Mechanism of Modified Biochar in Mitigating Carbon and Nitrogen Loss in Drought Soil with Green Manure Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of Experimental Area
2.2. Preparation and Characterization of Biochar and Green Manure
2.3. Experimental Design and Incubation Setup
2.3.1. Determination of Soil Water Holding Capacity (WHC)
2.3.2. Experimental Treatments and Incubation Setup
2.4. Characterization of Soil Indicators
2.4.1. Soil Physicochemical Characteristics
2.4.2. Soil Microbiological Analysis
2.5. Data Analysis
3. Results and Discussion
3.1. Properties of Biochar
3.2. Soil Emissions of N2O and CO2
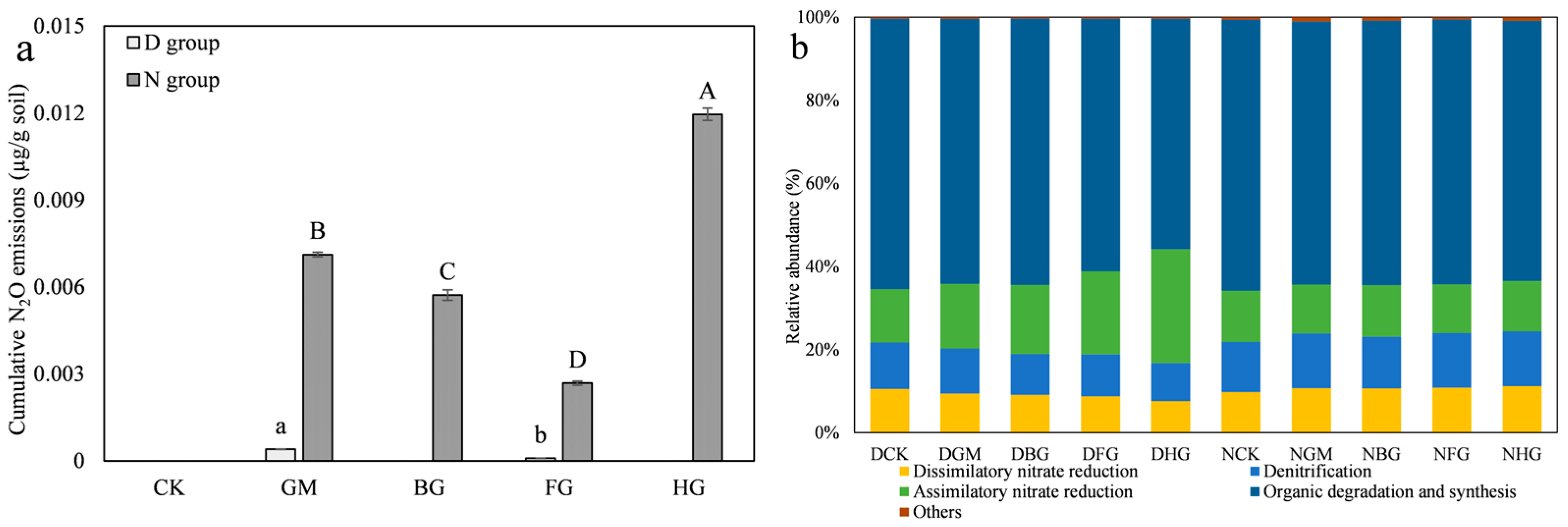
3.2.1. The Properties of Modified Biochar Determined N2O Emissions
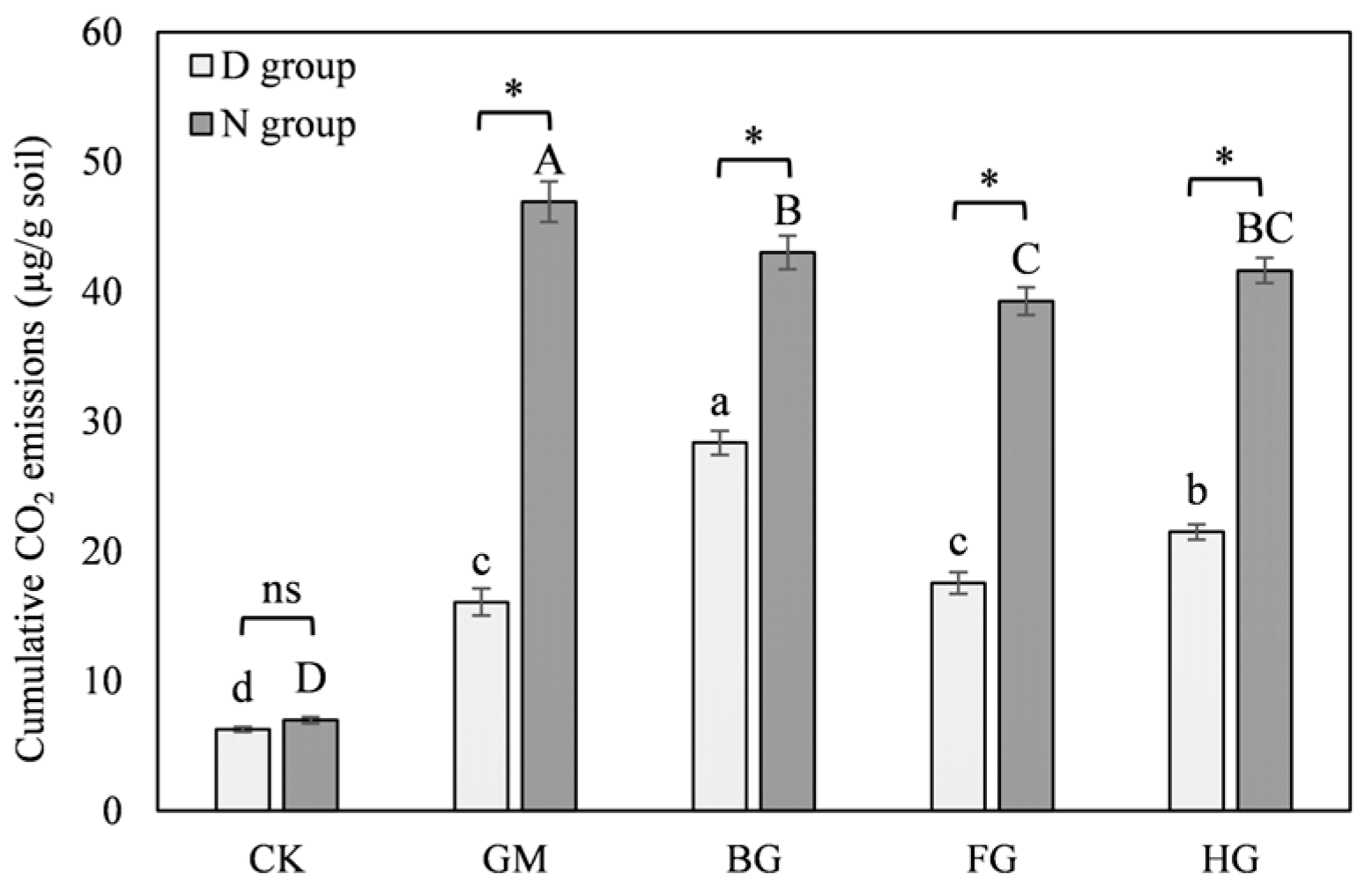
3.2.2. The Properties of Modified Biochar Reduce CO2 Emissions
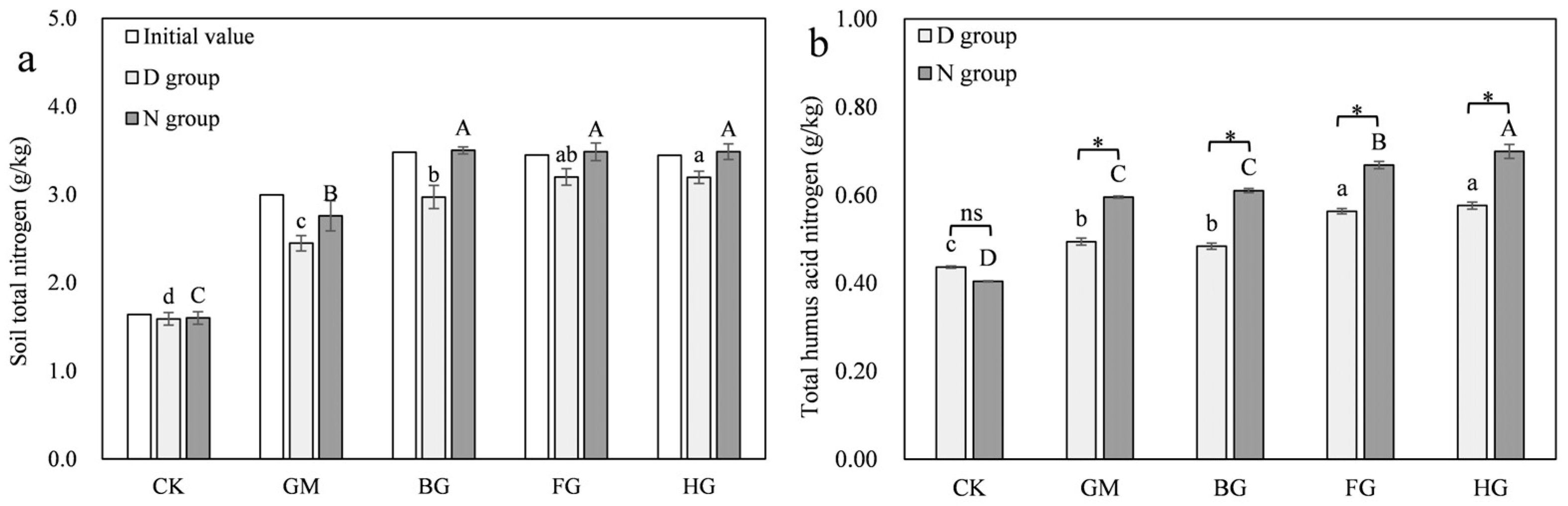
3.3. Changes in Soil Nitrogen
3.3.1. Modified Biochar Reduces Nitrogen Loss from Drought Soil by Minimizing N2O Leaching and NH3 Volatilization
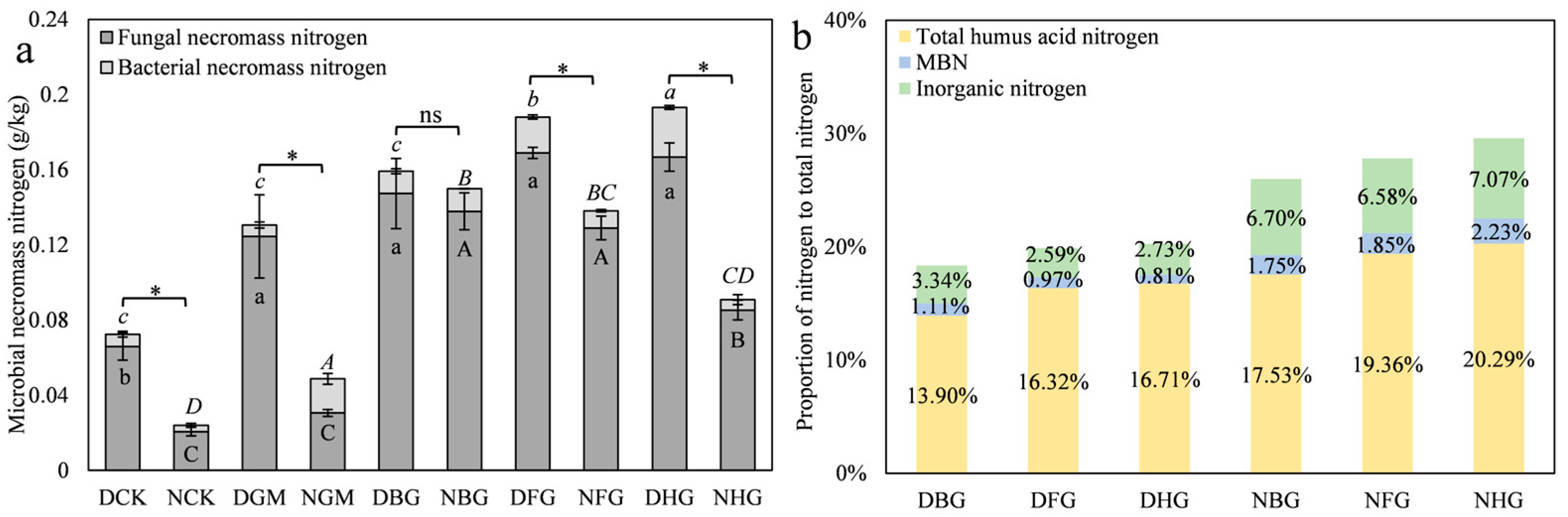
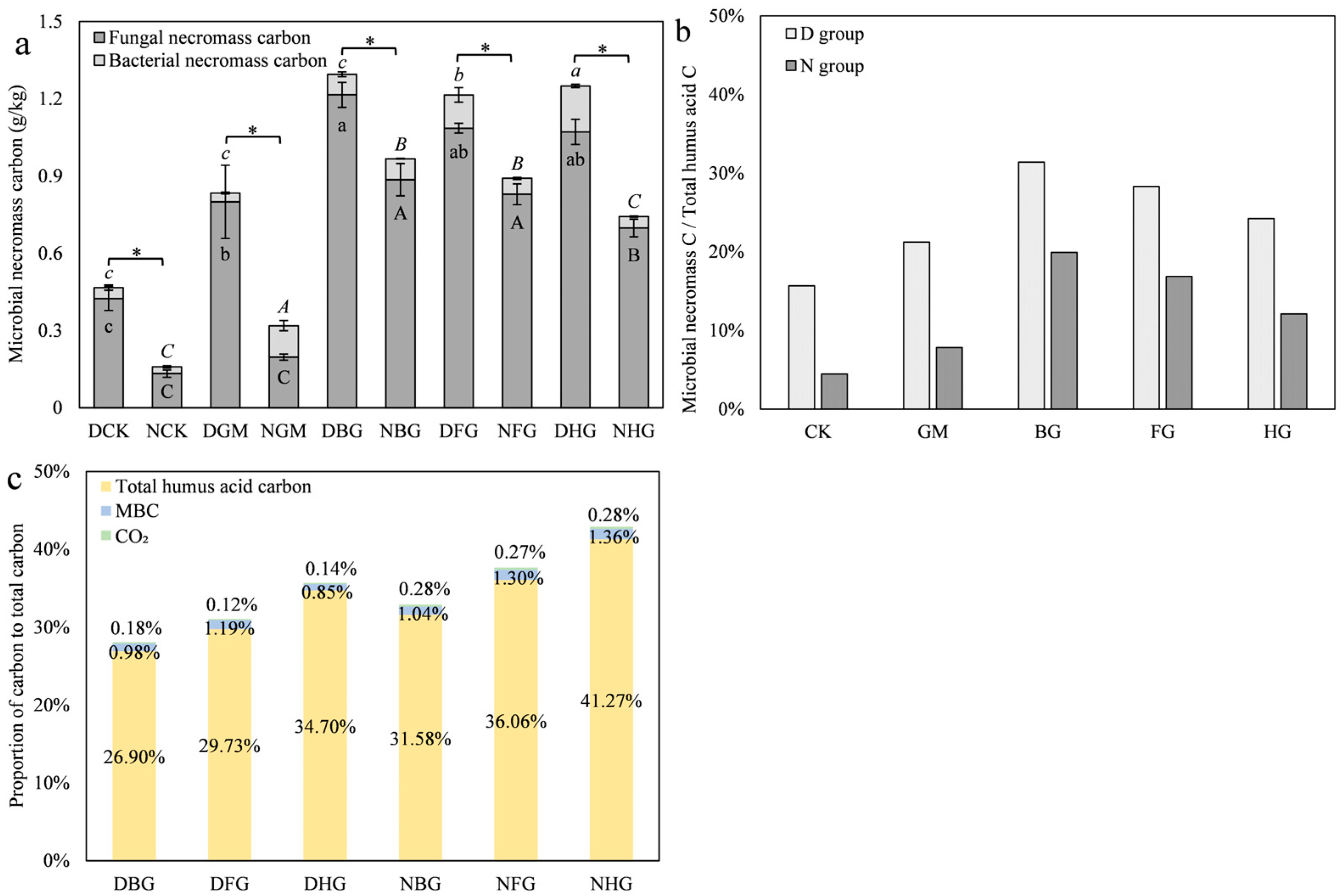
3.3.2. Modified Biochar Retains Nitrogen in Microbial Necromass to Reduce Nitrogen Loss Under Drought Conditions
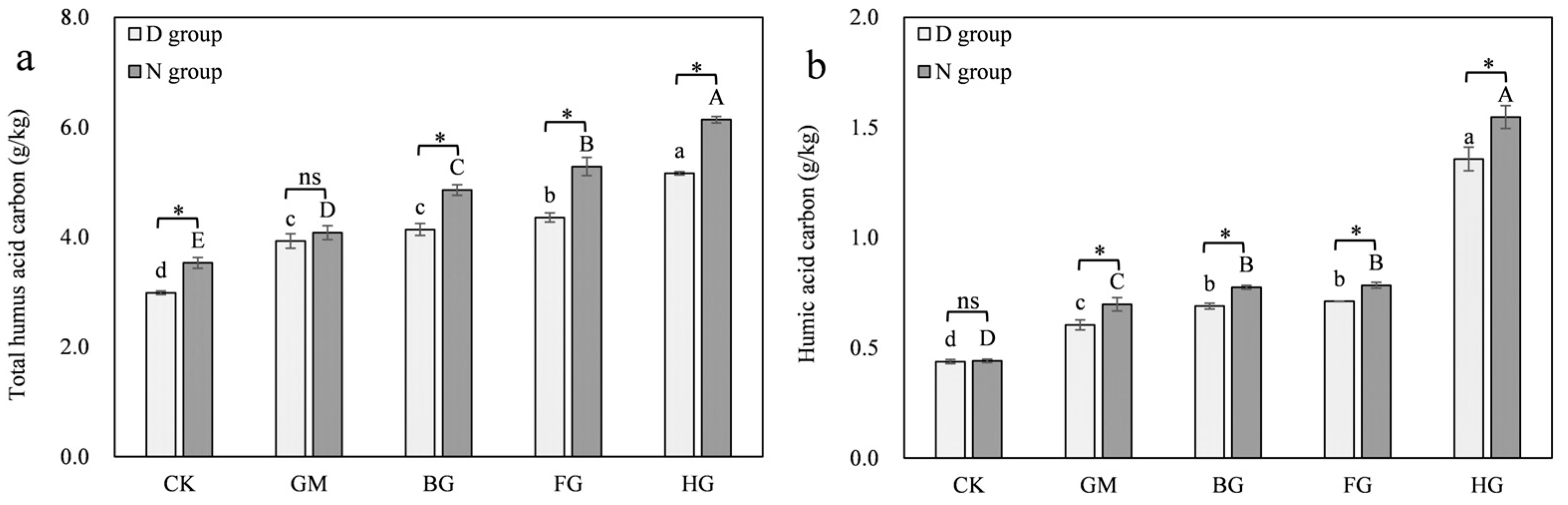
3.3.3. Moisture Promotes the Humification Process of Microbial Necromass
3.4. Changes in Soil Carbon
3.4.1. Modified Biochar Reduces Soil Carbon Losses
3.4.2. Modified Biochar Enhanced the Conversion of Microbial Necromass to Humic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baggs, E.M.; Watson, C.A.; Rees, R.M. The fate of nitrogen from incorporated cover crop and green manure residues. Nutr. Cycl. Agroecosys. 2000, 56, 153–163. [Google Scholar] [CrossRef]
- Gao, X.; Shi, D.; Lv, A.; Wang, S.; Yuan, S.; Zhou, P.; An, Y. Increase phosphorus availability from the use of alfalfa (Medicago sativa L) green manure in rice (Oryza sativa L.) agroecosystem. Sci. Rep. 2016, 6, 36981. [Google Scholar] [CrossRef]
- Tautges, N.E.; Borrelli, K.; Burke, I.C.; Fuerst, E.P. Nitrogen fertility effects of alfalfa, pea green manure, and poultry manure on organic wheat productivity in a semiarid climate. Agroecol. Sust. Food 2018, 42, 169–188. [Google Scholar] [CrossRef]
- de Ruijter, F.J.; Huijsmans, J.F.M.; Rutgers, B. Ammonia volatilization from crop residues and frozen green manure crops. Atmos. Environ. 2010, 44, 3362–3368. [Google Scholar] [CrossRef]
- Peng, S.; Yang, S.; Xu, J. Ammonia volatilization and its influence factors of paddy field under water-saving irrigation. Trans. Chin. Soc. Agric. Eng. 2009, 25, 35–39. [Google Scholar]
- Wu, D.; Wei, Z.; Well, R.; Shan, J.; Yan, X.; Bol, R.; Senbayram, M. Straw amendment with nitrate-N decreased N2O/(N2O+N2) ratio but increased soil N2O emission: A case study of direct soil-born N2 measurements. Soil Biol. Biochem. 2018, 127, 301–304. [Google Scholar] [CrossRef]
- Nelissen, V.; Rütting, T.; Huygens, D.; Staelens, J.; Ruysschaert, G.; Boeckx, P. Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol. Biochem. 2012, 55, 20–27. [Google Scholar] [CrossRef]
- Lei, K.; Dai, W.; Wang, J.; Li, Z.; Cheng, Y.; Jiang, Y.; Yin, W.; Wang, X.; Song, X.; Tang, Q. Biochar and straw amendments over a decade divergently alter soil organic carbon accumulation pathways. Agronomy 2024, 14, 2176. [Google Scholar] [CrossRef]
- Wang, W.; Lu, S. Influence of green manure rapes returning and biochar application on soil carbon and nitrogen statuts of newly built green houses. J. Soil Sci. Plant Nutr. 2024, 24, 2035–2047. [Google Scholar] [CrossRef]
- Kimani, S.M.; Bimantara, P.O.; Kautsar, V.; Tawaraya, K.; Cheng, W. Poultry litter biochar application in combination with chemical fertilizer and Azolla green manure improves rice grain yield and nitrogen use efficiency in paddy soil. Biochar 2021, 3, 591–602. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Q.; Chen, H.; Jia, X.; Gong, F.; Liu, X.; Chi, D. N-loaded clinoptilolite under water-saving irrigation mitigates ammonia volatilization while increasing grain yield and water-nitrogen use efficiency. Field Crops Res. 2023, 300, 109000. [Google Scholar] [CrossRef]
- Qi, S.; Ding, J.; Yang, S.; Jiang, Z.; Xu, Y. Impact of biochar application on ammonia volatilization from paddy fields under controlled irrigation. Sustainability 2022, 14, 1337. [Google Scholar] [CrossRef]
- Zarezadeh, S.; Zheng, Y.; Jenkins, S.N.; Mercer, G.D.; Moheimani, N.R.; Singh, P.; Mickan, B.S. Sustainable soil management in agriculture under drought stress: Utilising waste-derived organic soil amendments and beneficial impacts on soil bacterial processes. Appl. Soil Ecol. 2025, 206, 105870. [Google Scholar] [CrossRef]
- Saarnio, S.; Heimonen, K.; Kettunen, R. Biochar addition indirectly affects N2O emissions via soil moisture and plant N uptake. Soil Biol. Biochem. 2013, 58, 99–106. [Google Scholar] [CrossRef]
- Iqbal, S.; Xu, J.; Khan, S.; Worthy, F.R.; Khan, H.Z.; Nadir, S.; Ranjitkar, S. Regenerative fertilization strategies for climate-smart agriculture: Consequences for greenhouse gas emissions from global drylands. J Clean. Prod. 2023, 398, 136650. [Google Scholar] [CrossRef]
- Mandal, S.; Thangarajan, R.; Bolan, N.S.; Sarkar, B.; Khan, N.; Ok, Y.S.; Naidu, R. Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere 2016, 142, 120–127. [Google Scholar] [CrossRef]
- Egyir, M.; Luyima, D.; Park, S.-J.; Lee, K.S.; Oh, T.-K. Volatilisations of ammonia from the soils amended with modified and nitrogen-enriched biochars. Sci. Total Environ. 2022, 835, 155453. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, Z.; Li, M.; Clough, T.; Wrage-Mönnig, N.; Qin, S.; Ge, T.; Liao, H.; Zhou, S. Biochar’s role as an electron shuttle for mediating soil N2O emissions. Soil Biol. Biochem. 2019, 133, 94–96. [Google Scholar] [CrossRef]
- Chen, M.; Wu, L.; Ding, X.; Liu, L.; Li, Y.; Fei, C.; Zhang, S. Fe-modified biochar improved the stability of soil aggregates and organic carbon: Evidence from enzymatic activity and microbial composition. Land Degrad. Dev. 2024, 35, 732–743. [Google Scholar] [CrossRef]
- Liao, X.; Mao, S.; Shan, Y.; Gao, W.; Wang, S.; Malghani, S. Impact of iron-modified biochars on soil nitrous oxide emissions: Variations with iron salts and soil fertility. J. Environ. Manag. 2024, 356, 120571. [Google Scholar] [CrossRef]
- Wang, J.; Riaz, M.; Babar, S.; Xia, H.; Li, Y.; Xia, X.; Wang, X.; Jiang, C. Iron-modified biochar reduces nitrogen loss and improves nitrogen retention in Luvisols by adsorption and microbial regulation. Sci. Total Environ. 2023, 879, 163196. [Google Scholar] [CrossRef]
- Zhu, Z.; Duan, W.; Chang, Z.; Du, W.; Chen, F.; Li, F.; Oleszczuk, P. Stability of functionally modified biochar: The role of surface charges and surface homogeneity. Sustainability 2023, 15, 7745. [Google Scholar] [CrossRef]
- Bravo, C.; Toniolo, R.; Pellegrini, E.; Millo, C.; Covelli, S.; Contin, M.; Martin-Neto, L.; De Nobili, M. Electron donating properties of humic acids in saltmarsh soils reflect soil geochemical characteristics. Geoderma 2022, 419, 115872. [Google Scholar] [CrossRef]
- An, M.; Chang, D.; Hong, D.; Fan, H.; Wang, K. Metabolic regulation in soil microbial succession and niche differentiation by the polymer amendment under cadmium stress. J. Hazard. Mater. 2021, 416, 126094. [Google Scholar] [CrossRef]
- Xue, P. The Effect of Adding Different Residues of Corn on Amino Sugars and Microbial Necromass Nitrogen in Brown Soil Aggregates. Master’s Thesis, Shenyang Agricultural Uinversity, Shenyang, China, 2022. [Google Scholar]
- Wang, Y.; Liu, R. H2O2 treatment enhanced the heavy metals removal by manure biochar in aqueous solutions. Sci. Total Environ. 2018, 628–629, 1139–1148. [Google Scholar] [CrossRef]
- Weldon, S.; van der Veen, B.; Farkas, E.; Kocatürk-Schumacher, N.P.; Dieguez-Alonso, A.; Budai, A.; Rasse, D. A re-analysis of NH4+ sorption on biochar: Have expectations been too high? Chemosphere 2022, 301, 134662. [Google Scholar] [CrossRef]
- Long, L.; Xue, Y.; Hu, X.; Zhu, Y. Study on the influence of surface potential on the nitrate adsorption capacity of metal modified biochar. Environ. Sci. Pollut. 2019, 26, 3065–3074. [Google Scholar] [CrossRef]
- Li, L.; Hao, Y.; Wang, W.; Biederman, J.A.; Zheng, Z.; Zhang, B.; Wang, Y.; Song, X.; Cui, X.; Xu, Z. Seasonal timing of extreme drought regulates N2O fluxes in a semiarid grassland. Geoderma 2023, 436, 116530. [Google Scholar] [CrossRef]
- Hu, R.; Liu, S.; Huang, W.; Nan, Q.; Strong, P.J.; Saleem, M.; Zhou, Z.; Luo, Z.; Shu, F.; Yan, Q.; et al. Evidence for assimilatory nitrate reduction as a previously overlooked pathway of reactive nitrogen transformation in estuarine suspended particulate matter. Environ. Sci. Technol. 2022, 56, 14852–14866. [Google Scholar] [CrossRef]
- Nelissen, V.; Saha, B.K.; Ruysschaert, G.; Boeckx, P. Effect of different biochar and fertilizer types on N2O and NO emissions. Soil Biol. Biochem. 2014, 70, 244–255. [Google Scholar] [CrossRef]
- Zhou, G.-W.; Yang, X.-R.; Li, H.; Marshall, C.W.; Zheng, B.-X.; Yan, Y.; Su, J.-Q.; Zhu, Y.-G. Electron shuttles enhance anaerobic ammonium oxidation coupled to iron(III) reduction. Environ. Sci. Technol. 2016, 50, 9298–9307. [Google Scholar] [CrossRef]
- Wang, M.; Hu, R.; Zhao, J.; Kuzyakov, Y.; Liu, S. Iron oxidation affects nitrous oxide emissions via donating electrons to denitrification in paddy soils. Geoderma 2016, 271, 173–180. [Google Scholar] [CrossRef]
- Tiedje, J.M.; Sexstone, A.J.; Myrold, D.D.; Robinson, J.A. Denitrification: Ecological niches, competition and survival. Antonie Leeuwenhoek 1983, 48, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Case, S.D.C.; McNamara, N.P.; Reay, D.S.; Whitaker, J. Can biochar reduce soil greenhouse gas emissions from a Miscanthus bioenergy crop? GCB Bioenergy 2014, 6, 76–89. [Google Scholar] [CrossRef]
- Cooper, J.; Greenberg, I.; Ludwig, B.; Hippich, L.; Fischer, D.; Glaser, B.; Kaiser, M. Effect of biochar and compost on soil properties and organic matter in aggregate size fractions under field conditions. Agric. Ecosyst. Environ. 2020, 295, 106882. [Google Scholar] [CrossRef]
- Xiao, K.-Q.; Zhao, Y.; Liang, C.; Zhao, M.; Moore, O.W.; Otero-Fariña, A.; Zhu, Y.-G.; Johnson, K.; Peacock, C.L. Introducing the soil mineral carbon pump. Nat. Rev. Earth Environ. 2023, 4, 135–136. [Google Scholar] [CrossRef]
- Riaz, M.; Roohi, M.; Arif, M.S.; Hussain, Q.; Yasmeen, T.; Shahzad, T.; Shahzad, S.M.; Muhammad, H.F.; Arif, M.; Khalid, M. Corncob-derived biochar decelerates mineralization of native and added organic matter (AOM) in organic matter depleted alkaline soil. Geoderma 2017, 294, 19–28. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Zhou, F.; Zhang, X.; Luo, J.; Li, Y.; Lindsey, S.; Shi, Y.; He, H.; Zhang, X. Effects of maize residue return rate on nitrogen transformations and gaseous losses in an arable soil. Agric. Water Manag. 2019, 211, 132–141. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.; Deng, F.; Zou, X.; Ruan, H.; Chen, H.Y.H. Responses of soil microbial biomass, diversity and metabolic activity to biochar applications in managed poplar plantations on reclaimed coastal saline soil. Soil Use Manag. 2018, 34, 597–605. [Google Scholar] [CrossRef]
- Xie, M.; Lu, X.; Wang, H.; Fu, X.; Wang, L. Large-scale biochar incorporation does not necessarily promote the carbon sink of estuarine wetland soil. Sustainability 2023, 15, 16709. [Google Scholar] [CrossRef]
- Mielnik, L.; Hewelke, E.; Weber, J.; Oktaba, L.; Jonczak, J.; Podlasiński, M. Changes in the soil hydrophobicity and structure of humic substances in sandy soil taken out of cultivation. Agric. Ecosyst. Environ. 2021, 319, 107554. [Google Scholar] [CrossRef]
- Yang, X.; Li, R.; Li, Y.; Mazarji, M.; Wang, J.; Zhang, X.; Song, D.; Wang, Y.; Zhang, Z.; Yang, Y.; et al. Composting pig manure with nano-zero-valent iron amendment: Insights into the carbon cycle and balance. Bioresour. Technol. 2023, 371, 128615. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shao, C.; Wang, M.; Zhang, W.; Zhang, W.; Bai, H.; Zhang, J.; Mecha, C.A.; Li, R.-H. Mechanisms of integrated porous biochar and Fe3O4 in modulating carbon emissions and humification in co-composting of cattle manure and wheat stalk. Chem. Eng. J. 2025, 518, 164730. [Google Scholar] [CrossRef]
- Hu, J.; Huang, C.; Zhou, S.; Liu, X.; Dijkstra, F.A. Nitrogen addition increases microbial necromass in croplands and bacterial necromass in forests: A global meta-analysis. Soil Biol. Biochem. 2022, 165, 108500. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, X.; Ma, S.; Li, Y.; Zhu, M.; Zhang, W.; Li, J.; Liu, X.; Hu, G.; Wang, X.; et al. Soil microbial necromass regulation of long-term fertilizer N retention influenced by maize stover mulching. Geoderma 2023, 433, 116453. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, W.; Zhang, X.; Bao, X.; Xie, H.; Li, J.; He, H.; Liang, C.; Zhang, X. Dynamics of microbial necromass in response to reduced fertilizer application mediated by crop residue return. Soil Biol. Biochem. 2022, 165, 108512. [Google Scholar] [CrossRef]
- He, H.; Zhang, W.; Zhang, X.; Xie, H.; Zhuang, J. Temporal responses of soil microorganisms to substrate addition as indicated by amino sugar differentiation. Soil Biol. Biochem. 2011, 43, 1155–1161. [Google Scholar] [CrossRef]
- Wang, X. Study on the Carbon and Nitrogen Turnover and Stability Processes of Microbial Necromass in Temperate Forest Soils. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2020. [Google Scholar]
- Gao, X.; Tan, W.; Zhao, Y.; Wu, J.; Sun, Q.; Qi, H.; Xie, X.Y.; Wei, Z. Diversity in the mechanisms of humin formation during composting with different materials. Environ. Sci. Technol. 2019, 53, 3653–3662. [Google Scholar] [CrossRef]
- Jia, P.; Huang, Y.; Zhang, H.; Huang, Q.; Chen, J.; Feng, L.; Tuo, Y.; Yuan, L.; Xie, J. Variation of microbial necromass carbon and its potential relationship with humification during composting of chicken manure with and without biochar addition. Bioresour. Technol. 2024, 409, 131258. [Google Scholar] [CrossRef]
- He, H.; Liu, J.; Shu, Z.; Chen, Y.; Pan, Z.; Peng, C.; Wang, X.; Zhou, F.; Zhou, M.; Du, Z.; et al. Microbially driven iron cycling facilitates organic carbon accrual in decadal biochar-amended soil. Environ. Sci. Technol. 2024, 58, 12430–12440. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.; Ramanathan, A.L.; Bauddh, K.; Korstad, J. Humic substances: Structure, function and benefits for agroecosystems—A review. Pedosphere 2023, 33, 237–249. [Google Scholar] [CrossRef]
- Hu, J.; Wenming, Z.; Ahmed, S.; Wei, F.; Ling, S.; Jimei, S.; Lixia, W.; Wu, J. Properties of humus-like substances isolated from organic wastes and their effects on structures of soil humus by Fourier transform infrared spectroscopy. Arch. Agron. Soil Sci. 2021, 67, 1930–1943. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, B.; Zhao, L.; Zhao, C.; Yang, F. Biochar promotes compost humification by regulating bacterial and fungal communities. Front. Microbiol. 2024, 15, 1470930. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Peay, K.G.; Kennedy, P.G.; Talbot, J.M. Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Microbiol. 2016, 14, 434–447. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, X.; Chen, H.Y.H.; Delgado-Baquerizo, M.; Wang, C.; Zhang, C.; Ruan, H. Fungal necromass is reduced by intensive drought in subsoil but not in topsoil. Glob. Change Biol. 2023, 29, 7159–7172. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, K.; Yang, Y.; Gao, B.; Zheng, H. Effects of biochar on the accumulation of necromass-derived carbon, the physical protection and microbial mineralization of soil organic carbon. Crit. Rev. Environ. Sci. Technol. 2024, 54, 39–67. [Google Scholar] [CrossRef]
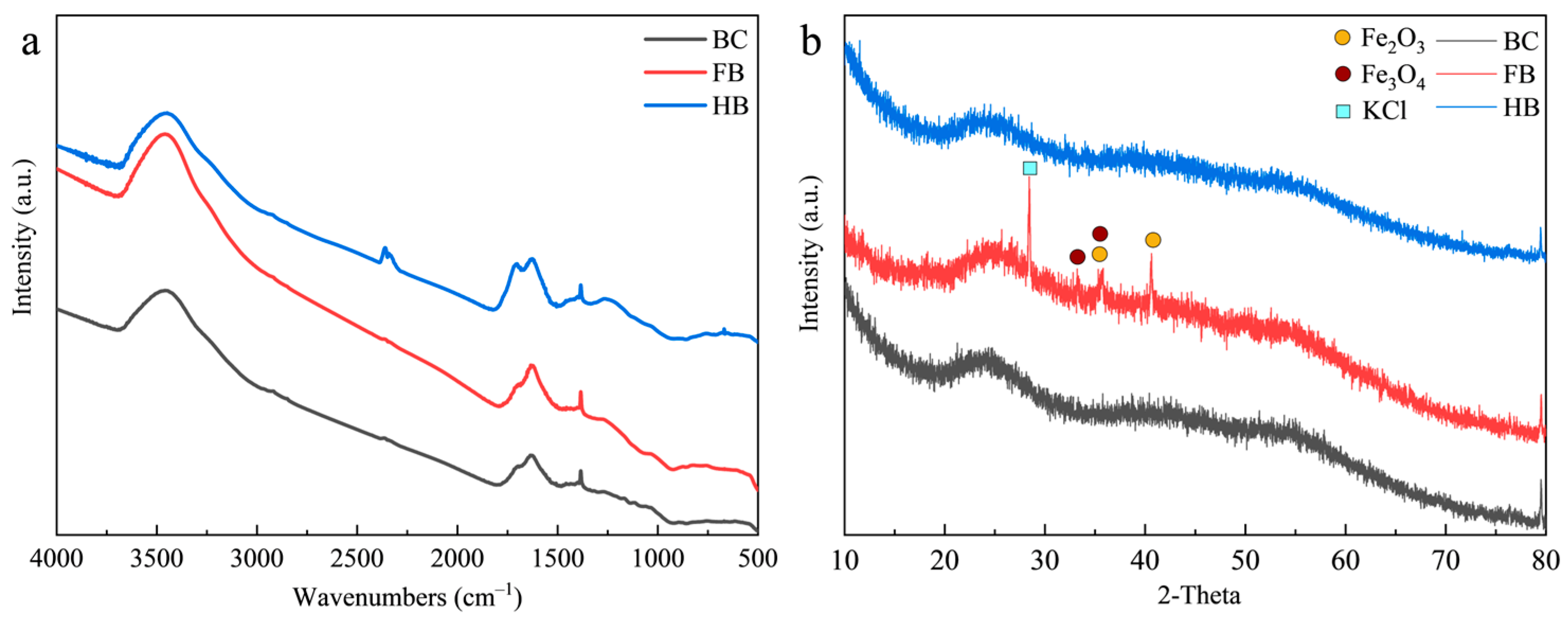

| Sample | C% | O% | N% | H% | (O+N)/C | H/C | pH | Electrical Conductivity (nS/cm) | Specific Surface Area (m2/g) |
|---|---|---|---|---|---|---|---|---|---|
| BC | 38.41 | 20.99 | 2.42 | 2.99 | 0.46 | 0.93 | 7.12 | 0.45 | 3.15 |
| FB | 34.79 | 18.46 | 2.26 | 2.49 | 0.45 | 0.86 | 3.04 | 1.34 | 3.81 |
| HB | 35.89 | 25.69 | 2.25 | 3.09 | 0.59 | 1.03 | 3.90 | 0.08 | 161.90 |
| GM | 23.47 | - | 8.49 | 5.02 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Zhang, L.; Chen, F.; Duan, W.; Li, F.; Zhang, D. Mechanism of Modified Biochar in Mitigating Carbon and Nitrogen Loss in Drought Soil with Green Manure Application. Agronomy 2025, 15, 2193. https://doi.org/10.3390/agronomy15092193
Zhu Z, Zhang L, Chen F, Duan W, Li F, Zhang D. Mechanism of Modified Biochar in Mitigating Carbon and Nitrogen Loss in Drought Soil with Green Manure Application. Agronomy. 2025; 15(9):2193. https://doi.org/10.3390/agronomy15092193
Chicago/Turabian StyleZhu, Ziyang, Lu Zhang, Fangyuan Chen, Wenyan Duan, Fangfang Li, and Di Zhang. 2025. "Mechanism of Modified Biochar in Mitigating Carbon and Nitrogen Loss in Drought Soil with Green Manure Application" Agronomy 15, no. 9: 2193. https://doi.org/10.3390/agronomy15092193
APA StyleZhu, Z., Zhang, L., Chen, F., Duan, W., Li, F., & Zhang, D. (2025). Mechanism of Modified Biochar in Mitigating Carbon and Nitrogen Loss in Drought Soil with Green Manure Application. Agronomy, 15(9), 2193. https://doi.org/10.3390/agronomy15092193






