M.; Shaheen, M.R.; Noor, M.A. Determination of physiomorphological characteristics of potato crop regulated by potassium management. Pak. J. Agric. Sci. 2013, 50, 611–615. [Google Scholar]
- Tariq, M.U.; Saeed, A.; Nisar, M.U.; Mian, I.A.; Afzal, M. Effect of potassium rates and sources on the growth performance and on chloride accumulation of maize in two different textured soils of Haripur, Hazara division. Sarhad J. Agric. 2011, 27, 415–422. [Google Scholar]
- Farhad, I.S.; Islam, M.N.; Hoque, S.; Bhuiyan, M.S. Role of potassium and sulphur on the growth, yield and oil content of soybean (Glycine max L.). Acad. J. Plant Sci. 2010, 3, 99–103. [Google Scholar]
- Tikkoo, A.; Yadav, S.S.; Kaushik, N. Effect of irrigation, nitrogen and potassium on seed yield and oil content of Jatropha curcas in coarse textured soils of northwest India. Soil Tillage Res. 2013, 134, 142–146. [Google Scholar] [CrossRef]
- Eshghi, S.; Safizadeh, M.R.; Jamali, B.; Sarseifi, M. Influence of foliar application of volk oil, dormex, gibberellic acid and potassium nitrate on vegetative growth and reproductive characteristics of strawberry cv. ‘Merak’. J. Biol. Environ. Sci. 2012, 6, 35–38. [Google Scholar]
- Goud, V.V.; Konde, N.M.; Mohod, P.V.; Kharche, V.K. Response of chickpea to potassium fertilization on yield, quality, soil fertility and economic in vertisols. Legum. Res. 2012, 37, 311–315. [Google Scholar] [CrossRef]
- Abbadi, J.; Gerendás, J.; Sattelmacher, B. Effects of potassium supply on growth and yield of safflower as compared to sunflower. J. Plant Nutr. Soil Sci. 2008, 171, 272–280. [Google Scholar] [CrossRef]
- Umar, S. Alleviating adverse effects of water stress on yield of sorghum, mustard, and groundnut by potassium application. Pak. J. Bot. 2006, 38, 1373–1380. [Google Scholar]
- Ng Kee Kwong, K.F. The effects of potassium on growth, development, yield and quality of sugarcane. In Potassium for Sustainable Crop Production, Proceedings of the International Symposium on the Role of Potassium in Nutrient Management for Sustainable Crop Production in India, New Delhi, India, 3–5 December 2001; Pasricha, N.S., Bansal, S.K., Eds.; Potash Research Institute of India (PRII) and International Potash Institute (IPI): Horgen, Switzerland, 2002; pp. 430–444. [Google Scholar]
- Abdel-Mawly, S.E.; Zanouny, I. Response of sugar beet (Beta vulgaris L.) to potassium application and irrigation with saline water. Assiut Univ. Bull Environ. Res. 2004, 7, 123–136. [Google Scholar]
- Abbas, G.; Aslam, M.; Malik, A.U.; Abbas, Z.; Ali, M.; Hussain, F. Potassium sulfate effects on growth and yield of mungbean (Vigna radiata L.) under arid climate. Int. J. Agric. Appl. Sci. 2011, 3, 72–75. [Google Scholar]
- Yang, S.; Fengmin, L.; Malhi, S.S.; Wang, P.; Suo, D.; Wang, J. Long-term fertilization efforts on crop yield and nitrate nitrogen accumulation in soil in northwestern China. Agron. J. 2004, 96, 1039–1049. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Relationship between insufficient potassium and crop maturity in cotton. Agron. J. 2003, 95, 1323–1329. [Google Scholar] [CrossRef]
- Ashfaq, A.; Hussain, N.; Athar, M. Role of potassium fertilizers in plant growth, crop yield and quality fiber production of cotton—An overview. FUUAST J. Biol. 2015, 5, 27–35. [Google Scholar]
- Mehrandish, M.; Moeini, M.J.; Armin, M. Sugar beet (Beta vulgaris L.) response to potassium application under full and deficit irrigation. Eur. J. Exp. Biol. 2012, 2, 2113–2119. [Google Scholar]
- Economakis, C.; Daskalaki, A. Effect of potassium nutrition on yield and quality of tomato plants grown with nutrient film technique under sodium chloride saline conditions. Acta Hortic. 2003, 609, 337–339. [Google Scholar] [CrossRef]
- Egilla, J.N.; Davies, F.T.; Drew, M.C. Effect of potassium on drought resistance of Hibiscus rosa-sinensis cv. Leprechaun: Plant growth, leaf macro- and micronutrient content and root longevity. Plant Soil 2001, 229, 213–224. [Google Scholar] [CrossRef]
- Römheld, V.; Kirkby, E.A. Research on potassium in agriculture: Needs and prospects. Plant Soil 2010, 335, 155–180. [Google Scholar] [CrossRef]
- Egilla, J.N.; Davies, F.T.; Boutton, T.W. Drought stress influences leaf water content, photosynthesis, and water-use efficiency of Hibiscus rosa-sinensis at three potassium concentrations. Photosynthetica 2005, 43, 135–140. [Google Scholar] [CrossRef]
- Zain, N.A.M.; Ismail, M.R.; Puteh, A.; Mahmood, M.; Islam, M.R. Drought tolerance and ion accumulation of rice following application of additional potassium fertilizer. Commun. Soil Sci. Plant Anal. 2014, 45, 2502–2514. [Google Scholar] [CrossRef]
- Fazeli, F.; Ghorbanli, M.; Niknam, V. Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol. Plant. 2007, 51, 98–103. [Google Scholar] [CrossRef]
- Degenkolbe, T.; Do, P.T.; Zuther, E.; Repsilber, D.; Walther, D.; Hincha, D.K.; Köhl, K.I. Expression profiling of rice cultivars differing in their tolerance to long-term drought stress. Plant Mol. Biol. 2009, 69, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Soleimanzadeh, H.; Habibi, D.; Ardakani, M.R.; Paknejad, F.; Rejali, F. Effect of potassium levels on antioxidant enzymes and malondialdehyde content under drought stress in sunflower (Helianthus annuus L.). Am. J. Agric. Biol. Sci. 2010, 5, 56–61. [Google Scholar] [CrossRef]
- Kanai, S.; Moghaieb, R.E.; El-Shemy, H.A.; Panigrahi, R.; Mohapatra, P.K.; Ito, J.; Nguyen, N.T.; Saneoka, H.; Fujita, K. Potassium deficiency affects water status and photosynthetic rate of the vegetative sink in green house tomato prior to its effects on source activity. Plant Sci. 2011, 180, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W.; Shen, Q.R.; Brueck, H. Effects of local nitrogen supply on water uptake of bean plants in a split root system. J. Integr. Plant Biol. 2007, 49, 472–480. [Google Scholar] [CrossRef]
- Oddo, E.; Inzerillo, S.; La Bella, F.; Grisafi, F.; Salleo, S.; Nardini, A. Short-term effects of potassium fertilization on the hydraulic conductance of Laurus nobilis L. Tree Physiol. 2011, 31, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Pereira, S. High salinity and drought act on an organ-dependant manner on potato glutamine synthetase expression and accumulation. Environ. Exp. Bot. 2007, 60, 121–126. [Google Scholar] [CrossRef]
- Pandey, R.; Agarwal, R.M.; Jeevaratnam, K.; Sharma, G.L. Osmotic stress induced alterations in rice (Oryza sativa L.) and recovery. Plant Growth Regul. 2004, 42, 79–87. [Google Scholar] [CrossRef]
- Din, J.; Khan, S.U.; Ali, I.; Gurmani, A.R. Physiological and agronomic response of canola varieties to drought stress. J. Anim. Plant Sci. 2011, 21, 78–83. [Google Scholar]
- Jatav, K.S.; Agarwal, R.M.; Singh, R.P.; Shrivastava, M. Growth and yield responses of wheat (Triticum aestivum L.) to suboptimal water supply and different potassium doses. J. Funct. Environ. Bot. 2012, 2, 39–51. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, M.; Li, S.; Alva, A.K.; Ashraf, M. Potassium fertilization mitigates the adverse effects of drought on selected Zea mays cultivars. Turk. J. Bot. 2014, 38, 713–723. [Google Scholar] [CrossRef]
- Ali, M.; Bakhat, J.; Khan, G.D. Effect of water deficiency and potassium application on plant growth, osmolytes and grain yield of Brassica napus L. cultivars. Acta Bot. Croat. 2014, 73, 299–314. [Google Scholar] [CrossRef]
- Zahoor, R.; Zhao, W.; Abid, M.; Dong, H.; Zhou, Z. Potassium application regulates nitrogen metabolism and osmotic adjustment in cotton (Gossypium hirsutum L.) functional leaf under drought stress. J. Plant Physiol. 2017, 215, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Premachandra, G.S.; Saneoka, H.; Ogata, S. Cell membrane stability and leaf water relationsas affected potassium nutrition of water-stressed maize. J. Exp. Bot. 1991, 42, 739–745. [Google Scholar] [CrossRef]
- Barman, T.S.; Baruah, U.; Saikia, J.K. Effects of potassium as antitranspirant on tea (Camellia sinensis L.) under drought. Two Bud 2011, 58, 70–73. [Google Scholar]
- Wei, J.; Li, C.; Li, Y.; Jiang, G.; Cheng, G.; Zheng, Y. Effects of external potassium (K) supply on drought tolerances of two contrastings winter wheat cultivars. PLoS ONE 2013, 8, 69737. [Google Scholar] [CrossRef] [PubMed]
- Fayez, K.A.; Bazaid, S.A. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J. Saudi Soc. Agric. Sci. 2014, 3, 45–55. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Luo, W.; Lin, W.; Ma, L.; Kabir, M.H. Model of cation transportation mediated by high-affinity potassium transporters (HKTs) in higher plants. Biol. Proced. Online 2015, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Dai, Z.; Li, S.; Xin, H. A novel system for evaluating drought–cold tolerance of grapevines using chlorophyll fluorescence. BMC Plant Biol. 2015, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.U.; Asraf, M.Y.; Khan, M.A.; Naqvi, M.H. Potassium induced salinity tolerance in wheat (Triticum aestivum L.). Int. J. Environ. Sci. Technol. 2005, 2, 233–236. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Psarras, G.; Vemmos, S.; Loupassaki, M.; Bertaki, M. Response of two olive cultivars to salt stress and potassium supplement. J. Plant Nutr. 2006, 29, 2063–2078. [Google Scholar] [CrossRef]
- Umar, S.; Diva, I.; Anjum, N.A.; Iqbal, M.; Ahmad, I.; Pereira, E. Potassium-induced alleviation of salinity stress in Brassica campestris L. Cent. Eur. J. Biol. 2011, 6, 1054–1063. [Google Scholar] [CrossRef]
- Abbasi, G.H.; Akhtar, J.; Anwar-Ul-Haq, M.; Ali, S.; Chen, Z.; Malik, W. Exogenous potassium differentially mitigates salt stress in tolerant and sensetive maize hybrids. Pak. J. Bot. 2014, 46, 135–146. [Google Scholar]
- Saida, C.; Houria, B.; Mebarek, B. Interactive effects of salinity and potassium on physio-morphological traits of tomato (Lycopersicon esculentum L.). Agric. Biol. J. N. Am. 2014, 5, 135–143. [Google Scholar]
- Chakraborty, K.; Bhaduri, D.; Meena, H.N. External potassium (K+) application improves salinity tolerance by promoting Na+-exclusion, K+-accumulation and osmotic adjustment in contrasting peanut cultivars. Plant Physiol. Biochem. 2016, 103, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Amjad, M.; Akhtar, J.; Murtaza, B.; Abbas, G.; Jawad, H. Differential accumulation of potassium results in varied salt-tolerance response in tomato (Solanum lycopersicum L.) cultivars. Hortic. Environ. Biotechnol. 2016, 57, 248–258. [Google Scholar] [CrossRef]
- Merwad, A.R.M.A. Efficiency of potassium fertilization and salicylic acid on yield and nutrient accumulation of sugar beet grown on saline soil. Commun. Soil Sci. Plant Anal. 2016, 47, 1184–1192. [Google Scholar] [CrossRef]
- Taffouo, V.D.; Wamba, O.F.; Youmbi, E.; Nono, G.V.; Akoa, A. Growth, yield, water status and ionic distribution response of three bambara groundnut (Vigna subterranea (L.) Verdc.) landraces grown under saline conditions. Int. J. Bot. 2010, 6, 53–58. [Google Scholar] [CrossRef]
- Shabala, S.N.; Lew, R.R. Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol. 2002, 129, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Shabala, L.; Zhang, J.; Pottosin, I.; Bose, J.; Zhu, M.; Fuglsan, A.T.; Velarde-Buendia, A.; Massart, A.; Hill, C.B.; Roessner, U. Cell-type specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare L.) to salinity stress. Plant Physiol. 2016, 172, 2445–2458. [Google Scholar] [CrossRef] [PubMed]
- Azedo-Silva, J.; Osorio, J.; Fonseca, F.; Correia, M.J. Effects of soil drying and subsequent re-watering on the activity of nitrate reductase in roots and leaves of Helianthus annuus. Funct. Plant Biol. 2004, 31, 611–621. [Google Scholar] [CrossRef]
- Dias, A.S.; Lidon, F.C. Bread and durum wheat tolerance under heat stress: A synoptical overview. Emir. J. Food Agric. 2010, 22, 412–436. [Google Scholar] [CrossRef]
- Meshah, E.A.E. Effect of irrigation regimes and foliar spraying of potassium on yield, yield components and water use efficiency of wheat in sandy soils. World J. Agric. Sci. 2009, 5, 662–669. [Google Scholar]
- Oosterhuis, D.M.; Loka, D.A.; Raper, T.B. Potassium and stress alleviation: Physiological functions and management of cotton. J. Plant Nutr. Soil Sci. 2013, 176, 331–343. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Heavy metals in the environment: Current status, toxic effects on plants and possible phytoremediation. In Phyto-technologies: Remediation of Environmental Contaminants; Anjum, N.A., Pereira, M.A., Ahmad, I., Duarte, A.C., Umar, S., Khan, N.A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 7–73. [Google Scholar]
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Sakran, A.M.; Basalah, M.O.; Ali, H.M. Effect of calcium and potassium on antioxidant system of Vicia faba L. under cadmium stress. Int. J. Mol. Sci. 2012, 13, 6604–6619. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Z.; Duan, C.L.; Guo, S.L.; Yang, Y.; Feng, Y.F.; Ma, R.J.; Yu, M.L. Potassium contributes to zinc stress tolerance in peach (Prunus persica) seedlings by enhancing photosynthesis and the antioxidant defense system. Genet. Mol. Res. 2015, 14, 8338–8351. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.M.; Yasin, N.A.; Ahmad, S.R.; Khan, W.U.; Ahmad, A.; Ali, A.; Rehman, S.U. Amelioration of cadmium stress in gladiolus (Gladiolus grandiflora L.) by application of potassium and silicon. J. Plant Nutr. 2017. [Google Scholar] [CrossRef]
- Cakmak, I. Tansley Review No. 111 Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Choi, S.; Jeong, S.; Jeong, W.; Kwon, S.; Chow, W.; Park, Y.I. Chloroplast Cu/Zn-superoxide dismutase is a highly sensitive site in cucumber leaves chilled in the light. Planta 2002, 216, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Meng, Z.; Zhang, G.; Qi, M.; Sun, Z.; Liu, Y.; Li, T. Sub-high temperature and high light intensity induced irreversible inhibition on photosynthesis system of tomato plant (Solanum lycopersicum L.). Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Schumann, T.; Paul, S.; Melzer, M.; Dörmann, P.; Jahns, P. Plant growth under natural light conditions provides highly flexible short-term acclimation properties toward high light stress. Front Plant Sci. 2017, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Mugnai, S.; Marras, A.M.; Mancuso, S. Effect of hypoxic acclimation on anoxia tolerance in Vitis roots: Response of metabolic activity and K+ fluxes. Plant Cell Physiol. 2011, 52, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Teakle, N.L.; Bazihizina, N.; Shabala, S.; Colmer, T.D.; Barrett-Lennard, E.G.; Rodrigo-Moreno, A.; Läuchli, A.E. Differential tolerance to combined salinity and O2 deficiency in the halophytic grasses Puccinellia ciliata and Thinopyrum ponticum: The importance of K+ retention in roots. Environ. Exp. Bot. 2013, 87, 69–78. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Kumar, S.; Bhakta, N.; Singh, S.K.; Rao, K.K.; Mishra, J.S.; Singh, A.K. Improvement of submergence tolerance in rice through efficient application of potassium under submergence-prone rainfed ecology of Indo-Gangetic Plain. Funct. Plant Biol. 2017, 44, 907–916. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant responses and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and Its Management: Perspectives and Strategies; Bandi, V., Shanker, A.K., Shanker, C., Mandapaka, M., Eds.; Springer: Berlin, Germany, 2012; pp. 261–316. [Google Scholar]
- Bhattacharjee, S. Sites of generation and physicochemical basis of formation of reactive oxygen species in plant cell. In Reactive Oxygen Species and Antioxidants in Higher Plants; Gupta, S.D., Ed.; CRC Press: New York, NY, USA, 2010; pp. 1–30. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarkli, M. Reactive oxygen species, oxidative damage, and antioxidant defense mechanism in plants under stressfull conditions: A review. J. Bot. 2012, 26. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.A.; Paul, M.J.; Foyer, C.H. Metabolite transport and associated sugar signalling systems underpinning source/sink interactions. Biochim. Biophys. Acta 2016, 1857, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Sangakkara, U.R.; Frehner, M.; Nösberger, J. Effect of soil moisture and potassium fertilizer on shoot water potential, photosynthesis and partitioning of carbon in mungbean and cowpea. J. Agron. Crop Sci. 2000, 185, 201–207. [Google Scholar] [CrossRef]
- Milford, G.F.J.; Johnston, A.E. Potassium and Nitrogen Interactions in Crop Production; International Fertiliser Society: York, UK, 2007; p. 615. [Google Scholar]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Salinity and programmed cell death: Unravelling mechanisms for ion specific signalling. J. Exp. Bot. 2009, 60, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.U.; Asif, S.M.; Khanzada, M.; Khan, M.A.; Ali, M.; Mumtaz, S.; Yousufzai, M.N.; Saif, M.S. Growth and ion accumulation in some wheat genotypes under NaCl stress. Pak. J. Biol. Sci. 2001, 4, 388–391. [Google Scholar]
- Bar-Tal, A.S.; Sparks, D.L.F. Potassium-salinity interaction in irrigated corn. Irrig. Sci. 2004, 12, 27–35. [Google Scholar] [CrossRef]
- Cha-um, S.; Siringam, K.; Juntawong, N.; Kirdmanee, C. Water relations, pigment stabilization, photosyntheticabilities and growth improvement in salt stressed rice plants treated with exogenous potassium nitrate application. Int. J. Plant Prod. 2010, 4, 187–198. [Google Scholar]
- Liang, T.B.; Wang, Z.L.; Wang, R.J.; Liu, L.L.; Shi, C.Y. Effects of potassium humate on ginger root growth and its active oxygen metabolism. Ying Yong Sheng Tai Xue Bao 2007, 18, 813–817. [Google Scholar] [PubMed]
- Zheng, Y.; Aijun, J.; Tangyuan, N.; Jialin, X.; Zengjia, L.; Gaoming, J. Potassium nitrate application alleviates sodium chloride stress in winter wheat cultivars differing in salt tolerance. J. Plant Physiol. 2008, 165, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.U.; Hadi, F.; Nawaz, M.A.; Rahman, K. Potassium and zinc increase tolerance to salt stress in wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2017, 116, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Kirnak, H.; Higgs, D. Enhancement of growth and normal growth parameters by foliar application of potassium and phosphorus in tomato cultivars grown at high (NaCl) salinity. J. Plant Nutr. 2001, 24, 357–367. [Google Scholar] [CrossRef]
- Peuke, A.D.; Jeschke, W.D.; Hartung, W. Flows of elements, ions and abscisic acid in Ricunus communis and site of nitrate reduction under potassium limitation. J. Exp. Bot. 2002, 53, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Kao, C.H. Abscisic acid induced changes in cell wall peroxidase activityand hydrogen peroxide level in roots of rice seedlings. Plant Sci. 2001, 160, 323–329. [Google Scholar] [CrossRef]
- Tu, B.; Liu, C.; Tian, B.; Zhang, Q.; Liu, X.; Herbert, S.J. Reduced abscisic acid content is responsible for enhanced sucrose accumulation by potassium nutrition in vegetable soybean seeds. J. Plant Res. 2017, 130, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; SkrumsagerMoller, I.; White, P. Function of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 135–189. [Google Scholar]
- Mengel, K. Potassium. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 91–120. [Google Scholar]
- Mengel, K. Principles of Plant Nutrition, 5th ed.; Kluwer Academic: Dordrecht, The Netherlands, 2001; pp. 481–509. [Google Scholar]
- Berg, W.K.; Cunningham, S.M.; Brouder, S.M.; Joern, B.C.; Johnson, K.D.; Volence, J.J. Influence of phosphorus and potassium on alfalfa yield, taproot C and N pools, and transcript levels of key genes after defoliation. Crop Sci. 2009, 49, 974–982. [Google Scholar] [CrossRef]
- Prasad, D.; Singh, R.; Singh, A. Management of sheath blight of rice with integrated nutrients. Indian Phytol. 2010, 63, 11–15. [Google Scholar]
- Wang, Y.; Wu, W.H. Plant sensing and signaling in response to K+ deficiency. Mol. Plant 2010, 3, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Ashley, M.K.; Grant, M.; Grabov, A. Plant responses to potassium deficiencies: A role for potassium transport proteins. J. Exp. Bot. 2006, 57, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Armengaud, P.; Sulpice, R.; Miller, A.J.; Stitt, M.; Amtmann, A.; Gibon, Y. Multilevel analysis of primary metabolism provides new insights into the role of potassium nutrition for glycolysis and nitrogen assimilation in Arabidopsis roots. Plant Physiol. 2009, 150, 772–785. [Google Scholar] [CrossRef] [PubMed]
- Shin, R.; Schachtman, D.P. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc. Natl. Acad. Sci. USA 2004, 101, 8827–8832. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Yu, H.Q.; Liu, N.; Yi, B.; Cao, M.J. Physiological characteristics of delaying leaf senescence in maize inbred lines tolerant to potassium deficiency. Acta Agron. Sin. 2012, 38, 1672–1679. [Google Scholar] [CrossRef]
- Jung, W.J.Y.; Shin, R.; Schachtmana, D.P. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell 2009, 21, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Hepper Doss, A.; Anand, S.P.; Keerthiga, M. Effect of foliar application of diammonium phosphate (DAP), potash (K) and naphthalene acetic acid (NAA) on growth, yield and some biochemical constituents of Vigna mungo (I.). Wudpecker J. Agric. Res. 2013, 2, 206–208. [Google Scholar]
- Kumar, N.; Dawson, J. Effect of different levels of nitrogen, potash and gibberellic acid (GA3) application on growth and yield attributes of rice (Oryza sativa L.). Progress. Res. 2013, 8, 197–198. [Google Scholar]
- Abd-El-Rhman, I.E.; Attia, M.F. Foliar spray with potassium nitrate and salicylic acid for improving growth, yield and nutrients uptake by olive trees under salinity stress conditions. Int. J. ChemTech Res. 2016, 9, 230–245. [Google Scholar]
- Troufflard, S.; Mullen, W.; Larson, T.R.; Graham, I.A.; Crozier, A.; Amtmann, A.; Armengaud, P. Potassium deficiency induces the biosynthesis of oxylipins and glucosinolates in Arabidopsis thaliana. BMC Plant Biol. 2010, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Richards, F.J.; Coleman, R.G. Occurrence of putrescine in potassium-deficient barley. Nature 1952, 170, 479–481. [Google Scholar] [CrossRef]
- Liu, K.; Fu, H.; Bei, Q.; Luan, S. Inward potassium channel in guard cells As a target for polyamine regulation of stomatal movements. Plant Physiol. 2000, 124, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, H.D.; Chen, L.Q.; Wang, Y.; Liu, L.L.; He, L.; Wu, W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Long, Y.; Schmitz-Thom, I.; Wang, X.P.; Zhang, C.; Li, H.; Wu, W.H. Two spatially and temporally distinct Ca2+ signals convey Arabidopsis thaliana responses to K+ deficiency. New Phytol. 2017, 213, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Ciani, S.; Schachtman, D.P. A peroxidase contributes to ROS production during Arabidopsis root response to potassium deficiency. Mol. Plant 2010, 3, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. Mechanisms and physiological roles of K+ efflux from root cells. J. Plant Physiol. 2014, 171, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P. The role of ethylene in plant responses to K+ deficiency. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, J.; Miller, A.J.; Luo, B.; Wang, M.; Zhu, Z.; Ouwerkerk, P.B. OsCHX14 is involved in the K+ homeostasis in rice (Oryza sativa) flowers. Plant Cell Physiol. 2016, 57, 1530–1543. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, W.; Deng, K.; Li, H.; Zhang, Z.; Zhang, L.; Chu, C. MicroRNA399 is involved in multiple nutrient starvation responses in rice. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
| Name of Crops | K Doses | Yield Improvement | References |
|---|---|---|---|
| Oryza sativa | 60 kg K2O ha−1 | Grain yield: 50% | [84] |
| O. sativa | 40 kg K2O ha−1 | Grain yield: 35% | [85] |
| O. sativa | 80 kg K2O ha−1 | Grain yield: 78.47% | [86] |
| O. sativa | 93.96–112.03 kg K2O ha−1 | Grain yield: 32.17% | [77] |
| Triticum aestivum | 108.42 kg K2O ha−1 | Grain yield: 10.66% | [87] |
| O. sativa | 60 kg K2O ha−1 | Grain yield: 13% | [84] |
| O. sativa | 40 kg K2O ha−1 | Grain yield: 21% | [85] |
| O. sativa | 80 kg K2O ha−1 | Grain yield: 41.16% | [88] |
| Lens culinaris | 42.16 kg K2O ha−1 | Grain yield: 34.16% | [89] |
| Capsicum annuum | 476.19 kg K2O ha−1 | Pod/fruit yield: 22.20% | [90] |
| Gossypium hirsutum | 112 kg K2O ha−1 | Lint yield: 10.18% | [56] |
| G. hirsutum | 150 kg K2O ha−1 | Lint yield: 13.79% | [91] |
| G. hirsutum | 180.70 kg K2O ha−1 | Lint yield: 5.7% | [92] |
| Solanum tuberosum | 225 kg K2O ha−1 | Tuber weight: 13.34% | [93] |
| S. tuberosum | 225 kg K2O ha−1 | Tuber yield: 78.11% | [94] |
| S. tuberosum | 150 kg K2O ha−1 | Tuber yield: 22.41% | [95] |
| Zea mays | 150 kg K2O ha−1 | Grain yield: 36.33% | [96] |
| Z. mays | 48.19 kg K2O ha−1 | Grain yield: 33.12% | [97] |
| Jatropha curcas | 60 kg K2O ha−1 | Oil yield: 17.30% | [98] |
| Fragaria × ananassa | 64 kg K2O ha−1 | Weight of primary fruits: 6.2% | [99] |
| Fragaria × ananassa | 64 kg K2O ha−1 | Weight of secondary fruits: 6.95% | [99] |
| Cicer arietinum | 95.23 kg K2O ha−1 | Grain yield: 34.50% | [100] |
| Carthamus tinctorius | 95.5 kg K2O ha−1 | Oil yield: 86.84% | [101] |
| Arachis hypogaea | 90.35 kg K2O ha−1 | Seed yield: 44.2% | [102] |
| Saccharum officinarum | 722.82 kg K2O ha−1 | Sugar yield: 30.17% | [103] |
| Betavulgaris | 171.42 kg K2O ha−1 | Root yield: 24.83% | [104] |
| Vigna radiata | 37.5 kg K2O ha−1 | Seed yield: 28.29% | [105] |
| Lycopersicon esculentum | 120 kg K2O ha−1 | Seed yield: 30.9% | [83] |
| Species and Cultivars | Drought Dose and Duration | K Doses | Protective Effects | References |
|---|---|---|---|---|
| Z. mays | Withholding water 31 days (d) after planting | 300 kg ha−1 |
| [128] |
| Hibiscus rosa-sinensis | Water deficit, 21 days | 10 mM |
| [111] |
| Helianthus annuus | Withholding irrigation at the end of growing period | 100 kg ha−1 |
| [117] |
| Camellia sinensis | Field capacity, 5 days | 2% KCl |
| [129] |
| T. aestivum | 15% PEG | 10 mM K2O |
| [124] |
| T. aestivum | 20% PEG, 7 days | 7.5 mM K2CO3 |
| [130] |
| Z. mays | 65 ± 5% water holding capacity of soil | 0.42 g kg−1 soil |
| [125] |
| Hordeum vulgare | 50% soil water content | 10 mM K2CO3 |
| [131] |
| O. sativa | Withholding irrigation 30 days after transplanting, 10 days | 120 kg ha−1 |
| [114] |
| G. hirsutum | At flowering stage withholding water for 8 days followed by 75 ± 5% soil relative water content | 300 kg ha−1 |
| [127] |
| Plant Species | Salinity (NaCl) Doses | K Doses | Protective Effects | References |
|---|---|---|---|---|
| T. aestivum | 100 mM | 10 mM |
| [136] |
| Olea europaea | 100 mM | 100 mM |
| [137] |
| Brassica campestris | 80 mM | 0.54 mM |
| [138] |
| Z. mays | 70 mM | 9 mM |
| [139] |
| H. vulgare | 150 mM | 10 mM |
| [131] |
| L. esculentum | 150 mM | 2.39 mM |
| [140] |
| A. hypogaea | 20 and 40 mM | 30 kg ha−1 |
| [141] |
| S. lycopersicum | 75 mM | 9 mM |
| [142] |
| B. vulgaris | 76 mM | 200 kg ha−1 |
| [143] |
| Plant Species | Metal Doses | K Doses | Protective Effects of K | References |
|---|---|---|---|---|
| Vicia faba | 200 μM Cd, 7 days | 6 mM K, 7 days |
| [155] |
| Prunus persica | 2 mM ZnCl2, 10 days | 10 mM KCl, 10 days |
| [156] |
| Gladiolus grandiflora | 50 mg kg−1 CdSO4·8H2O, 60 days | 200 mg L−1 K along with 200 mg L−1 Si, 60 days |
| [157] |
| Plant Species | Levels of Stresses | K Doses | Protective Effects | References |
|---|---|---|---|---|
| Vigna radiata | Drought (25% and 50% field capacity) | 3 mM |
| [173] |
| L. esculentus | 60 mM NaCl | 5 mM |
| [183] |
| H. rosa-sinensis | Water deficit after 54 days of transplanting | 10 mM |
| [113] |
| T. aestivum | 100 mM NaCl | 16 mM |
| [181] |
| O. sativa | 200 mM NaCl | 11.8 mM |
| [179] |
| T. aestivum | Water deficit at milking stage | 1.5% |
| [81] |
| Z. mays | 70 mM NaCl | 9 mM |
| [139] |
| Solanum lycopersicum | 150 mM NaCl | 9 mM |
| [142] |
| T. aestivum | 8.56 mM NaCl | 0.49 mM |
| [182] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. https://doi.org/10.3390/agronomy8030031
Hasanuzzaman M, Bhuyan MHMB, Nahar K, Hossain MS, Mahmud JA, Hossen MS, Masud AAC, Moumita, Fujita M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy. 2018; 8(3):31. https://doi.org/10.3390/agronomy8030031
Chicago/Turabian StyleHasanuzzaman, Mirza, M. H. M. Borhannuddin Bhuyan, Kamrun Nahar, Md. Shahadat Hossain, Jubayer Al Mahmud, Md. Shahadat Hossen, Abdul Awal Chowdhury Masud, Moumita, and Masayuki Fujita. 2018. "Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses" Agronomy 8, no. 3: 31. https://doi.org/10.3390/agronomy8030031
APA StyleHasanuzzaman, M., Bhuyan, M. H. M. B., Nahar, K., Hossain, M. S., Mahmud, J. A., Hossen, M. S., Masud, A. A. C., Moumita, & Fujita, M. (2018). Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy, 8(3), 31. https://doi.org/10.3390/agronomy8030031










