Abstract
The low fertility of tropical Oxisols challenges sustainable agriculture. While biochar-based granular fertilizers (BBGFs) offer a solution, the influence of different organic binders is unclear. This study investigated how BBGFs formulated with bio-oil (BO), pyroligneous extract (PE), and cassava wastewater (CW) impact soil enzyme activities and nutrient dynamics over time. Eucalyptus biochar (B) and natural phosphate (NP) were granulated with three binders at four doses. These treatments, plus controls (unfertilized soil, NP, B with NP, and B alone), were incubated in an Oxisol, assessing soil samples after 10 and 40 days of incubation. All BBGFs significantly enhanced β-glucosidase, acid phosphatase, and arylsulfatase activities over controls, with increases exceeding 8%. While the BBGFs-BO formulation sustained the highest enzymatic activity, BBGFs-PE at 125% maximized acid phosphatase at 10 days, with a subsequent decline, and inhibited arylsulfatase at the 150% dose. BBGFs-CW was most effective for increasing P availability (up to 24.0 mg kg−1). BBGFs-BO and BBGFs-PE also enhanced soil organic carbon and cation exchange capacity by up to 430% and 163%, respectively. The BBGFs-BO at 150% dose is the most effective and stable formulation to enhance nutrient cycling and soil health, offering a viable pathway to convert agricultural residues into high-value fertilizers.
1. Introduction
Sustainable soil management is crucial to meeting modern agricultural demands, particularly in the context of climate change and growing pressure for more efficient practices [1]. Tropical soils, such as Oxisols, are highly weathered and characterized by low natural fertility due to high acidity and low nutrient and organic matter contents [2,3]. Intensive agriculture in tropical soils favors the mineralization of organic matter and, consequently, increased acidity, aluminum toxicity, and nutrient leaching. The reduction in organic matter also affects soil particle aggregation, aggregate stability, and water retention and storage capacity [4]. Furthermore, the predominance of Fe and Al oxyhydroxides and kaolinite in the clay fraction, combined with high acidity, favors phosphorus fixation reactions, significantly reducing phosphorus availability and the efficiency of phosphate fertilizers [5].
Improving soil organic matter through carbon-rich amendments represents a critical strategy to counteract these limitations and restore fertility in highly weathered tropical soils [6]. In this context, biochar emerges as a particularly promising amendment due to its ability to enhance key soil properties, boost agricultural productivity, and provide environmental benefits, including mitigation of greenhouse gas emissions and reduced carbon footprint [7].
Biochar is a solid carbonaceous product obtained through the pyrolysis of organic materials under low or no oxygen conditions, exhibiting significant properties such as high carbon stability, porosity, and specific surface area [4]. These characteristics improve soil chemical, physical, and biological conditions, including increased cation exchange capacity (CEC), carbon sequestration, elevation of pH in acidic and highly weathered soils, enhanced nutrient cycling, and reduced nutrient leaching losses [6,8,9]. Furthermore, biochar application has been associated with increased soil microbial activity, a key indicator for assessing soil ecosystem health [10,11].
Biochar offers several benefits for soil properties and for the remediation of soils and waters contaminated by potentially toxic elements, as heavy metals [12] and organic pollutants, especially emerging pollutants such as perfluoroalkyl and polyfluoroalkyl substances (PFASs), which pose serious risks to the ecosystem and human health [13]. However, there is a risk that biochar itself may add pollutants to the soil, resulting from the raw material and the pyrolysis process, with negative effects on the environment, plants, animals, microorganisms, and even human health [14]. Given this scenario, it is important to monitor the potential impacts of biochar-based fertilizers using indicators sensitive to environmental pollution, such as soil enzyme activity.
Soil enzymatic activity, mediated by microbial extracellular enzymes such as β-glucosidase, phosphatases, and arylsulfatase, plays a pivotal role in organic matter decomposition and nutrient cycling, catalyzing oxidative and hydrolytic reactions [15]. These processes are directly influenced by soil management practices and input applications, making them widely used as indicators to evaluate the impacts of fertilization strategies and land use [16,17].
β-Glucosidase catalyzes the hydrolysis of maltose and cellobiose, yielding glucose. Phosphatases facilitate phosphorus mineralization by hydrolyzing esters and anhydrides of phosphoric acid, while arylsulfatase hydrolyzes organic sulfate esters in the soil, contributing to the release of bioavailable sulfur forms [18,19]. In Brazil, the enzymes β-glucosidase and arylsulfatase are used by farmers to evaluate agricultural soil quality, as they are related, directly or indirectly, to the productive potential and sustainability of land use [16,17].
The production of biochar-based granular fertilizers (BBGFs) using alternative binders such as bio-oil (BO), pyroligneous extract (PE)—byproducts of charcoal production—and cassava wastewater (CW), a residue from cassava starch production, represents a promising approach for tropical soils [20]. CW is a residue produced in large volumes in the cassava starch agroindustry, rich in nutrients and with great polluting potential if disposed of inappropriately. In addition to increasing fertilizer granule stability to improve application quality, alternative binders have the potential to reduce production costs, promote controlled nutrient release, and improve plant nutrient use efficiency [20,21,22]. This strategy increases soil nutrient bioavailability and contributes to the sustainability of agricultural systems.
Although the impact of biochar on soil microbiota is not yet fully understood, its physicochemical properties are known to vary depending on feedstock materials and pyrolysis conditions [9]. These properties directly influence microorganisms, which are sensitive to environmental changes and serve as indicators of soil quality [17]. Thus, investigating how biochar, particularly in BBGFs formulations, affects microbial activity is essential for optimizing its application in sustainable soil fertility management.
We hypothesize that incorporating alternative binders, such as CW, PE, and BO, into BBGFs alters soil enzymatic activity and chemical properties. Although these binders provide carbon and nutrients, PE and BO may contain toxic pyrolysis-derived compounds that harm soil microorganisms, while CW may undergo rapid mineralization. To evaluate the toxicity of PE and BO, soil enzymes were evaluated in comparison to CW, which is supposedly non-toxic and favors soil microbial activity. Soil enzymes are widely used as indicators of soil health due to their strong correlation with soil quality, and are significantly influenced by different agricultural practices, such as organic amendments [10,23]. Thus, the objective of this study was to evaluate alternative binders to produce BBGFs and their respective effects on soil chemical properties and enzymatic activity, as indicators of soil health over time.
2. Materials and Methods
2.1. Biochar Production
Biochar was produced through slow pyrolysis of Eucalyptus urograndis wood in a kiln-furnace system (Figure S1A) located at the Institute of Agricultural Sciences, Federal University of Minas Gerais, Montes Claros, MG, Brazil, whose geographical coordinates are 16°41′2.03″ S, 43°50′19.28″ W, altitude 646 m. The biochar consisted of fines generated during the pyrolysis process, conducted at 450 °C with a heating rate of 0.1 °C min−1 and a residence time of six days (Figure S1B). Furnace temperatures were monitored using an infrared sensor every 30 min during the heating and drying stages and every 8 h during the cooling stage [24].
2.2. Physical and Chemical Characteristics of Biochar and Binders
After mechanical grinding, the biochar was sieved through a 0.25 mm mesh to standardize particle size. The chemical and physical properties of the biochar were characterized, as shown in Table 1, with methodological details described by [20]. Briefly, pH and electrical conductivity (EC) were determined in a biochar:deionized water solution (1:20, w/v) after shaking for 1.5 h on an orbital shaker. Bulk density was calculated by filling a 10 mL graduated cylinder with biochar, compacting it by shaking for 1 min, and weighing the sample. Moisture, volatile matter, ash, and fixed carbon (FC) contents were determined by heating in a muffle furnace according to the protocol in ASTM D1762-84 [25]. Total carbon and nitrogen contents were analyzed using the wet oxidation method with potassium dichromate followed by titration with ferrous sulfate and the Kjeldahl method, respectively [26]. Oxygen and hydrogen contents were estimated based on correlations established by [27]. Samples were subjected to acid digestion with a 3:1 (v/v) nitric-perchloric acid solution in a digestion block for nutrient determination. Phosphorus was quantified using the phosphomolybdovanadate method, potassium, and sodium by flame photometry (B 462, Micronal S.A., Osasco, SP, Brazil), sulfur by the turbidimetric method with barium chloride, and other nutrients by atomic absorption spectrometry (Varian AAS 240 FS, Agilent Technologies, Palo Alto, CA, USA).

Table 1.
Proximate, ultimate analysis and physicochemical characteristics of biochar obtained from eucalyptus wood fines generated as byproducts after pyrolysis.
The three binders used in this study (CW, PE and BO) were obtained from different agro-industrial processes. The CW is a liquid residue acquired from local agro-industries in Montes Claros, MG, Brazil, generated during the processing of cassava roots for starch production.
The PE and BO binders were obtained as byproducts from the same slow pyrolysis process in the kiln-furnace system used for biochar production. The vapors released during wood pyrolysis were channeled, cooled, and condensed into a raw liquid. This liquid was then stored and left undisturbed for a six-month period, allowing for spontaneous gravitational separation of its phases. After decantation, two distinct fractions were separated: the dense, viscous, water-insoluble phase (pyrolysis tar) that settled at the bottom was collected as BO, while the overlying aqueous phase was collected as PE.
The binders were characterized for pH, EC, and total solids (Table 2). The pH and EC were measured directly in liquid samples of CW and PE. For BO samples (liquid-pasty consistency), a 1:5 (BO:distilled water, v/v) ratio was used, with measurements performed on the aqueous extract [26]. Total solids were determined according to the protocol in ASTM E1756-08 [28], involving heating 50 mL of the sample in porcelain crucibles in a water bath at 65 °C for 16 h, followed by drying in an oven at 103 °C for 24 h. After drying, the crucibles were cooled in desiccators to determine the mass of solids.

Table 2.
Basic properties of binders used to produce biochar-based granular fertilizers.
Fourier-transform infrared (FTIR) spectroscopy was employed to identify the main functional groups present in the biochar and binders. Spectra were recorded using a Cary 640 FTIR spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA), operating in the 4000–500 cm−1 range, with a resolution of 2 cm−1 and 64 scans per sample. The potassium bromide (KBr)-pressed pellet technique was applied, in which 1 mg of dried sample was mixed with 100 mg of spectroscopic-grade KBr (Merck and Co., Rahway, NJ, USA) and compressed into a pellet for analysis.
2.3. Biochar-Based Fertilizers Production
Biochar-based granular fertilizers (BBGFs) were produced using a disk granulator, employing a 3:1 (w/w) ratio of biochar to reactive natural phosphate (NP) sourced from phosphorites containing 27% total P2O5 and 8.1% P2O5 soluble in 2% citric acid. Binders were applied by spraying onto the biochar-NP mixture, followed by a homogenization process lasting approximately 40 min at 32.98 rad s−1.
The binder quantity was determined as a percentage of the physical mixture (biochar + NP) weight, at proportions of 75, 100, 125, and 150%. For the 75% dose, 1 kg of the mixture (750 g biochar + 250 g NP) was combined with 750 mL of binder, with increments of 250 mL for each 25% increase. After granulation, the BBGFs were dried in an oven at 65 ± 5 °C for approximately 72 h until constant weight. The granules were cooled to room temperature and stored in plastic containers.
2.4. Experimental Design
Two experiments were conducted to evaluate the activities of β-glucosidase, acid phosphatase, and arylsulfatase enzymes, as well as the chemical properties of soil fertilized with BBGFs. Experiment 1 involved a 10-day incubation period of the fertilizers in the soil, while Experiment 2 extended to 40 days (Figure 1).
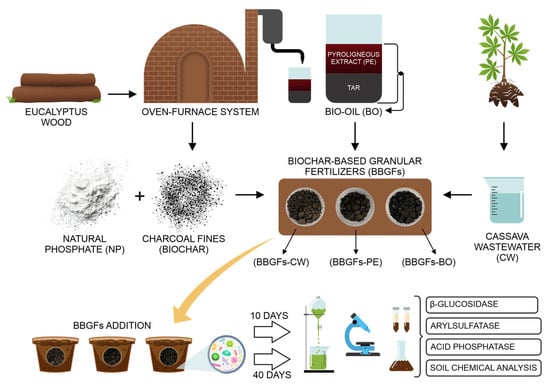
Figure 1.
Experimental methodology for testing biochar-based granular fertilizers (BBGFs) in a tropical Oxisol.
Both experiments were conducted in a completely randomized design, with treatments arranged in a 4 × 3 + 4 factorial scheme with three replications, totaling 48 experimental units. The treatments comprised four binder proportions for granule production (75, 100, 125, and 150%), three binder types (CW, PE, and BO), and four additional treatments: natural soil or unfertilized soil (NS), reactive natural phosphate (NP), biochar and natural phosphate (B + NP), and biochar alone (B).
2.5. Soil Enzyme Activity and Chemical Analysis
In both experiments, pots were filled with 100 cm3 of a dystrophic Oxisol (Red Yellow Latosol, according to the Brazilian Soil Classification System) collected from the 0–20 cm layer under Cerrado biome (largest savanna region in South America). Soil samples were air-dried and sieved through 2.0 mm. The soil exhibited low fertility, with available P and K+ contents of 0.9 and 13.7 mg dm−3 (Mehlich-1 method), respectively, pH 5.2 (water), and exchangeable Ca2+ and Mg2+ concentrations of 0.3 and 0.1 cmolc kg−1 (1 M KCl extraction), respectively. Soil organic carbon (SOC) was 0.1 dag kg−1, measured via loss on ignition according to [29].
Soil acidity was corrected with a mixture of calcium carbonate and magnesium carbonate in a molar ratio of 3:1 (Ca:Mg) to increase soil base saturation to 70%. The soil of each experimental plot was incubated with the carbonate mixture for 30 days, maintaining soil moisture levels close to field capacity. After this period, the different BBGFs were mixed and homogenized with the soil of each experimental unit (pots containing 100 cm3 of soil).
The amount of BBGFs applied to the soil was determined based on the dry mass produced from the granule preparation mixture, considering the respective binder doses for BBGFs-CW, BBGFs-PE, and BBGFs-BO, to supply 200 mg dm−3 of phosphorus to the soil [30]. This calculation accounted for the composition of the natural phosphate, containing 27% P2O5.
The additional treatments containing B and NP, applied individually or in combination, were incorporated into the soil conventionally (non-granulated). For the combined application, a 3:1 (B:NP, w/w) ratio was used, consistent with that adopted for BBGFs production.
At the end of Experiment 1 (10 days) and Experiment 2 (40 days), 30 g soil samples were collected from each experimental unit for enzymatic analyses. Samples were sieved through a 1 mm mesh to remove roots and plant debris, stored in plastic bags, and kept at 4 °C until analysis, which occurred within approximately one week of collection [10,15]. In both experiments, soil samples from each treatment were chemically characterized for pH in water, P-Meh (Mehlich-1 P), P-Res (P-Resin), SOC, K+, Ca2+, Mg2+, S, Fe2+, and Zn2+, following the methodologies of [31]. The CEC was calculated from the results obtained.
The activities of β-glucosidase, acid phosphatase, and arylsulfatase were assessed in triplicate, including a control, according to the method described by [19]. This method involves incubating soil samples for 1 h at 37 °C in buffered solutions containing the substrates p-nitrophenyl-β-D-glucoside, p-nitrophenyl disodium phosphate, and p-nitrophenyl sulfate, respectively. Due to the short incubation period, toluene was omitted from the analyses [16]. After incubation, the amount of p-nitrophenol (PNP) released from the enzymatic reaction was determined, expressed as mg PNP kg−1 dry soil h−1, using UV-visible spectrophotometry.
2.6. Statistical Analysis
The Shapiro–Wilk test was used to verify data normality. Data were subjected to analysis of variance (ANOVA) using the F-test (p < 0.05). In cases of significance among binder doses, regression analysis was performed. The best models were selected based on the significance of model parameters and the highest coefficient of determination (R2). Additional treatments were compared with other treatments, regardless of doses, using Dunnett’s test (p < 0.05). Additionally, soil chemical and enzymatic variables were subjected to Principal Component Analysis (PCA) and Pearson’s correlation matrix. Statistical analyses were conducted using R software, version 4.2.2.
3. Results
3.1. Physical and Chemical Analysis
The main physico-chemical properties of the eucalyptus biochar are presented in Table 1. The biochar exhibited an alkaline pH in water (8.9) and a high ash content of 50.6%. The ultimate analysis showed a total carbon content of 50.7% and a high C/N ratio of 255. Among the mineral elements, Ca was the most abundant at 50.6 g kg−1, followed by Fe at 17.5 g kg−1 and Mg at 4.2 g kg−1.
The FTIR spectra identified the main functional groups present in the biochar and binders (Figure 2). A broad O-H stretching band, characteristic of hydroxyl groups, was observed in all materials between 3450 and 3300 cm−1. Aliphatic C-H stretching was also evident around 3000–2900 cm−1. The binders, particularly PE and BO, were distinguished by a strong C=O stretching peak in the 1750–1600 cm−1 region, associated with ketone and carbonyl groups from the thermal decomposition of cellulose and lignin. The BO spectrum also showed more pronounced peaks in the C-O stretching and aromatic C-H bending region (1100–700 cm−1), confirming a more complex and aromatic structure.
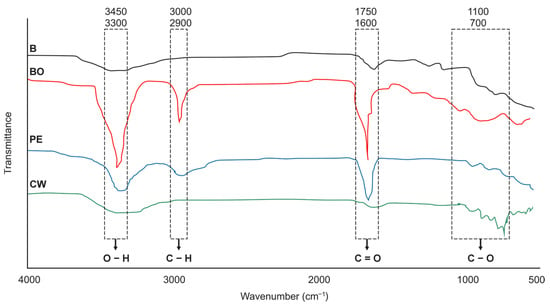
Figure 2.
Fourier-transform infrared (FTIR) spectra of biochar (B), cassava wastewater (CW), pyroligneous extract (PE), and bio-oil (BO). The peak assignments are noted above their respective peaks.
3.2. β-Glucosidase
The activity of β-glucosidase in soil amended with BBGFs increased linearly with binder doses after 10 and 40 days of incubation (Figure 3A,B). At 10 days, the highest activities were observed at the 150% dose, with values of 65.38, 105.16, and 134.13 mg PNP kg−1 soil h−1 for BBGFs-CW, BBGFs-PE, and BBGFs-BO, respectively (Figure 3A). After 40 days, the activity decreased across all treatments, but BBGFs-BO at 150% maintained the highest value (134.58 mg PNP kg−1 soil h−1), while BBGFs-CW and BBGFs-PE showed lower activities (47.64 and 58.87 mg PNP kg−1 soil h−1, respectively) (Figure 3B).
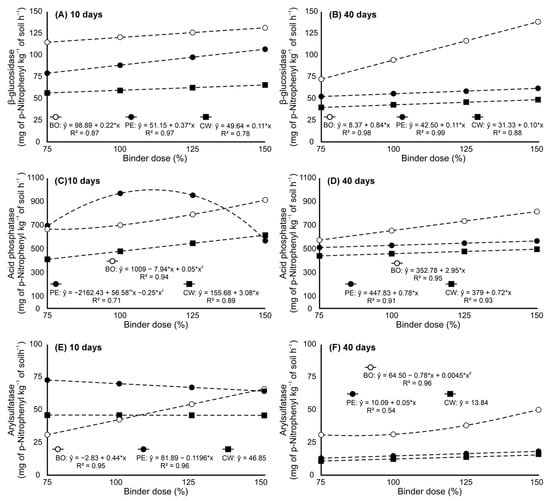
Figure 3.
Soil enzyme activity in response to increasing doses of different binders in biochar-based granular fertilizers (BBGFs). The experiment was conducted in a tropical Oxisol, with measurements taken after 10 and 40 days of incubation. The panels show the activity of: (A) β-glucosidase at 10 days; (B) β-glucosidase at 40 days; (C) acid phosphatase at 10 days; (D) acid phosphatase at 40 days; (E) arylsulfatase at 10 days; (F) arylsulfatase at 40 days. * significant by t-test (p-value < 0.05).
3.3. Acid Phosphatase
Acid phosphatase activity varied depending on the binder type and dose (Figure 3C,D). Enzymatic activity increased linearly with application dose of BBGFs-CW after 10 days of soil incubation, reaching 645.52 mg PNP kg−1 soil h−1 at 150% (Figure 3C). In contrast, BBGFs-PE and BBGFs-BO amendments produced quadratic dose–response curves in soil enzyme activity, with a peak activity of 1082.22 mg PNP kg−1 soil h−1 at 125% for BBGFs-PE, followed by a decrease to 569.84 mg PNP kg−1 soil h−1 at 150%, while the highest values for treatments with BBGFs-BO were observed at the 150% dose (914.62 mg PNP kg−1 soil h−1) (Figure 3C). After 40 days of incubation, soil enzyme activity increased linearly with binder dose for all BBGFs formulations, with BO-amended soils at 150% showing the highest activity (811.54 mg PNP kg−1 soil h−1), followed by BBGFs-PE- (558.62 mg PNP kg−1 soil h−1) and BBGFs-CW-treated soils (490.20 mg PNP kg−1 soil h−1) (Figure 3D).
3.4. Arylsulfatase
After 10 days of incubation, soils fertilized with BBGFs-CW maintained stable arylsulfatase activity (46.85 mg PNP kg−1 soil h−1) regardless of application dose (Figure 3E). In contrast, increasing doses of BBGFs-PE significantly reduced arylsulfatase activity in soils from 72.55 mg PNP kg−1 soil h−1 (75% dose) to 64.17 mg PNP kg−1 soil h−1 (150% dose) (Figure 3E). After 40 days, soils with BBGFs-BO exhibited a quadratic response, peaking at 50.27 mg PNP kg−1 soil h−1 at 150%, and soils with BBGFs-PE demonstrated a linear increase in the levels of arylsulfatase activity, reaching peak values at the 150% dose (18.53 mg PNP kg−1 soil h−1), while treatments with BBGFs-CW showed no significant dose effect, with mean levels of 13.84 mg PNP kg−1 soil h−1 (Figure 3F).
3.5. Comparative Effects of BBGFs on Soil Enzyme Activities
All BBGFs significantly enhanced soil enzyme activities for β-glucosidase, acid phosphatase and arylsulfatase, compared to the control treatments, with higher values observed at 10 days than at 40 days across all treatments and enzymes (Figure 4A–C). The mean β-glucosidase activity across binder doses was significantly higher in soils with BBGFs-BO (123.47 and 102.64 mg PNP kg−1 soil h−1 at 10 and 40 days, respectively) compared to BBGFs-PE (92.43 and 54.70 mg PNP kg−1 soil h−1) and BBGFs-CW (61.99 and 42.98 mg PNP kg−1 soil h−1) (Figure 4A). For acid phosphatase, the highest values were observed in soils fertilized with BBGFs-PE (793.36 and 535.78 mg PNP kg−1 soil h−1 at 10 and 40 days, respectively) and BBGFs-BO (764.50 and 685.08 mg PNP kg−1 soil h−1) compared to BBGFs-CW (502.61 and 459.66 mg PNP kg−1 soil h−1) (Figure 4B). Arylsulfatase activity was highest in BBGFs-PE treated soils at 10 days (68.43 mg PNP kg−1 soil h−1), while BBGFs-BO achieved greater activity at 40 days (37.75 mg PNP kg−1 soil h−1) (Figure 4C).
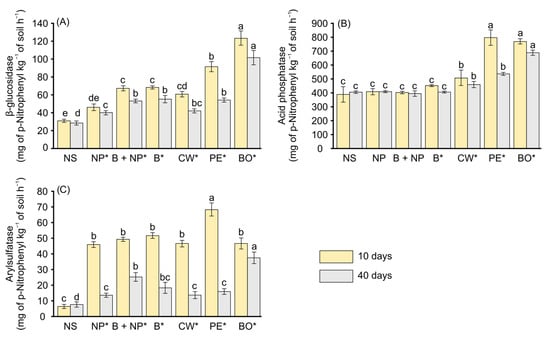
Figure 4.
Mean enzymatic activity in a tropical Oxisol treated with biochar-based granular fertilizers (BBGFs) using different binders and controls (unfertilized soil, NS; natural phosphate, NP; biochar, B; biochar + natural phosphate, B + NP) after 10 and 40 days of incubation. The panels show the activity of: (A) β-glucosidase; (B) acid phosphatase; (C) arylsulfatase. Different letters on bars of the same color indicate significant differences among treatments (Tukey’s test, p < 0.05). An asterisk (*) indicates significant difference between incubation periods (10 and 40 days) for the same treatment (F-test, p < 0.05).
3.6. Principal Component Analysis of Soil Properties and Enzyme Activities over Time
PCA was performed to establish relationships between different BBGFs, soil chemical properties, and soil enzymatic activity, considering incubation periods of 10 and 40 days. Regarding soil chemical properties, the first two principal components (PC1 and PC2) accounted for approximately 70% of the total variation after 10 days of incubation (Figure 5A), while at 40 days, these components explained 65.50% of the variability (Figure 5B). Concerning soil enzymatic activity, it was observed that, in both incubation periods, the first two principal components explained more than 78% of the total variation (Figure 5C,D).
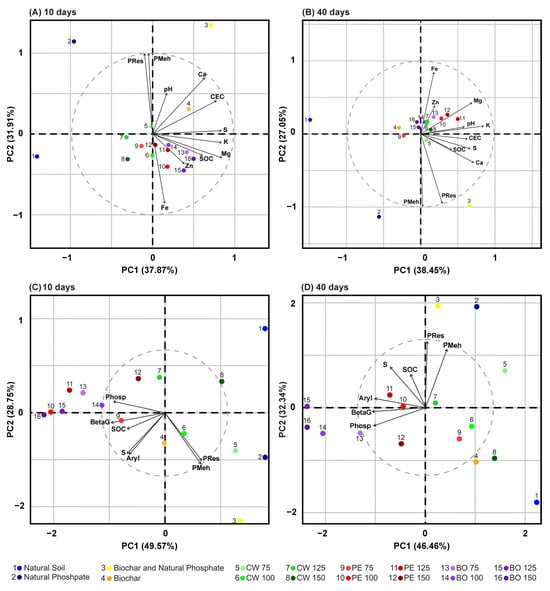
Figure 5.
Principal component analysis (PCA) of the effects of different fertilizer treatments on soil properties in a tropical Oxisol. The analysis includes biochar-based granular fertilizers (BBGFs) with cassava wastewater (CW), pyroligneous extract (PE), or bio-oil (BO) binders, and control treatments (unfertilized soil, NS; natural phosphate, NP; biochar, B; biochar + natural phosphate, B + NP). The panels display the effects on: (A) soil nutrient dynamics at 10 days; (B) soil nutrient dynamics at 40 days; (C) enzyme activity at 10 days; (D) enzyme activity at 40 days.
At 10 days of incubation, PCA revealed that soils treated with BBGFs-PE at doses of 100, 125, and 150% and BBGFs-BO at doses of 75 to 150% showed an association with SOC, Mg, Fe, Zn, S and K (Figure 5A). Treatments with BBGFs-CW at a dose of 75% exhibited an association with P-Meh and P-Res. Soils receiving additional treatments with biochar, either alone or combined with NP, were associated with Ca, P-Meh, P-Res, CEC, and pH (Figure 5A). Furthermore, significant positive correlations were found among the studied soil chemical properties at 10 days of incubation: P-Meh and P-Res (r = 0.89); CEC and S (r = 0.84); K and S (r = 0.65); K and Ca (r = 0.61); K and Mg (r = 0.72); Ca and CEC (r = 0.73); S and Mg (r = 0.69); and SOC and Mg (r = 0.55) (Figure 6A).
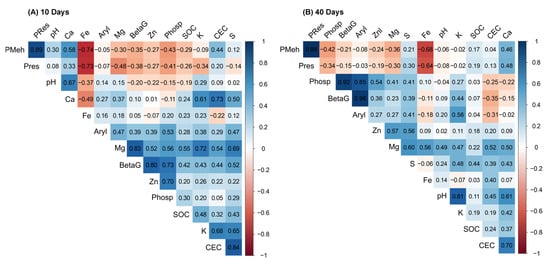
Figure 6.
Pearson correlation matrix of the relationships between soil chemical properties and enzymatic activities in a tropical Oxisol after the application of different biochar-based granular fertilizers (BBGFs). Positive correlations are shown in blue and negative correlations in red, with color intensity being proportional to the correlation coefficient (r). The panels display the correlations at: (A) 10 days of incubation; and (B) 40 days of incubation.
At 40 days of incubation, PCA showed that soils treated with BBGFs containing PE at doses of 100, 125, and 150%, BO at doses of 75 and 100%, and CW at doses of 100, 125, and 150% were associated with K, Mg, Zn, Fe, pH, and CEC (Figure 5B). Additionally, soils incorporated with BBGFs-CW at a 75% dose exhibited an association with SOC, P-Res, P-Meh, Ca, S, and CEC (Figure 5B). Most soils with BBGFs displayed a negative correlation with P-Meh and P-Res, in contrast to soils treated with NP, applied either alone or combined with biochar, and those amended with BBGFs-CW at the 75% dose (Figure 5A,B). The Pearson correlation analysis of soil chemical properties at 40 days revealed the following significant positive associations: P-Meh and P-Res (r = 0.99); Ca and pH (r = 0.61); K and pH (r = 0.81); CEC and Ca (r = 0.70); Mg and Zn (r = 0.57); Mg and Ca (r = 0.50); Mg and CEC (r = 0.52); Mg and S (r = 0.60); and S and Zn (r = 0.56) (Figure 6B).
According to the biplot, overall, at 10 and 40 days of incubation, soils treated with BBGFs containing PE and BO showed a stronger association with the activity of the enzymes β-glucosidase, acid phosphatase and arylsulfatase, except for soils amended with BBGFs-PE at a 75% dose, which exhibited a negative correlation with these enzymes at 40 days (Figure 5C,D). The analysis of the Pearson’s correlation matrix showed a positive correlation between acid phosphatase and β-glucosidase (r ≥ 0.73), arylsulfatase (r ≥ 0.53), and Zn (r ≥ 0.54). In contrast, a negative correlation was found between Fe and both P-Meh and P-Res (r ≤ −0.64), and between acid phosphatase activity and P-Meh (r ≤ −0.42), across both incubation periods (Figure 6A,B).
4. Discussion
The linear increase in β-glucosidase activity with increasing doses of BBGFs-CW, BBGFs-PE, and BBGFs-BO in granulated fertilizers, observed at both incubation periods, suggests that these materials release a wide variety of organic compounds, including phenolic compounds, aldehydes, ketones, alcohols, organic acids, and readily accessible substrates such as carbohydrates and proteins. These compounds likely stimulate soil microbial activity, enhancing enzyme production [32,33,34,35].
The wide variety of organic compounds is supported by the FTIR spectra of the biochar and binders, which exhibit characteristic functional groups related to such compounds. The spectra exhibit typical O-H stretching bands in the range of 3450–3300 cm−1 (Figure 2), attributed to hydroxyl groups present in phenols, alcohols, carboxylic acids, or adsorbed water molecules [3,36]. The vibrational region between 3000 and 2900 cm−1 (Figure 2) corresponds to C-H stretching, indicative of aliphatic compounds, and the region between 1750 and 1600 cm−1 is associated with ketone groups (C=O) and aromatic structures [37].
These findings align with the observations of [10], who reported elevated β-glucosidase activity in soils amended with eucalyptus-derived biochar, a response attributed to labile carbon inputs. Furthermore, cellulose-rich biochars (e.g., wood-derived biochars) enhance soil enzymatic activity through microbial hydrolysis of cellulose into glucose, releasing labile carbon that feeds microbial metabolism [18].
The superior performance of BBGFs-BO, particularly at 150% application, can be attributed to its high content of recalcitrant carbon compounds, such as aromatic hydrocarbons and heterocyclic structures, which serve as a sustained substrate for β-glucosidase activity [38,39]. This is further supported by the more pronounced vibrational peaks observed for the BO binder around 1600 cm−1 and 900–700 cm−1 (Figure 2), which are consistent with C=C stretching of aromatic components [36] and out-of-plane C-H bending vibrations in aromatic rings [40], respectively.
BBGFs-PE similarly enhances enzymatic activity through labile carbon release, though mediated by different organic compounds, particularly its carboxaldehydes, hydroxyl groups and organic acids (Figure 2). These water-soluble components rapidly modify critical soil properties including pH, water retention capacity and nutrient availability [33,41,42].
Three possible hypotheses may help explain how granular BBGFs formulations mitigate the potential toxicity of polyaromatic compounds in BO and PE binders: (1) Physical encapsulation: the biochar matrix can physically trap toxic compounds, slowing their release into the soil and preventing microbial inhibition [32]; (2) Chemical adsorption: oxygen-containing functional groups on the biochar surfaces can effectively immobilize harmful compounds through adsorption [43,44]; and (3) Microbial adaptation: over time, soil microbial communities can adapt and gradually degrade recalcitrant aromatics, reducing their concentration and impact in the soil environment [45,46]. These combined mechanisms enhance the efficacy of BO and PE as binders while minimizing their associated environmental risks.
The variable response of acid phosphatase to binder doses reflects complex interactions between phosphorus availability, soil chemistry, and binder properties. The quadratic response of BBGFs-PE at 10 days, peaking at 125% and declining at 150%, is likely driven by two concurrent factors: (1) the high EC of raw PE, measured at 9963.3 μS cm−1 (Table 2), which forces microorganisms to divert energy from enzymatic synthesis to osmoregulation [47], and (2) adsorption of polar substrates to biochar and PE’s negatively charged functional groups, reducing substrate availability [48]. This salt stress effect is particularly evident for acid phosphatase and arylsulfatase [49]. Notably, PE-amended granules still demonstrate lower salinity impacts than conventional fertilizers [50], as biochar’s charged surfaces adsorb saline ions, partially mitigating osmotic stress [51].
In contrast, the increase in BO and CW binder doses in the BBGFs applied to the soil corresponded with elevated acid phosphatase activity, which suggests these binders create a favorable microenvironment through their lower salinity (Table 2) and ability to maintain moderately acidic to near neutral pH (5.4–6.3; Table S1).
The decrease in arylsulfatase enzyme activity in soil by BBGFs-PE with increasing doses may be associated with the salinity-induced osmotic stress on sulfate-reducing microbes, competitive adsorption of enzyme substrates and products by functional groups, and hydrophobic sequestration, where PE’s nonpolar domains trap reaction components in poorly accessible microenvironments, limiting their diffusion and contact [48,52]. These effects are amplified by pH deviations from the enzymatic optimum, particularly when values exceed 6.2 (Table S1), disrupting both microbial enzyme production and catalytic efficiency [19,35]. The similar inhibition of acid phosphatase confirms that amendment chemistry and pH jointly impair anion-hydrolyzing enzymes in soils.
The significant increase in soil arylsulfatase activity following BBGFs-BO addition at 10 and 40 days likely results from the gradual microbial breakdown of complex sulfur-containing compounds, particularly heterocyclic structures [39]. In BBGFs-BO at 150% binder dose, for instance, soil S content reached 4.7 mg kg−1 at 10 days, followed by a decline to 3.3 mg kg−1 at 40 days (Table S2), indicating early mineralization of labile S forms and possible limited degradation of recalcitrant compounds over time.
In contrast, BBGFs-CW maintained stable activity at 10 days but showed reduced values by 40 days, consistent with lower arylsulfatase expression under sulfur availability in the binder (<1.0%; Table S3) [22]. Arylsulfatase serves as both a crucial biocatalyst in the soil sulfur cycle and a sensitive indicator of microbial metabolic activity. This enzyme catalyzes the mineralization of organic sulfur compounds by cleaving sulfate esters, thereby releasing plant-available sulfate (SO42−). The released sulfate supports soil fertility by enabling the synthesis of sulfur-containing amino acids, coenzymes, and functional proteins [53].
The higher enzymatic activity in soils at 10 days compared to 40 days across all BBGFs indicates a positive priming effect from readily degradable carbon, which diminishes as labile substrates are depleted [54]. Furthermore, the increased β-glucosidase activity likely stimulated other soil enzymes, since this enzyme catalyzes the release of low-molecular-weight sugars that serve as energy sources for soil microorganisms [55].
This priming effect dynamics follows a characteristic pattern: initial stimulation of microbial activity by labile carbon shifts toward negative priming as persistent organic matter becomes protected through biochar’s surface interactions and pore entrapment [48]. Concurrently, prolonged amendment exposure drives microbial community succession, favoring taxa adapted to the modified soil environment [54,56]. Biochar can increase the diversity of the soil microbial community, as demonstrated by the increase in the Shannon Index, since, in addition to improving the chemical and physical properties of the soil, biochar particles have a high specific surface area and porosity, which provides a favorable habitat for microorganisms [11].
The greater enzymatic activity observed during the shorter incubation period (10 days) likely reflects an immediate microbial response to the organic substrates supplied by the amendments, which is consistent with the higher SOC values recorded at this time point (Table S1). This peak activity across all treatments with biochar, followed by subsequent decline, aligns with typical substrate utilization dynamics where microbial activity diminishes as available substrates are depleted [48,54]. Furthermore, studies have indicated that interactions between extracellular enzymes and surface charges of soil minerals may reduce or inactivate their activity due to conformational changes in their structure [52,57,58].
In this study, the observed decrease in acid phosphatase activity over time could potentially be attributed to phosphorus input from the biochar amendment. Soil phosphatase enzymes, synthesized by both plants and soil microbiota, generally exhibit enhanced activity under conditions of phosphorus limitation [19]. This inverse relationship reflects feedback inhibition, where increased soluble phosphate represses enzyme synthesis in microorganisms and, similarly, downregulates phosphatase production in plant roots [59]. Similar results were found by [18], where the addition of wheat straw biochar to soil reduced acid phosphatase activity due to increased soluble phosphate availability. Likewise, PE binder, a component of our BBGFs, has been shown to increase phosphorus availability and consequently decrease soil enzyme activity [42].
The biochar used in this study contained high ash content (50.6%; Table 1), which contributes to pH elevation in tropical soils [20]. This alkaline effect likely explains the observed decrease in acid phosphatase activity, as this enzyme exhibits optimal function between pH 4–6 [60]. By day 40, all biochar-amended treatments had reached pH values exceeding this optimal range (Table S1). The biochar immobilizes P from natural phosphates through its Mg, Ca, and Fe constituents (Table 1), competing with P fixation by Al/Fe (oxy)hydroxides (goethite, hematite, gibbsite) and kaolinite in the clay fraction of weathered tropical soils. This mechanism enhances P bioavailability in the soil system, and the resulting increased soluble P content reduce acid phosphatase activity [3,10].
Overall, the PCA enabled an integrated assessment of most variables analyzed, demonstrating that BBGFs influenced soil nutrient dynamics (Tables S1 and S2). At 10 days of incubation, soils amended with BBGFs-PE and BBGFs-BO were associated with SOC, K, Mg, S, Zn, and Fe, likely due to elevated enzymatic activity during this period. This increase may reflect negative priming effect, where microbial preference for biochar compounds preserved native SOC while promoting the mineralization of biochar-derived carbon and nutrients. The initial incorporation of BBGFs into the soil appears to have stimulated microbial utilization of readily available carbon components from the biochar [61]. Although the biochar exhibited a high C/N ratio (Table 1), its combination with acidic binders (Table 2) and reactive natural phosphate likely enhanced biochar mineralization at 10 days, mitigating nutrient limitations [62].
The addition of BBGFs formulated with 75% CW increased soil P-Meh and P-Res, while increasing Ca content, CEC, and pH. These results paralleled those observed with non-granulated biochar treatments, applied alone or with NP, and support [20] findings on CW’s hydrophilic characteristics. The 75% CW binder dosage directly influenced granule structure by reducing interparticle cohesive forces, increasing fertilizer solubility compared to higher doses. In this scenario, a negative effect of biochar on acid phosphatase activity was observed in the BBGFs-CW treatment at the 75% binder dose, associated with a negative correlation in the PCA between soil P levels and enzyme activity. This suggests that native soil microorganisms converted organic phosphates from the fertilizer primarily into bioavailable forms, reducing the demand for phosphatase activity, as previously reported [10,18].
After 40 days, soils amended with BBGFs showed up to a 62.7% increase in CEC (Table S1), as both the biochar components and binding agents provided oxygen-containing functional groups. This aligns with studies showing biochar’s capacity to modify soil properties, improving water retention, nutrient availability and soil structure while regulating pH levels and reducing nutrient leaching [8,63]. The observed CEC enhancement in BBGFs-treated soils further confirms biochar’s role in promoting carbon sequestration and optimizing nutrient cycling efficiency. According to [64], CEC is favored by biochars produced at moderate temperatures (<500 °C) from ash-rich feedstocks. Furthermore, biochar application can directly enhance soil CEC in addition to preserving native SOC from microbial mineralization [65].
At 40 days of incubation, as the labile carbon fractions from biochar were depleted, reducing the availability of readily accessible compounds, it is presumed that the increased investment of carbon in enzymes targeting recalcitrant compounds sustained microbial metabolism while decreasing enzymatic activity over time, due to higher energy expenditure [35,61]. As a carbon-rich and recalcitrant additive, biochar contributes to increasing the soil carbon stock, even under positive priming conditions. This reflects the stabilization of biochar-derived carbon, which offsets losses from the mineralization of native SOC [56,66]. On the other hand, soils amended with the BBGFs-CW (75%) treatment maintained a negative priming effect, owing to the lower recalcitrance of this fertilizer, as discussed earlier, which likely contributed to an increase in soil carbon stock.
At 10 and 40 days of soil incubation with BBGFs-PE and BBGFs-BO, the activities of β-glucosidase and arylsulfatase exhibited strong relationships with SOC and S levels, as evidenced by previous research, which demonstrated that these enzymes served as effective bioindicators for detecting changes in soil management systems (such as conservation agriculture in relation to conventional tillage) [18,35,67,68]. However, these associations do not necessarily imply a direct causal link between enzyme activity and soil chemical characteristics. Rather, they likely indicate a broader context in which enhanced soil quality, resulting from effective management practices, boosts nutrient availability, organic matter content, and biological activity, as noted by [69].
Soils treated with BBGFs-PE and BBGFs-BO exhibited a negative correlation with P-Res and P-Meh at 10 and 40 days of incubation, as these fertilizers generally have low water solubility, resulting in limited P availability in the short term, although this availability may increase over time due to the gradual dissolution of the fertilizer and the activity of acid phosphatase enzyme [2,20]. In contrast, soluble phosphate fertilizers release P immediately upon application, but their availability decreases over time due to adsorption by soil particles and conversion into less soluble chemical forms [20,70].
Soil microorganisms perform multiple enzyme-mediated processes that support plant growth and development, including nutrient cycling, pollutant decomposition, soil aggregate formation, and pathogen control [16]. These processes contribute to the sustainability of agricultural production, as the enhancement of soil biological activity improves nutrient recycling and optimizes the utilization of applied fertilizers, leading to greater agronomic efficiency of fertilization [67,69]. Although soil enzymes may originate from plant and animal residues, microbial biomass constitutes their predominant source. Consequently, an increase in soil enzymatic activity can be interpreted as a reflection of heightened biological vitality [19].
This study focused on enzymatic activities as key functional indicators of the soil’s biochemical response to the BBGFs. While the results clearly demonstrate the overall impact of the formulations, future research could provide a deeper mechanistic understanding. For instance, linking the observed enzymatic functions to shifts in the microbial community structure would be a valuable next step. Furthermore, future investigations could build upon our findings by including direct quantification of specific compounds, such as polycyclic aromatic hydrocarbons, to fully characterize the environmental interactions of these novel fertilizers.
5. Conclusions
The application of BBGFs formulated with alternative binders (BO, PE, and CW) up to a dose of 150% significantly increased the activity of β-glucosidase, acid phosphatase, and arylsulfatase in the soil compared to the controls, particularly within the initial 10-day period. However, enzymatic activity declined by 40 days across all binder treatments, indicating a temporal shift in microbial response.
Incorporating these agro-industrial binders into BBGFs supports sustainable soil management by the valorization of agricultural residues, improvement of soil pH and CEC, and reduction in nutrient losses due to enhanced microbial activity. Such practices contribute substantially to environmentally friendly agriculture in tropical soils.
The use of BO as a binder in BBGFs consistently enhanced the enzymatic activity of β-glucosidase, acid phosphatase, and arylsulfatase, maintaining higher and more stable values over time compared to BBGFs-PE and BBGFs-CW, highlighting its potential as a slow-release amendment for sustainable nutrient cycling in tropical Oxisols. In contrast, BBGFs-PE showed a pronounced initial boost in acid phosphatase activity, while BBGFs-CW exhibited the least impact on enzyme activity.
BO at a 150% dose is recommended both for the production and agricultural application of BBGFs, given its superior capacity to increase β-glucosidase, acid phosphatase, and arylsulfatase activities, as well as improve SOC, CEC, and nutrient availability.
Future research should build upon these findings through long-term field trials to verify their agronomic performance and broader environmental impacts. Such investigations would also benefit from integrating microbial community analysis and direct toxicological assessments to provide a deeper mechanistic understanding of these BBGF formulations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15092230/s1. Figure S1. (A) Kiln-furnace system. (B) Charcoal fines (biochar) from pyrolysis at 450 °C; Table S1. Soil chemical properties after 10 and 40 days of incubation with biochar-based granular fertilizers (BBGFs) produced with different doses of binders’ cassava wastewater (CW), pyroligneous extract (PE) and bio-oil (BO). The treatments natural soil (NS), natural phosphate (NP), biochar and natural phosphate (B+NP) and biochar (B) were used as a reference for comparison with the other treatments; Table S2. Soil chemical properties after 10 and 40 days of incubation with biochar-based granular fertilizers (BBGFs) produced with different doses of binders’ cassava wastewater (CW), pyroligneous extract (PE) and bio-oil (BO). The treatments natural soil (NS), natural phosphate (NP), biochar and natural phosphate (B+NP) and biochar (B) were used as a reference for comparison with the other treatments; Table S3. Chemical composition of cassava wastewater (CW) used as binder in biochar-based granular fertilizers (BBGFs). References [31,71] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, J.M.d.S.J. and L.A.F. (Luiz Arnaldo Fernandes); methodology, J.M.d.S.J., L.A.F. (Luiz Arnaldo Fernandes), F.C., L.A.F. (Leidivan Almeida Frazão) and R.F.P.; validation, J.M.d.S.J., L.A.F. (Luiz Arnaldo Fernandes), F.C., L.A.F. (Leidivan Almeida Frazão) and R.F.P.; formal analysis, J.M.d.S.J. and L.A.F. (Luiz Arnaldo Fernandes); investigation, J.M.d.S.J.; resources, L.A.F. (Luiz Arnaldo Fernandes), F.C. and L.A.F. (Leidivan Almeida Frazão); data curation, J.M.d.S.J. and L.A.F. (Luiz Arnaldo Fernandes); writing—original draft preparation, J.M.d.S.J.; writing—review and editing, J.M.d.S.J., L.A.F. (Luiz Arnaldo Fernandes), F.C., L.A.F. (Leidivan Almeida Frazão) and R.F.P.; visualization, J.M.d.S.J.; supervision, L.A.F. (Luiz Arnaldo Fernandes), F.C. and L.A.F. (Leidivan Almeida Frazão); project administration, L.A.F. (Luiz Arnaldo Fernandes); funding acquisition, L.A.F. (Luiz Arnaldo Fernandes). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil), the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG/Brazil) and the Center for Carbon Research in Tropical Agriculture (CCARBON) at the Universidade de São Paulo (USP/Brazil), sponsored by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP/Brazil) under grant 2021/10573-4.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil) and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG/Brazil) for financial support and scholarships. Special thanks to CNPq for the fellowship for the second author (grants #304127/2023-0), to the Pró-Reitoria de Pesquisa (PRPQ) da Universidade Federal de Minas Gerais (UFMG/Brazil) and to the Laboratório de Química Instrumental (LQI/UFMG).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Igalavithana, A.D.; Choi, S.W.; Shang, J.; Hanif, A.; Dissanayake, P.D.; Tsang, D.C.W.; Kwon, J.H.; Lee, K.B.; Ok, Y.S. Carbon dioxide capture in biochar produced from pine sawdust and paper mill sludge: Effect of porous structure and surface chemistry. Sci. Total Environ. 2020, 739, 139845. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.S.S.; Ribeiro, I.C.A.; Nardis, B.O.; Barbosa, C.F.; Lustosa Filho, J.F.; Melo, L.C.A. Long-term effect of biochar-based fertilizers application in tropical soil: Agronomic efficiency and phosphorus availability. Sci. Total Environ. 2021, 760, 143955. [Google Scholar] [CrossRef] [PubMed]
- Pogorzelski, D.; Lustosa Filho, J.F.; Matias, P.C.; Santos, W.O.; Vergütz, L.; Melo, L.C.A. Biochar as composite of phosphate fertilizer: Characterization and agronomic effectiveness. Sci. Total Environ. 2020, 743, 140604. [Google Scholar] [CrossRef]
- Basak, B.B.; Sarkar, B.; Saha, A.; Sarkar, A.; Mandal, S.; Biswas, J.K.; Wang, H.; Bolan, N.S. Revamping highly weathered soils in the tropics with biochar application: What we know and what is needed. Sci. Total Environ. 2022, 822, 153461. [Google Scholar] [CrossRef] [PubMed]
- Novais, R.F.; Smyth, T.J. Fósforo em Solo e Planta em Condições Tropicais; Univesidade Federal de Viçosa: Viçosa, Brazil, 1999; 399p. [Google Scholar]
- Santos Júnior, J.M.; Colen, F.; Pegoraro, R.F.; Heinrichs, R.; Frazão, L.A.; Sampaio, R.A.; Fernandes, L.A. Biochar pellets as soil conditioner on the growth of Urochloa brizantha BRS Paiaguás. Rev. Ciênc. Agron. 2024, 55, e20238690. [Google Scholar] [CrossRef]
- Shoudho, K.N.; Khan, T.H.; Ara, U.R.; Khan, M.R.; Shawon, Z.B.Z.; Hoque, M.E. Biochar in global carbon cycle: Towards sustainable development goals. Curr. Res. Green. Sustain. Chem. 2024, 8, 100409. [Google Scholar] [CrossRef]
- Adhikari, S.; Moon, E.; Timms, W. Identifying biochar production variables to maximize exchangeable cations and increase nutrient availability in soils. J. Clean. Prod. 2024, 446, 141454. [Google Scholar] [CrossRef]
- Otoni, J.P.; Matoso, S.C.G.; Pérez, X.L.O.; Silva, V.B. Potential for agronomic and environmental use of biochars derived from different organic waste. J. Clean. Prod. 2024, 449, 141826. [Google Scholar] [CrossRef]
- Lopes, E.M.G.; Reis, M.M.; Frazão, L.A.; Terra, L.E.M.; Lopes, E.F.; Santos, M.M.; Fernandes, L.A. Biochar increases enzyme activity and total microbial quality of soil grown with sugarcane. Environ. Technol. Innov. 2021, 21, 101270. [Google Scholar] [CrossRef]
- Lopes, E.M.G.; Mota, M.F.C.; Santos Júnior, J.M.; Reis, M.M.; Frazão, L.A.; Fernandes, L.A. Biochar alters the soil microbiological activity of sugarcane fields over time. Sci. Agric. 2024, 81, e20230289. [Google Scholar] [CrossRef]
- Mir, N.R.; Mir, B.A.; Mavi, M.S.; Kapoor, N. Revitalizing soils: Biochar’s battle against heavy metal menace in plants—A review. Pedosphere, 2025; in press. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; He, L.; Wang, J.; Wang, S.; Shi, X.; Zhang, X.; Wang, H.; He, F. Environmental behavior of per- and polyfluoroalkyl substances (PFASs) and the potential role of biochar for its remediation: A review. Biochar 2025, 7, 14. [Google Scholar] [CrossRef]
- Dong, M.; Jiang, M.; He, L.; Zhang, Z.; Gustave, W.; Vithanage, M.; Niazi, N.K.; Chen, B.; Zhang, X.; Wang, H.; et al. Challenges in safe environmental applications of biochar: Identifying risks and unintended consequence. Biochar 2025, 7, 12. [Google Scholar] [CrossRef]
- Mendes, I.C.; Souza, L.M.; Sousa, D.M.G.; Lopes, A.A.C.; Reis Júnior, F.B.; Lacerda, M.P.C.; Malaquias, J.V. Critical limits for microbial indicators in tropical Oxisols at post-harvest: The FERTBIO soil sample concept. Appl. Soil Ecol. 2019, 139, 85–93. [Google Scholar] [CrossRef]
- Mendes, I.C.; Sousa, D.M.G.; Dantas, O.D.; Lopes, A.A.C.; Reis Júnior, F.B.; Oliveira, M.I.; Chaer, G.M. Soil quality and grain yield: A win–win combination in clayey tropical oxisols. Geoderma 2021, 388, 114880. [Google Scholar] [CrossRef]
- Barbosa, J.Z.; Poggere, G.; Corrêa, R.S.; Hungria, M.; Mendes, I.C. Soil enzymatic activity in Brazilian biomes under native vegetation and contrasting cropping and management. Appl. Soil Ecol. 2023, 190, 105014. [Google Scholar] [CrossRef]
- Song, X.; Razavi, B.S.; Ludwig, B.; Zamanian, K.; Zang, H.; Kuzyakov, Y.; Dippold, M.A.; Gunina, A. Combined biochar and nitrogen application stimulates enzyme activity and root plasticity. Sci. Total Environ. 2020, 735, 139393. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil. Analysis: Microbiological and Biochemical Properties; Mickelson, S.H., Bigham, J.M., Eds.; Soil Science Society of America Inc.: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Santos Júnior, J.M.; Colen, F.; Frazão, L.A.; Pegoraro, R.F.; Fernandes, L.A. Granulated organomineral fertilizers from by-products of the agricultural and forestry sector. Sci. Agric. 2025, 82, e20240132. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, L.; Meulien, E.S.; Bi, X.T.; Lim, J.C.; Chen, W. Waste Plastics as an Effective Binder for Biochar Pelletization. Energy Fuels 2021, 35, 13840–13846. [Google Scholar] [CrossRef]
- Schmidt, V.K.O.; Vasconscelos, G.M.D.; Vicente, R.; Carvalho, J.S.; Della-Flora, I.K.; Degang, L.; Oliveira, D.; Andrade, C.J. Cassava wastewater valorization for the production of biosurfactants: Surfactin, rhamnolipids, and mannosileritritol lipids. World J. Microbiol. Biotechnol. 2023, 39, 65. [Google Scholar]
- Genova, G.; Borruso, L.; Signorini, M.; Mitterer, M.; Niedrist, G.; Cesco, S.; Felderer, B.; Cavani, L.; Mimmo, T. Analyzing soil enzymes to assess soil quality parameters in long-term copper accumulation through a machine learning approach. Appl. Soil Ecol. 2024, 195, e105261. [Google Scholar] [CrossRef]
- Schettini, B.L.S.; Jacovine, L.A.G.; Torres, C.M.M.E.; Carneiro, A.C.O.; Villanova, P.H.; Rocha, S.J.S.S.; Rufino, M.P.M.X.; Silva, L.B.; Castro, R.V.O. Furnace-kiln system: How does the use of new technologies in charcoal production affect the carbon balance? Ind. Crop. Prod. 2022, 187, 115330. [Google Scholar] [CrossRef]
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2021; 2p.
- Tedesco, M.J.; Gianello, C.; Bissani, C.A.; Bohnen, H.; Volkweiss, S.J. Soil, Plants and Other Materials Analysis, 2nd ed.; Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 1995; 174p. [Google Scholar]
- Klasson, K.T. Biochar characterization and a method for estimating biochar quality from proximate analysis results. Biomass Bioenergy 2017, 96, 50–58. [Google Scholar] [CrossRef]
- ASTM E1756-08; Standard Test Method for Determination of Total Solids in Biomass. ASTM International: West Conshohocken, PA, USA, 2020; 2p.
- Schulte, E.E.; Hoskins, B. Recommended soil organic matter tests. In Recommended Soil Testing Procedures for the Northeastern United States, 3rd ed.; Sims, J.T., Wolf, A., Eds.; University of Delaware: Newark, NJ, USA, 2011; pp. 63–74. [Google Scholar]
- Malavolta, E. Elements of Plant Mineral Nutrition; Agronômica Ceres: São Paulo, Brazil, 1980; 252p. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual of Soil Analysis Methods, 3rd ed.; Embrapa Solos: Rio de Janeiro, Brazil, 2017; 573p. [Google Scholar]
- Cheng, J.; Liao, Z.; Hu, S.; Geng, Z.; Zhu, M.; Xu, W. Synthesis of an environmentally friendly binding material using pyrolysis byproducts and modified starch binder for slow-release fertilizers. Sci. Total Environ. 2022, 819, 153146. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Chin, K.L.; Khoo, P.S.; Hafizuddin, M.S.; H’ng, P.S. Production and Potential Application of Pyroligneous Acids from Rubberwood and Oil Palm Trunk as Wood Preservatives through Vacuum-Pressure Impregnation Treatment. Polymers 2022, 14, 3863. [Google Scholar] [CrossRef] [PubMed]
- Mafaldo, I.M.; Araújo, L.M.; Cabral, L.; Barão, C.E.; Noronha, M.F.; Fink, J.R.; Albuquerque, T.M.R.; Lima, M.S.; Vidal, H.; Pimentel, T.C.; et al. Cassava (Manihot esculenta) Brazilian cultivars have different chemical compositions, present prebiotic potential, and beneficial effects on the colonic microbiota of celiac individuals. Food Res. Int. 2024, 195, 114909. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, M.; Khadem, A. The effects of biochar on soil extra and intracellular enzymes activity. Biomass Convers. Biorefinery 2024, 14, 21993–22005. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- McCall, M.A.; Watson, J.S.; Tan, J.S.W.; Sephton, M.A. Biochar Stability Revealed by FTIR and Machine Learning. ACS Sustain. Resour. Manag. 2025, 2, 842–852. [Google Scholar] [CrossRef]
- Frainetti, A.J.; Klinghoffer, N.B. Recent experimental advances on the utilization of biochar as a tar reforming catalyst: A review. Int. J. Hydrogen Energy 2023, 48, 8022–8044. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Martins-Vieira, J.C.; Missau, J.; Anshu, K.; Siakpebru, O.K.; Thengane, S.K.; Morais, A.R.C.; Tanabe, E.H.; Bertuol, D.A. Review on Biomass Pyrolysis with a Focus on Bio-Oil Upgrading Techniques. Analytica 2023, 4, 182–205. [Google Scholar] [CrossRef]
- Ibarra, J.V.; Moliner, R.; Bonet, A.J. FT-i.r. investigation on char formation during the early stages of coal pyrolysis. Fuel 1994, 73, 918–924. [Google Scholar] [CrossRef]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Yuan, Y.; Kong, Q.; Zheng, Y.; Zheng, H.; Liu, Y.; Cheng, Y.; Zhang, X.; Li, Z.; You, X.; Li, Y. Co-application of biochar and pyroligneous acid improved peanut production and nutritional quality in a coastal soil. Environ. Technol. Innov. 2022, 28, 102886. [Google Scholar] [CrossRef]
- El-Naggar, A.; Mosa, A.; Ahmed, N.; Niazi, N.K.; Yousaf, B.; Sarkar, B.; Rinklebe, J.; Cai, Y.; Chang, S.X. Modified and pristine biochars for remediation of chromium contamination in soil and aquatic systems. Chemosphere 2022, 303, 134942. [Google Scholar] [CrossRef]
- Freddo, A.; Cai, C.; Reid, B.J. Environmental contextualization of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ. Pollut. 2012, 171, 18–24. [Google Scholar] [CrossRef]
- Bolan, S.; Sharma, S.; Mukherjee, S.; Kumar, M.; Rao, C.S.; Nataraj, K.C.; Singh, G.; Vinu, A.; Bhowmik, A.; Sharma, H.; et al. Biochar modulating soil biological health: A review. Sci. Total Environ. 2024, 914, 169585. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Xiong, X.; Zhu, H.; Xu, H.; Leng, P.; Li, J.; Tang, C.; Xu, J. Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 2021, 3, 239–254. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Z.; Yang, K.; Gu, P.; Liu, S.; Jia, Y.; Zhang, Z.; Wang, T.; Yin, J.; Miao, H. Deeper insight into the effect of salinity on the relationship of enzymatic activity, microbial community and key metabolic pathway during the anaerobic digestion of high strength organic wastewater. Bioresour. Technol. 2022, 363, 127978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wang, N.; Jiang, S.; Li, F.; Luo, S.; Chen, A.; Li, H.; Lin, X.; Zhang, J.; Zhang, L.; et al. Potential implications of biochar and compost on the stoichiometry-based assessments of soil enzyme activity in heavy metal-polluted soils. Carbon Res. 2022, 1, 29. [Google Scholar] [CrossRef]
- Sritongon, N.; Sarin, P.; Theerakulpisut, P.; Riddech, N. The effect of salinity on soil chemical characteristics, enzyme activity and bacterial community composition in rice rhizospheres in Northeastern Thailand. Sci. Rep. 2022, 12, 20360. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Timmer, V.R. Fertilizer-induced Changes in Rhizosphere Electrical Conductivity: Relation to Forest Tree Seedling Root System Growth and Function. New For. 2005, 30, 147–166. [Google Scholar] [CrossRef]
- Din, M.M.U.; Khan, M.I.; Azam, M.; Ali, M.H.; Qadri, R.; Naveed, M.; Nasir, A. Effect of Biochar and Compost Addition on Mitigating Salinity Stress and Improving Fruit Quality of Tomato. Agronomy 2023, 13, 2197. [Google Scholar] [CrossRef]
- Zeng, L.; Zimmerman, A.R.; Huang, R. Adsorption of extracellular enzymes by biochar: Impacts of enzyme and biochar properties. Geoderma 2024, 451, 117082. [Google Scholar] [CrossRef]
- Zaid, F.; Al-Awwal, N.; Yang, J.; Anderson, S.H.; Alsunuse, B.T.B. Effects of Biochar-Amended Composts on Selected Enzyme Activities in Soils. Processes 2024, 12, 1678. [Google Scholar] [CrossRef]
- Liu, X.A.; Finley, B.K.; Mau, R.L.; Schwartz, E.; Dijkstra, P.; Bowker, M.A.; Hungate, B.A. The soil priming effect: Consistent across ecosystems, elusive mechanisms. Soil Biol. Biochem. 2020, 140, 107617. [Google Scholar] [CrossRef]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of Soil Microbiota Enzymes in Soil Health and Activity Changes Depending on Climate Change and the Type of Soil Ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef]
- Lu, W.; Zha, Q.; Zhang, H.; Chen, H.Y.H.; Yu, J.; Tu, F.; Ruan, H. Changes in soil microbial communities and priming effects induced by rice straw pyrogenic organic matter produced at two temperatures. Geoderma 2021, 400, 115217. [Google Scholar] [CrossRef]
- Li, Y.; Koopal, L.K.; Chen, Y.; Shen, A.; Tan, W. Conformational modifications of lysozyme caused by interaction with humic acid studied with spectroscopy. Sci. Total Environ. 2021, 768, 144858. [Google Scholar] [CrossRef]
- Zang, Y.; Liu, F.; Li, X.; Sheng, A.; Zhai, J.; Liu, J. Adsorption kinetics, conformational change, and enzymatic activity of β-glucosidase on hematite (α-Fe2O3) surfaces. Colloids Surf. B Biointerfaces 2020, 193, 111115. [Google Scholar] [CrossRef]
- Reid, M.S.; Bieleski, R.L. Response of Spirodela oligorrhiza to Phosphorus Deficiency. Plant Physiol. 1970, 46, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A. Soil Enzyme Activity Response under the Amendment of Different Types of Biochar. Agronomy 2022, 12, 569. [Google Scholar] [CrossRef]
- Zhang, Y.; Dang, Y.; Wang, J.; Huang, Q.; Wang, X.; Yao, L.; Vinay, N.; Yu, K.; Wen, X.; Xiong, Y.; et al. A synthesis of soil organic carbon mineralization in response to biochar amendment. Soil Biol. Biochem. 2022, 175, 108851. [Google Scholar] [CrossRef]
- Abban-Baidoo, E.; Manka’abusi, D.; Apuri, L.; Marschner, B.; Frimpong, K.A. Biochar addition influences C and N dynamics during biochar co-composting and the nutrient content of the biochar co-compost. Sci. Rep. 2024, 14, 23781. [Google Scholar] [CrossRef]
- Dey, S.; Purakayastha, T.J.; Sarkar, B.; Rinklebe, J.; Kumar, S.; Chakraborty, R.; Datta, A.; Lal, K.; Shivay, Y.S. Enhancing cation and anion exchange capacity of rice straw biochar by chemical modification for increased plant nutrient retention. Sci. Total Environ. 2023, 886, 163681. [Google Scholar] [CrossRef]
- Domingues, R.R.; Sánchez-Monedero, M.A.; Spokas, K.A.; Melo, L.C.A.; Trugilho, P.F.; Valenciano, M.N.; Silva, C.A. Enhancing Cation Exchange Capacity of Weathered Soils Using Biochar: Feedstock, Pyrolysis Conditions and Addition Rate. Agronomy 2020, 10, 824. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Awad, Y.M.; Yang, X.; Ryu, C.; Rizwan, M.; Rinklebe, J.; Tsang, D.C.W.; Ok, Y.S. Influence of soil properties and feedstocks on biochar potential for carbon mineralization and improvement of infertile soils. Geoderma 2018, 332, 100–118. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Liu, C.; Blagodatskaya, E.; Kuzyakov, Y.; Zeng, Z.; Jones, D.L.; Zang, H. Quantifying apparent and real priming effects based on inverse labelling. Appl. Soil Ecol. 2024, 195, 105234. [Google Scholar] [CrossRef]
- Chaer, G.M.; Mendes, I.C.; Dantas, O.D.; Malaquias, J.V.; Reis Júnior, F.B.; Oliveira, M.I.L. Evaluating C trends in clayey Cerrado Oxisols using a four-quadrant model based on specific arylsulfatase and β-glucosidase activities. Appl. Soil Ecol. 2023, 183, 104742. [Google Scholar] [CrossRef]
- Passinato, J.H.; Amado, T.J.C.; Kassam, A.; Acosta, J.A.A.; Amaral, L.P. Soil Health Check-Up of Conservation Agriculture Farming Systems in Brazil. Agronomy 2021, 11, 2410. [Google Scholar] [CrossRef]
- Pawlowski, E.; Sobucki, L.; Barbosa, J.G.P.; Handte, V.G.; Vieira, I.B.; Brunetto, G.; Mendes, I.C.; Jacques, R.J.S. Relationships between yield, enzymatic activity, and chemical properties across different soil layers and phenological stages of grapevines in southern Brazil. Appl. Soil Ecol. 2024, 204, 105732. [Google Scholar] [CrossRef]
- Lustosa Filho, J.F.; Carneiro, J.S.S.; Barbosa, C.F.; Lima, K.P.; Leite, A.A.; Melo, L.C.A. Aging of biochar-based fertilizers in soil: Effects on phosphorus pools and availability to Urochloa brizantha grass. Sci. Total Environ. 2020, 709, 136028. [Google Scholar] [CrossRef]
- Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Manual de Métodos Analíticos Oficiais para Fertilizantes e Corretivos; MAPA: Brasília, Brazil, 2017; 240p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).