Polymer-Coated Urea Delays Growth and Accumulation of Key Nutrients in Aerobic Rice but Does Not Affect Grain Mineral Concentrations
Abstract
:1. Introduction
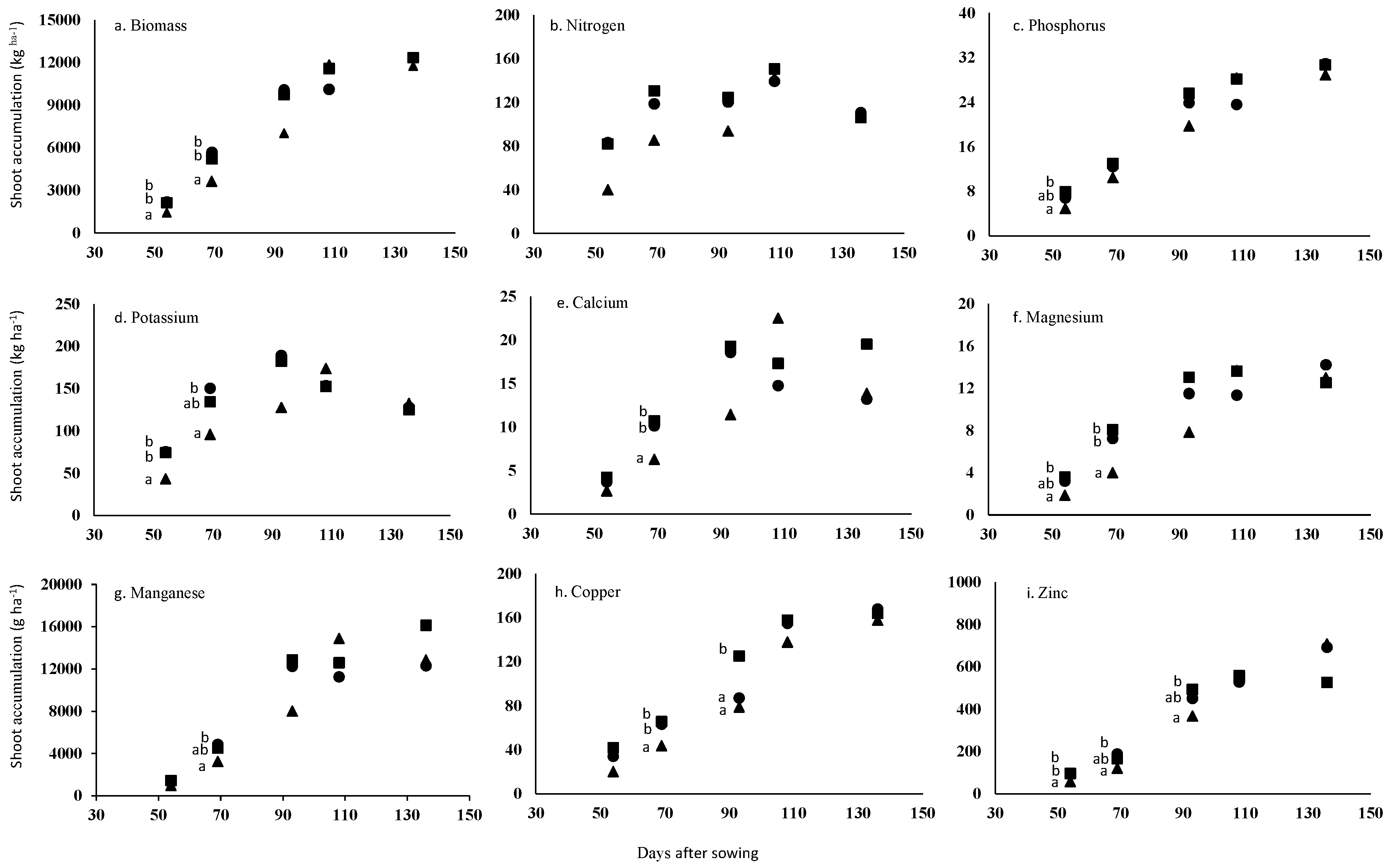
2. Results
| Grain Yield (t·ha−1) | Macronutrients (mg·g−1) | Micronutrients (mg·kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | Cu | Mn | Zn | ||
| Grain nutrient concentration | 5.08 | 12.4 | 3.44 | 4.03 | 0.22 | 1.23 | 20.6 | 197 | 36.1 |
| Nutrient harvest index | 0.42 | 0.55 | 0.58 | 0.16 | 0.07 | 0.47 | 0.64 | 0.07 | 0.29 |
3. Discussion
4. Experimental Section
| Soil Depth (mm) | ||||
|---|---|---|---|---|
| Property | 0–100 | 100–300 | 300–600 | 600–900 |
| Basic texture | clay loam | clay | clay | clay |
| Total carbon (%) | 2.3 | 2.1 | 1.1 | 2.1 |
| Total nitrogen (%) | 0.18 | 0.16 | 0.09 | 0.21 |
| KCl extractable ammonium (mg·kg−1) | 9.9 | 11.3 | 12.5 | 12.2 |
| KCl extractable nitrate (mg·kg−1) | 0.90 | 1.3 | 0.9 | 1.2 |
| pH (1:5 water) | 5.57 | 5.50 | 5.35 | 5.50 |
| EC (dS·m−1) | 0.04 | 0.05 | 0.06 | 0.07 |
| Bray 1 phosphorus (mg·kg−1) | 14.8 | 6.0 | 5.9 | 1.5 |
| Total acid extractable sulfur (mg·kg−1) | 411 | 427 | 806 | 379 |
| Cation exchange capacity (cmol+·kg−1) | 27.3 | 27.3 | 25.0 | 26.3 |
| Base cations (%) | ||||
| Calcium | 54.3 | 52.0 | 42.0 | 41.7 |
| Magnesium | 36.8 | 36.8 | 40.9 | 40.0 |
| Potassium | 2.3 | 1.8 | 1.5 | 1.2 |
| Sodium | 1.3 | 1.6 | 2.3 | 4.9 |
| Aluminium | 3.2 | 4.7 | 8.6 | 8.3 |
| DPTA-extractable micronutrients | ||||
| Zinc (mg·kg−1) | 2.5 | 1.3 | 1.3 | 0.9 |
| Manganese (mg·kg−1) | 13 | 9 | 8 | 4 |
| Iron (mg·kg−1) | 221 | 209 | 177 | 127 |
| Copper (mg·kg−1) | 1.0 | 0.6 | 0.9 | not detectable |
4.1. Measurements
4.2. Statistical Analyses
5. Conclusions
Conflicts of Interest
References
- Timilsena, Y.P.; Adhikari, R.; Casey, P.; Muster, T.; Gill, H.; Adhikari, B. Enhanced efficiency fertilizers: A review of formulation and nutrient release patterns. J. Sci. Food Agric. 2015, 95, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Suter, H.; Islam, A.; Edis, R.; Freney, J.R.; Walker, C.N. Prospects of improving efficiency of fertilizer nitrogen in Australian agriculture: A review of enhanced efficiency fertilizers. Soil Res. 2008, 46, 289–301. [Google Scholar] [CrossRef]
- Burkitt, L.L. A review of nitrogen losses due to leaching and surface runoff under intensive pasture management in Australia. Soil Res. 2014, 52, 621–636. [Google Scholar] [CrossRef]
- Zhou, M.; Butterbach-Bahl, K. Assessment of nitrate leaching loss on a yield-scaled basis from maize and wheat cropping systems. Plant Soil 2014, 374, 977–991. [Google Scholar] [CrossRef]
- Macdonald, B.C.T.; Denmead, O.T.; White, I. Quantification of NOx and NH3 emissions from two sugarcane fields. Soil Res. 2014, 52, 833–840. [Google Scholar] [CrossRef]
- Schwenke, G.D.; Manning, W.; Haigh, B.M. Ammonia volatilisation from nitrogen fertilizers surface-applied to bare fallows, wheat crops and perennial-grass-based pastures on Vertosols. Soil Res. 2014, 52, 805–821. [Google Scholar] [CrossRef]
- Nelson, K.A.; Motavalli, P.P.; Nathan, M. Nitrogen fertilizer sources and application timing affects wheat and inter-seeded red clover yields on claypan soils. Agronomy 2014, 4, 497–513. [Google Scholar] [CrossRef]
- Shenwu, L.; Wang, X.; Liu, G. A simple and reasonable calculation equation of balanced fertilization. Agronomy 2015, 5, 180–187. [Google Scholar]
- Mahli, S.S.; Soon, Y.K.; Grant, C.A.; Lemke, R.; Lupwayi, N. Influence of controlled-release urea on seed yield and N concentration and N use efficiency of small grain crops grown on Dark Gray Luvisols. Can. J. Soil Sci. 2010, 90, 363–372. [Google Scholar]
- Grant, C.A.; Wu, R.; Selles, F.; Harker, F.N.; Clayton, G.W.; Bittman, S.; Zebarth, B.J.; Lupwayi, N.Z. Crop yield and nitrogen concentration with controlled release urea and split applications of nitrogen as compared to non-coated urea applied at seeding. Field Crop Res. 2012, 127, 170–180. [Google Scholar] [CrossRef]
- Gao, X.; Asgedom, H.; Tenuta, M.; Flaten, D.N. Enhanced efficiency urea sources and placement effects on nitrous oxide emissions. Agron. J. 2015, 107, 265–277. [Google Scholar] [CrossRef]
- Rose, T.J.; Bloomfield, C.; Raymond, C.; King, G.J. Perturbation of nutrient source-sink relationships by post-anthesis stresses results in differential accumulation of key nutrients into wheat grain. J. Plant Nutr. Soil Sci. 2015, 178, 89–98. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Chalk, P.M.; Craswell, E.T.; Polidoro, J.C.; Chen, D. Fate and efficiency of 15N-labelled slow- and controlled release fertilizers. Nutr. Cycl. Agroecosyst. 2015. [Google Scholar] [CrossRef]
- Dunn, B.W.; Dunn, T.S.; Beecher, H.G. Nitrogen timing and rate effects on growth and grain yield of delayed permanent-water rice in south-eastern Australia. Crop Pasture Sci. 2014, 65, 878–887. [Google Scholar] [CrossRef]
- Chen, D.; Suter, H.; Islam, A.; Edis, R. Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea. Soil Biol. Biochem. 2010, 42, 660–664. [Google Scholar] [CrossRef]
- Weiske, A.; Benckiser, G.; Ottow, J.C.G. Effect of the new nitrification inhibitor DMPP in comparison to DCD on nitrous oxide (N2O) emissions and methane (CH4) oxidation during 3 years of repeated applications in field experiments. Nutr. Cycl. Agroecosyst. 2001, 60, 57–64. [Google Scholar] [CrossRef]
- Garcia, A.G.; Douradeo-Neto, D.; Basanta, M.D.V.; Ovejero, R.F.L.; Favarin, J.L. Logistic rice model for dry matter and nutrient uptake. Sci. Agric. 2003, 60, 481–488. [Google Scholar]
- Rose, T.J.; Rengel, Z.; Bowden, J.; Ma, Q. Differential accumulation of phosphorus and potassium by canola cultivars compared to wheat. J. Soil Sci. Plant Nutr. 2007, 170, 404–411. [Google Scholar] [CrossRef]
- Dobermann, A.; Fairhurst, T.H. Nutrient Disorders and Nutrient Management; Potash and Phosphate Institute, Potash and Phosphate Institute of Canada and International Rice Research Institute: Singapore, 2000. [Google Scholar]
- Ma, Q.; Rengel, Z.; Rose, T.J. The effectiveness of deep placement of fertilizers is determined by crop species and edaphic conditions in Mediterranean-type environments: A review. Aust. J. Soil Res. 2009, 47, 19–32. [Google Scholar] [CrossRef]
- Rayment, G.E.; Lyons, D.J. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata Press: Port Melbourne, Australia, 2011. [Google Scholar]
- Huang, J.; Nhan, T.; Wong, V.N.L.; Johnston, S.G.; Lark, M.; Triantafilis, J. Digital soil mapping of a coastal acid sulfate soil landscape. Soil Res. 2014, 52, 327–339. [Google Scholar] [CrossRef]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rose, T.J. Polymer-Coated Urea Delays Growth and Accumulation of Key Nutrients in Aerobic Rice but Does Not Affect Grain Mineral Concentrations. Agronomy 2016, 6, 9. https://doi.org/10.3390/agronomy6010009
Rose TJ. Polymer-Coated Urea Delays Growth and Accumulation of Key Nutrients in Aerobic Rice but Does Not Affect Grain Mineral Concentrations. Agronomy. 2016; 6(1):9. https://doi.org/10.3390/agronomy6010009
Chicago/Turabian StyleRose, Terry J. 2016. "Polymer-Coated Urea Delays Growth and Accumulation of Key Nutrients in Aerobic Rice but Does Not Affect Grain Mineral Concentrations" Agronomy 6, no. 1: 9. https://doi.org/10.3390/agronomy6010009
APA StyleRose, T. J. (2016). Polymer-Coated Urea Delays Growth and Accumulation of Key Nutrients in Aerobic Rice but Does Not Affect Grain Mineral Concentrations. Agronomy, 6(1), 9. https://doi.org/10.3390/agronomy6010009






